Sevinch Siddiqova1* , Doniyor Azizov 2
, Doniyor Azizov 2 , Azamat Eshbekov1
, Azamat Eshbekov1 , Salixjan Maulyanov1
, Salixjan Maulyanov1 , Nilufar Elova3 and Aziza Azimova1
, Nilufar Elova3 and Aziza Azimova1
1Department. of Chemistry Natural compounds, National University of Uzbekistan, Uzbekistan, Tashkent
2Department of Pharmacy and Chemistry, Alfraganus University, Uzbekistan, Tashkent
3 Laboratory of Microbiology and biotechnology of probiotics, Institute of microbiology, Uzbekistan, Tashkent
Corresponding Author E-mail:sevinch.siddikova@inbox.ru
DOI : https://dx.doi.org/10.13005/bpj/3065
Abstract
The growing global population has increased the demand for food and created challenges in managing agricultural waste. Melon (Cucumis melo L.) peels, a byproduct of melon processing, hold potential as a source of biologically active compounds, including polysaccharides and phenolic acids, with antimicrobial, antioxidant, and prebiotic properties. This study aims to isolate and characterize polysaccharides from melon peels and evaluate their biological activities, providing a sustainable approach to waste utilization. Melon peel samples of the Torpedo variety were collected during the ripening period in Uzbekistan. Sequential extractions were performed using solvents (chloroform, ethanol, ammonium oxalate, and potassium hydroxide) to isolate water-soluble polysaccharides (WSPS), pectic substances (PS), and hemicellulose (HMC). Monosaccharide composition was analyzed using paper chromatography (PCh), gas chromatography (GC), and high-performance liquid chromatography (HPLC). Antimicrobial activity was assessed against opportunistic bacteria, and prebiotic activity was evaluated using Pediococcus acidilactici as a model strain. The extraction yielded 8.0 g of WSPS, 5.0 g of PS, and 2.0 g of HMC per 100 g of raw melon peel. Monosaccharide analysis revealed the presence of galactose, glucose, mannose, xylose, arabinose, and rhamnose. The antimicrobial assay demonstrated significant inhibition zones for pathogenic bacteria, suggesting strong antibacterial activity of melon peel polysaccharides. Additionally, prebiotic activity assays showed enhanced growth of Pediococcus acidilactici, indicating the potential of melon peel polysaccharides as prebiotic agents. The results highlight the potential of melon peel polysaccharides as functional biomolecules with antimicrobial and prebiotic properties. Their ability to inhibit bacterial growth and promote probiotic strains suggests applications in food preservation and gut health. Further studies on their structural characterization and bioactivity could enhance their utilization as natural additives or therapeutic agents, contributing to sustainable waste management and value addition in agriculture.
Keywords
Cucumis melo (Melon); Hemicellulose; Infrared (IR) Spectroscopy; Neutral Sugars; Pectic Substances; Uronic Acids; Water-Soluble Polysaccharides
Download this article as:| Copy the following to cite this article: Siddiqova S, Azizov D, Eshbekov A, Maulyanov S, Elova N, Azimova A. Polysaccharides of the Peel of Two Types of Сucumis Melo (Torpedo, Obi-Navvat) and Their Antimicrobial and Prebiotic Activity. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Siddiqova S, Azizov D, Eshbekov A, Maulyanov S, Elova N, Azimova A. Polysaccharides of the Peel of Two Types of Сucumis Melo (Torpedo, Obi-Navvat) and Their Antimicrobial and Prebiotic Activity. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3W15lKo |
Introduction
Melon (Cucumis melo) is a widely consumed fruit that generates significant agricultural by-products, including peels, which are often discarded as waste1. Recent studies have demonstrated that melon peels are rich in bioactive compounds such as polysaccharides, phenolic substances, and essential minerals, highlighting their potential for value-added applications in food, pharmaceutical, and nutraceutical industries2,3. Polysaccharides derived from plant by-products have gained considerable attention due to their diverse biological activities, including prebiotic, antioxidant, and antimicrobial properties. In particular, water-soluble polysaccharides (WSPS), pectin substances (PS), and hemicelluloses (HMC) are of interest for their structural diversity and functional potential4,5. However, the composition, structure, and bioactivity of polysaccharides from melon peels remain underexplored, especially in lesser-known varieties such as Torpedo and Obi-navvat. Despite the growing interest in sustainable utilization of agricultural by-products, there is a lack of comprehensive studies investigating the biochemical composition and biological activities of polysaccharides isolated from melon peels6,7. Understanding their structural properties, degree of esterification, and functional roles can provide insights into their potential applications as prebiotic agents or antimicrobial compounds. The present study aims to: Characterize the composition and structure of polysaccharides extracted from Torpedo melon peels, including alcohol-soluble sugars (ASP), water-soluble polysaccharides (WSPS), pectin substances (PS), and hemicelluloses (HMC). Analyze the degree of esterification and structural changes of pectin substances. Evaluate the biological activities of the isolated polysaccharides, focusing on their antibacterial and bifidogenic potential8,9. By addressing these objectives, this study seeks to provide a scientific basis for the utilization of melon peel-derived polysaccharides in industrial and therapeutic applications, contributing to the sustainable valorization of agricultural waste10.
Materials and Methods
Chemicals
All chemicals used were of analytical grade. Ethanol, methanol, sulfuric acid, and sodium hydroxide were obtained from Sigma-Aldrich. Monosaccharide standards (D-galacturonic acid, glucose, mannose, rhamnose) were purchased from Fluka. Bacterial strains (Serratia marcescens, Escherichia coli, Pseudomonas aeruginosa) and Pediococcus acidilactici were sourced from microbial culture collections. Double-distilled water was used for all experiments.
Bark of Cucumis melo was used To isolate the carbohydrate complex, the, collected during the ripening period: Torpedo in September 2023 in the Syrdarya area of the Republic of Uzbekistan. Chloroform: Analytical grade, used for inactivation of raw materials.Ethanol (96%): Analytical grade, used for primary extraction. Both ammonium oxalate and oxalic acid (0.5% solution): used for extraction of pectic substances. Potassium hydroxide (5%): used for hemicellulose. Sulfuric acid: used for hydrolysis of polysaccharides. Butanol-pyridine water 6:4:3 system: used for paper chromatography.
Technique for raw material inactivation. To get rid of coloring and low molecular weight components, 100 g of dried and crushed Cucumis melo raw materials were treated twice with boiling chloroform for an hour at a 1:4 hydromodule. After that, boiling 82° ethanol was used twice to extract alcohol-soluble sugars (1:4, 1:3). In a 6:4:3 butanol-n-pyridine-water system, alcohol extracts were separated by filtration, mixed, and evaporated to a small volume before being examined using paper chromatography (PCh). Hexose was identified using aniline acid phthalate (1), and ketosis was indicated using a 5% alcoholic solution of urea-hydrochloric acid (2).
WSPS isolation. The remaining raw material was extracted twice at a hydromodule of 1:4 using cold water at room temperature for one and a half hours. Filtration was used to separate the extracts, which were then somewhat evaporated and precipitated using three times as much ethyl alcohol. After centrifuging the precipitate for 10 minutes at 5000 ob/min, it was cleaned and dehydrated using alcohol. WSPS yields 8.0 g, while WSPS1 yields 7.7 g. The remaining raw material was then extracted twice using water at a hydromodule of 1:3, 1:2, and a temperature of 80–85 °C for 1.5 hours. Alcohol was used to mix, evaporate, and precipitate the extracts. The precipitate was handled as previously mentioned. WSPS1-h yields 1.4 g, while WSPS2-h yields 5.1 g. Pectin substances (PS) are isolated. Following the isolation of the GMC sum, the meal was extracted twice using a hydromodule of 1:4, 1:3, and an equal mixture of 0.5% solutions of oxalic acid and ammonium oxalate at 75 °C. Filtration was used to separate the extract, which was then dialized against running water, evaporated, and precipitated using three times as much alcohol. The precipitate was handled in the same manner as previously mentioned. From air-dry raw materials, 5.0 g of PS and 4.1 g of PS2 are produced.
HMC isolation. Following PS isolation, the remaining raw material was subjected to two treatments using a 1:3 hydromodule and a 5% KOH solution at room temperature for 1.5–2 hours. After filtering the KOH solution, the extracts were dialized to remove salts, neutralized with CH3COOH, evaporated to a density, and precipitated with three times the volume of alcohol. After centrifugation, the precipitated HMC precipitate was cleaned and dried with alcohol, yielding 2.0 g of HMC and 1.8 g of HMC1.
Pectic acid production. After dissolving 0.5 g of PS in 50 ml of 0.1 n. NaOH and allowing it to sit at room temperature for 6 hours, 0.3 n. HCl was added until full precipitation occurred. Centrifugation was used to separate the precipitate, which was then cleaned with alcohol and dried. The result is 0.31 grams.
Obtaining galacturonan. After dissolving 0.5 g of PS in 10 ml of water, 10 ml of 2 n. H2SO4 was added. The reaction mixture was cooled, the precipitate was separated by centrifugation, and it was cleaned and dried with ethyl alcohol. Hydrolysis was then conducted for two hours at 90 ° C. 0.18 g of galacturonan is the yield.
Full hydrolysis of polysaccharides in acid. The WSPS, PS, and HMC samples were hydrolyzed with 1 hour of H2SO4 at 100 °C for 8 hours and 2 hours of H2SO4 at 100 °C for 20 hours. Barium carbonate was used to neutralize the hydrolysates, followed by KU-2(H+) cationite deionization and evaporation. Using known witnesses on Filtrak FN-12 paper, PCh investigated the qualitative monosaccharide composition of PS in the butanol-pyridine water 6:4:3 system, developers 1, 2.
Polysaccharide antimicrobial action. Aqueous solutions of the polysaccharides extracted from melon were made at a concentration of 100 mg/ml prior to the operation commencing. For testing, conditionally pathogenic bacteria were cultivated on a nutrient medium for 24 hours at 37 °C in a thermostat. A 0.9% saline solution was diluted from the opportunistic microbe cultures that had developed till the turbidity index reached 107.
25 ml of dissolved nutrient medium was poured onto the horizontal surface of a Petri dish and dried in a thermostat. Then, a suspension of test microorganisms prepared on the surface of the nutrient medium using sterilized cotton swabs was planted by lubrication and depressions with a diameter of 6 mm were formed using a sterilized perforator. Solutions of the corresponding substances in the amount of 100 µl were poured into the recesses (before filling the recesses). + 40°C was kept for 1-2 hours, and then the opportunistic bacterium was incubated in a thermostat (36 ± 1)°C 24 hours. The zones limiting bacterial growth were measured using a ruler in diameter (mm)11-12.
Prebiotic activity of polysaccharides. A local strain of lactobacilli Pediococcus acidilactici was used to study the prebiotic activity. MRS broth (Hi-Media, India) serves as the lactobacilli’s nutritional medium. A sterile nutritional solution was mixed with the prebiotic under study. For 24 hours at 38 °C, 1 ml of the lactobacilli-grown inoculate was added to MRS broth for every 9.0 ml of nutritional medium. The strains were cultivated for 24 hours at 38 ± 1 °C. One milliliter of the culture medium’s live cell count (lg KOE/ml) was considered. Five iterations of the trials were conducted13.
GC analysis. Shimadzu GC-2010 chromatograph with flame ionization detector, Shimadzu Rxi-624Sil MS quartz capillary column (30mx0.25mmx1.40mkm), injector temperature 260°C, detector temperature 280°C, and column temperature 230°C were used to perform the GC analysis of the samples. Aldononitrile acetates were used to collect the samples14.
High-performance liquid chromatography (HPLC) was used to quantify the monosaccharide content of polysaccharides. RID-20A detector, 4.6×250 mm aminopropyl column, 75:25 acetonitrile water as the mobile phase, 1 ml/min, autostasis 20-40 µl, and 40 °C15. An IR Fourier spectrometer, System 2000 (Perkin-Elmer), was used to record the materials infrared spectra in potassium bromide tablets. There are one hundred scans. The titrimetric approach was used to determine PS’s degree of esterification16.
Statistics
Statistical significance between control and experimental groups was evaluated using a paired t-test for combined values and an unpaired t-test for separate comparisons. A significance threshold of P<0.001 was used to denote statistically significant differences.
Result
Alcohol-soluble sugars (ASP), water-soluble polysaccharides (WSPS), pectin compounds (PS), and hemicelluloses (HMC) are among the polysaccharides that we have successively separated from melon crusts. Glucose and fructose constitute the ASP according to the PCh (system 1, developer 1, 2). Two methods were used to isolate water-soluble polysaccharides (WSPS): first, raw materials were extracted using cold water (WSPS-c), which is room temperature, and second, hot water at a temperature of 80–90 °C (WSPS-h). A combination of 0.5% solutions of oxalic acid and ammonium oxalate, hemicellulose (HMC), and 5% KOH solution was used to isolate pectin components (PS). Table 1 displays the yield of polysaccharides along with the makeup of their monosaccharides.
Table 1: The combination of monosaccharides and the percentage of numerous polysaccharide groups in Cucumis Melo crusts n=4 p<0.001***
|
Type of melons |
Type of carbohydrates |
Yield, % |
The ratio of monosaccharides, GCh,HPLC |
||||||
|
Rha |
Ara |
Xyl |
Man |
Glu |
Gal |
UAc,% |
|||
|
Torpedо |
WSPS-c |
8,0 |
1,0 |
38,5 |
2,4 |
4,3 |
52,7 |
21,2 |
+ |
|
WSPS-h |
1,4 |
1,0 |
61,7 |
5,9 |
7,3 |
21,7 |
1,6 |
+ |
|
|
PS |
5,0 |
1,8 |
44,6 |
1,7 |
2,6 |
1,0 |
4,4 |
+ |
|
|
HMC |
2,0 |
6,0 |
80,0 |
6,0 |
3,0 |
1,0 |
3,0 |
+ |
|
|
Obi-navvat |
WSPS1-c |
7,7 |
16,0 |
15,7 |
3,8 |
41,0 |
9,5 |
14,0 |
+ |
|
WSPS1-h |
5,1 |
9,2 |
13,5 |
7,4 |
53,5 |
8,4 |
8,0 |
+ |
|
|
PS1 |
4,1 |
17,5 |
16,3 |
3,3 |
52,0 |
8,5 |
2,0 |
+ |
|
|
HMC1 |
1,8 |
23,0 |
17,0 |
5,5 |
35,5 |
9,3 |
9,6 |
+ |
|
As Table 1 demonstrates, WSPS and PS accumulate more in the peel of the Torpedo melon, their content is 8% and 5%, respectively. The monosaccharide compositions of WSPS-c and WSPS-h do not differ dramatically in their qualitative composition. but there are differences in the amount of individual monosaccharides. Upon analyzing the WSPS’s monosaccharide composition, it was discovered that the predominant monosaccharides were rhamnose (16%), WSPS1 mannose (41%), and glucose (52.7%) and arabinose.(Table 1). PS, PS 1 also differed in monosaccharide composition. The presence of arabinose (44.6%) and PS1 – mannose (52.0%) and rhamnose (17.5%) is observed in PS (Fig. 1,2).
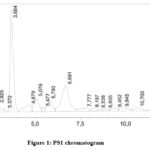 |
Figure 1: PS1 chromatogram
|
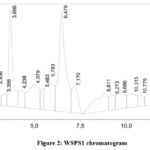 |
Figure 2. WSPS1 chromatogram
|
As can be seen from table 1, the amount of glucose is significantly higher in WSPS. This suggests that a high starch synthesis is observed in the peel of the Torpedo melon. Consequently, climatic conditions and the time of harvesting plants affect the synthesis of polysaccharides.
Table 2: Physico-chemical characteristics of pectin substances of Cucumis melo peel n=4 p<0.001***
|
Substance and variety of melon |
Number free COOH groups. % |
Number esterified COOH groups, % |
Total number of COOH groups,% |
Degree of esterefication,% |
|
PS (Torpedo) |
5,04 |
5,67 |
10,71 |
52,94 |
|
PS1 (Obi-navvat) |
3,9 |
4,6 |
8,5 |
54,11 |
Todetermine the degree of esterification, atitrimetricanalysis was performed,theresults of whichrevealed the content of carboxylicandesterifiedgroups:According to titrimetricanalysis, the content of free(Cs)carboxylicgroupsinPSis5.04and the content of esterified(Ce) is 5.67,while the degree of esterificationis52.94%,whichallows us to attribute the studiedPSto highly esterifiedpectins. Since PS1’s degree of esterification is 54.11% and its Cs and Ce amounts are 3.9 and 4.6%, respectively, PS1 is similarly greatly esterified. (Table2).
The isolated pectin substances belong to highly esterified pectins, however, the PS isolated from the Torpedo variety has a high number of free groups and a large number of methoxylated carboxyl groups17.
HMC is an amorphous powder of light cream color18-19,partiallysolubleinwater,completelyindilutealkalisolutions.As is known, the mainchain of pectinsubstancesconsistsofpolygalacturonans.To do this, weisolatedpecticacidand galacturonane fromPS.WhentreatingCucumismeloPS with alkali, saponification of methoxylgroupsoccursandpecticacid(62.0%) is formed,which, comparedwith the initialPS, has alreadylostsolubilityinwater.During the hydrolysis of PV(2hH2SO4,90°C,2h), apolysaccharidewith a yield of 36%-galacturonane,consistingonlyofD-galacturonicacidresidues, was obtained.
Table 3: Data from the IR spectra of PV, pectic acid and galacturonane. n=4 p<0.001
|
Frequency , sm–1 |
Existing fluctuations |
Frequency , sm–1 |
Existing fluctuations |
|
Pectic substances |
|||
|
3295 2934 1733 1630 1423 |
ν (ОН)с ν (СН)к ν (С=О)Е δ (Н2О) δas (CH3)Е |
1370 1144 1072 1047, 1013 952, 829, 755 |
δs (CH3)Е ν (C-O-C) ν, δ (C-OH)k ν (C-C), (C-O)K Triplet α-1,4-glycoside bond |
|
Pectic acid |
|||
|
3311 2929 1729 1329 1232
|
ν (ОН)с ν (СН)к ν (С=О)Е δ (CH)K δ (CH)K, δ (OH)C, δ (OH)Λ, ν (C-O-C)E |
1143 1095, 1046, 1012 910, 884, 831 791, 739, 625
|
ν (C-O-C) ν (C-C), (C-O)K α-1,4-glycoside triplet bond Pyranous ring vibrations |
|
Galacturonan |
|||
|
3332 2930 1727 1641 1412 1329 |
ν (ОН)с ν (СН)к ν (С=О)Е δ (Н2О) δas (CH)K δ (CH)K |
1225 1143 1073, 1011 948, 888, 881 738, 623, 531 |
δ (CH)K, δ (OH)C, δ (OH)Λ, ν (C-O-C)E ν (C-O-C) ν (C-C), (C-O)K α-1,4-glycoside triplet bond Pyranous ring vibrations |
Analysis of IR spectra (Table 3) PS, pectic acid and galacturonane showed the presence of a number of absorption bands inherent in pectin substances20. The most characteristic absorption bands should be noted, in particular, at 1727-1733 cm-1 – valence vibrations of the carbonyl of the carboxyl group21. In the IR spectrum of pectic acid and galacturonane, these bands were quite intense compared to those in the initial PS, since methyl groups were cleaved off, and the absorption band of 1370 cm-1, which exerts the presence of a methyl group, was completely absent. The absorption bands of the triplet in the region 828-835; 870-875 and 890-910 cm-1 were present in the spectra of these compounds, indicating the presence of an α-1,4-glycoside bond between the residues of D-galacturonic acid22.
When tested against bacteria including Escherichia coli 002673/477, Pseudomonas aeruginosa 003841/114, Stayphlococcus aerus, Bacillus subtilis BKM, Proteus mirabilis, Klebsiella oxitoca, and Serratia marcescens, the polysaccharides extracted from Torpedo melon did not exhibit antibacterial action.
Polysaccharides extracted from the examined melon, including WSPS-c Torpedo, have demonstrated an antibacterial activity against the conditionally pathogenic bacterium Serratia marcescens. The damaging microorganism’s growth inhibition zone reached 20 mm in diameter (Figure 3.)
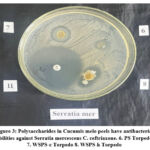 |
Figure 3: Polysaccharides in Cucumis melo peels have antibacterial abilities against Serratia mercescens C. ceftriaxone. 6. PS Torpedo 7. WSPS-c Torpedo 8. WSPS-h Torpedo
|
In vitro tests were used to examine the bifidogenic potential of water-soluble polysaccharides and pectin compounds found in two varieties of Cucumis melo peel (Obi-navvat and Torpedo). Pediococcus acidilactici, the manufacturing stock, was employed as a prebiotic. By assessing how carbohydrates affected the growth and reproduction dynamics of bifidobacteria grown in an in vitro MRC medium (Hi-Media, India), the bifidogenic activity of polysaccharides was investigated. Table 4 shows that, in comparison to the control, every chemical had an impact that stimulated the proliferation of bifidobacteria. Notably, PS, WSPS-c, and WSPS1-c were among the polysaccharides mentioned above that significantly aided in the growth of bifidobacteria. The amount of bifidobacteria in the 2% volume after 48 hours of growing on a PS-enriched medium was 1011, 1011, and 5×1010 CFU/g, respectively. This is higher than the control group (Table 4).
Table 4: Results of prebiotic activity of isolated polysaccharides
|
№ |
Polysaccharides |
Titer of live lactobacillus cells on the viability of Pediococcus acidilactici n=4 |
p-value (Significance) |
|
1 |
Sample |
109 |
Reference |
|
2 |
WSPS-c (Torpedo) |
1011 |
Significant vs Sample p<0.001 |
|
3 |
WSPS-h (Torpedo) |
108 |
Not significant vs Sample p=0.013 |
|
3 |
PS (Torpedo) |
1011 |
Significant vs Sample p<0.001 |
|
4 |
WSPS1-c (Obi-navvat) |
5 x 1010 |
Significant vs Sample p<0.001 |
|
5 |
PS1 (Obi-navvat) |
3 x 108 |
Not significant vs Sample p = 7.94 x 10-6 |
Discussion
The current study highlights the structural diversity, biochemical composition, and functional properties of polysaccharides isolated from the peels of Cucumis melo varieties, specifically Torpedo and Obi-navvat. These findings emphasize the significant potential of melon-derived polysaccharides for industrial and therapeutic applications.
Polysaccharide Composition and Yield
The extraction and analysis of alcohol-soluble sugars (ASP), water-soluble polysaccharides (WSPS), pectin substances (PS), and hemicelluloses (HMC) revealed notable differences in polysaccharide yield and monosaccharide composition between the melon varieties. Torpedo melon peels exhibited higher yields of WSPS and PS (8% and 5%, respectively) compared to Obi-navvat, with glucose identified as the predominant monosaccharide. This suggests a high rate of starch synthesis, potentially influenced by climatic conditions and harvest timing. The variation in monosaccharide composition, including the prevalence of rhamnose, mannose, and glucose, underscores the diversity of polysaccharides in melon peels and their adaptability to environmental factors.
Structural Properties of Pectin Substances
The degree of esterification (DE) analysis classified the pectin substances (PS and PS1) as highly esterified, with DE values exceeding 50%. The high number of methoxylated carboxyl groups and free carboxylic groups in Torpedo melon PS suggests enhanced functional properties. Saponification of PS yielded pectic acid, which exhibited reduced water solubility due to the removal of methoxyl groups. Hydrolysis of PS further confirmed the presence of D-galacturonic acid residues in galacturonane, reinforcing the structural integrity and bioactivity of these pectin derivatives.
The IR spectroscopic analysis supported these findings by identifying characteristic absorption bands associated with carboxyl and glycoside bonds. The absence of methyl group bands in pectic acid and galacturonane spectra validated the successful cleavage of methoxyl groups during chemical treatment. These results demonstrate the potential of pectin substances as functional biopolymers in various applications.
Biological Activities
Antibacterial Properties
The antibacterial activity of melon-derived polysaccharides was limited, with WSPS-c from Torpedo melon showing selective inhibition against Serratia marcescens. The inhibition zone of 20 mm indicates a promising avenue for further investigation into the mechanism of action and potential for targeted antimicrobial applications. However, the lack of activity against other bacterial strains suggests that these polysaccharides may have specific or narrow-spectrum bioactivity.
Prebiotic Potential
The bifidogenic activity of WSPS and PS was particularly notable, with significant stimulation of Pediococcus acidilactici growth in vitro. Polysaccharides such as PS, WSPS-c, and WSPS1-c demonstrated superior prebiotic effects, enhancing bifidobacteria proliferation to levels significantly higher than the control. This indicates their potential as functional ingredients in gut health formulations and probiotic-enriched food products. The ability of these polysaccharides to promote beneficial microbiota growth underscores their relevance in developing nutraceuticals for digestive health.
Industrial and Therapeutic Implications
The structural diversity, high degree of esterification, and selective bioactivity of melon-derived polysaccharides position them as valuable candidates for industrial and therapeutic applications. The prebiotic potential of these compounds offers opportunities for incorporating them into dietary supplements and functional foods, while their selective antibacterial activity could be harnessed for developing targeted antimicrobial agents. Additionally, the presence of glucose-rich polysaccharides highlights their potential as a source for starch-based products.
Future Perspectives
While this study provides a comprehensive characterization of melon-derived polysaccharides, further research is required to explore their structure-function relationships and in vivo efficacy. Optimizing extraction methods to enhance yield and bioactivity is essential for commercial viability. Moreover, investigating the interactions of these polysaccharides with other bioactive compounds could uncover novel applications in food, pharmaceuticals, and nutraceuticals. Expanding the scope of antibacterial and prebiotic testing to include additional strains and conditions will also provide deeper insights into their functional potential.
In conclusion, the polysaccharides isolated from Torpedo and Obi-navvat melon peels exhibit diverse structural and functional properties, making them promising candidates for a range of applications. This study lays the groundwork for further exploration of melon-derived polysaccharides as sustainable and versatile biopolymers.
Conclusion
The study successfully isolated alcohol-soluble sugars, water-soluble polysaccharides, highly esterified pectin substances, and hemicelluloses from Cucumis melo peels, providing a comprehensive overview of their qualitative and quantitative characteristics. Analysis via IR spectroscopy further revealed the structural details of the isolated polysaccharides. Despite the polysaccharides’ lack of antibacterial efficacy against the seven conditionally harmful microorganisms that were tested, certain substances, such as WSPS-c Torpedo, demonstrated antimicrobial effects against Serratia marcescens, highlighting the strain-dependent nature of antimicrobial activity. Additionally, the prebiotic potential of these polysaccharides was confirmed, suggesting their promising role in the development of functional food products and biologically active food additives. This study lays the groundwork for further exploration into the application of melon-derived polysaccharides in the food and health industries.
Acknowledgement
The work was done by PhD student in order to defense. We would like to thank for E.N. Arashovna and “Microbiology and biotechnology of probiotics” laboratory and R.K.Rakhmanberdiyva , M.Kh.Malikova, D.Sh.Azizova and “Chemistry of high molecular weight plant substances” laboratory for their contribution.
Conflict of interest
The authors do not have any conflict of interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author Contributions
Sevinch Siddiqova Conceptualization, methodology, investigation, and writing the original draft. Doniyor Azizov methodology, data analysis and editing. Azamat Eshbekov investigation, manuscript preparation. Salixjan Maulyanov supervision and resources. Nilufar Elova investigation and interpretation. Aziza Azimova data management.
References
- Rahim, E. F. A., Leong, S. S., Sarbini, S. R., Latif, K., & Malahubban, M. Banana (Musa acuminata), orange (Citrus reticulata), and watermelon (Citrullus lanatus) shells as prebiotic. Borneo Journal of Resource Science and Technology, 2022, 12(1), 81–94.
CrossRef - Хакимов, Р. А., & Халимова, М. У. Селекция дыни на устойчивость к болезням в Республике Узбекистан. Овощи России, 2022, 4, 28–32.
- Yang, W., Yang, Z., Zou, Y., Sun, X., & Huang, G. Extraction and deproteinization process of polysaccharide from purple sweet potato. Chem. Biol. Drug. Diss., 2022, 99, 111–117.
CrossRef - Мавлянова, Р., Рустамов, А., Хакимов, Р., Хакимов, А., Турдиева, М., & Падулоси, С. Дыни Узбекистана. Субрегиональный офис IPGRI для Центральной Азии, 2005, Ташкент, 13–24.
- Parle, M. Musk melon is eat-must melon. International Research Journal of Pharmacy, 2021, 2(8), 52–57.
- Silva, M. A., Albuquerque, T. G., Alves, R. C., Oliveira, M. B. P. P., & Costa, H. S. Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods. Trends in Food Science & Technology, 2020, 98, 181–189.
CrossRef - Morais, D. R., Rotta, E. M., Sargi, S. C., Bonafé, E. G. Proximate composition, mineral contents, and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. Journal of the Brazilian Chemical Society, 2016, 28(2), 308–318.
CrossRef - El-Tantawy, M. E., Haggag, E. G., Kamal, A. M., & Lithy, R. M. Phytochemical and biological evaluation of banana, cantaloupe, and guava waste parts. Journal of Pharmacy Research, 2006, 10(5), 308–318.
- Liu, Y., Huang, G., & Hu, J. Extraction, characterization and antioxidant activity of polysaccharides from Chinese watermelon. International Journal of Biological Macromolecules, 2018.
CrossRef - Zoirovich OS, Ugli AI, Raxmatillayevich ID. The effect of Ájuga Turkestánica on the rat aortic smooth muscle ion channels[J]. Biomed Pharmacol J, 2024, 17(2).
CrossRef - Abdullaev AA, Inamjanov DR, Abduazimova DS. Sílybum Mariánum’s impact on physiological alterations and oxidative stress in diabetic rats[J]. Biomed Pharmacol J, 2024, 17(2).
CrossRef - Лысак, В. В. Микробиология: учеб. пособие. Минск: БГУ, 2007. 000 с. ISBN 985-485-709-3.
CrossRef - Islamova, Z. I., Ogay, D. K., Abramenko, O. I., Lim, A. L., Abduazimov, B. B., Malikova, M. Kh., Rakhmanberdiyva, R. K., Khushbaktova, Z. A., & Syrov, V. N. Comparative assessment of the prebiotic activity of some pectin polysaccharides. Chemical and Pharmaceutical Journal, India, 2017, 51, 41–44.
CrossRef - Erkulov, Z. E., Malikova, M. K., & Rakhmanberdyeva, R. K. Carbohydrates from the aerial part of Ferula kuhistanica and F. tenuisecta. Chem. Nat. Compd., 2011, 47, 182–184.
CrossRef - Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. Q., & Smith, F. Anal. Chem., 1956, 28(3), 50–56.
CrossRef - Филлипов, М. Х. Инфракрасные спектры пектиновых веществ. Щтиинца, Кишинев, 1978, 14–22.
- Rakhmanberdyeva, R. K., & Filippov, M. P. Infrared spectroscopy study of Gleditsia macracantha seeds. Chem. Nat. Compd., 2011, 47, 179–181.
CrossRef - Fonteles, T. V., Costa, M. G. M., Tibério de Jesus, A. L., Fontes, C. P. M. L., Fernandes, F. A. N., & Rodrigues, S. Stability and quality parameters of probiotic cantaloupe melon juice produced with sonicated juice. Food and Bioprocess Technology, 2013, 6(10), 2860–2869.
CrossRef - Цзи, С. Л., Хоу, С. И., Ши, М. М., Ян, Ю. З., & Лю, Ю. К. Экскурсия в исследования женьшеня. Food Rev, 2022, 38(6), 49–65.
- Азизова, Д. Ш., Рахманбердиева, Р. К., Маликова, М. Х., Эшбеков, А. Э. Углеводный состав надземной части Solanum tuberosum L. Фарм. Журнал, 2023, 3, 6–11.
- Маликова, М. Х., Ахмедова, X. X., Рахманбердыева, Р. К., Жаунбаева, К. С. Пектиновые вещества Ferula kuhistanica и F. tenuisecta. Хим. природ. соед., 2018, 1, 13–15.
- Simpson, R., & Morris, G. A. The antidiabetic potential of polysaccharides extracted from members of the cucurbit family. Bioactive Carbohydrates and Dietary Fibre, 2014, 3(2), 106–114.
CrossRef








