Fairuz Fatema Priya 1, 2 , Abu Zobayed 1
, Abu Zobayed 1 , Md. Abu Sayeed 1
, Md. Abu Sayeed 1 , Md. Mizanur Rahman Moghal1*
, Md. Mizanur Rahman Moghal1*
1Department of Pharmacy, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh.
2Department of Pharmacy, City University, Khagan, Birulia, Savar, Dhaka, Bangladesh.
Corresponding Author E-mail: mizan.phar@mbstu.ac.bd
DOI : https://dx.doi.org/10.13005/bpj/3068
Abstract
Curcuma zedoaria (Christm.) Roscoe, a traditional herb used to treat helminthiasis, cardiovascular issues, and cancer, lacks scientific evidence to support its therapeutic value. This study intended to explore the scientific rationale for its traditional usage, by phytochemical assessment and pharmacological evaluation of its methanolic extract (ME). The phytochemical analysis of ME was performed using conventional approaches, for qualitative detection. In vitro antioxidant capacity was then measured using DPPH free radical scavenging assay, and the percentage of inhibition (IC50) of both standard ascorbic acid (AA) and ME was calculated. The anthelmintic activity was investigated at doses 50, 100, and 150 mg/ml by comparing the effects of ME and albendazole on the paralysis and death of earthworms. Furthermore, cyclophosphamide (CP) induced cardiotoxic rats were used to evaluate the in vivo cardioprotective ability of ME at doses of 150 and 300 mg/kg body weight. Subsequently, serum cardiac biomarkers including aspartate aminotransferase, creatine kinase-MB, lactate dehydrogenase, as well as lipid profiles (triglycerides, and total cholesterols) were assessed. The phytochemicals assessment demonstrated the diversity of compounds' presence. The antioxidant assay demonstrated a significant (p<0.01) dose-response relationship of ME (IC50 33.132 μg/ml) compared to AA (IC50 20.276 μg/ml). Besides, the anthelmintic study thereafter revealed a significant (p<0.01) dose-dependent paralysis (2.824 ± 0.037 minutes) and mortality (3.732 ± 0.031 min.) of worms at 50 mg/ml dose compared to the standard control. Furthermore, the cardioprotective indicators and lipid profiles which CP elevated, have significantly (p<0.001) returned to nearly normal levels in serum due to the ME treatment at both 150 and 300 mg/kg doses. In addition, rats’ CP intoxicated heart weight (0.904 ± 0.019 gm) was significantly (p<0.01) reduced (0.860 ± 0.016 gm) by ME at 150 mg/kg dose. Therefore, C. zedoaria rhizome possesses powerful antioxidant, anthelmintic, and cardioprotective efficacy. The outcomes paved the way for more exploration to develop novel therapies from this herb.
Keywords
Anthelmintic; cardioprotective; Curcuma zedoaria; methanolic extract; phytochemicals
Download this article as:| Copy the following to cite this article: Priya F. F, Zobayed A, Sayeed M. A, Moghal M. M. R. Phytochemical Assessment and Pharmacological Evaluation of Curcuma zedoaria (Christm.) Roscoe Methanolic Extract – Preliminary Study. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Priya F. F, Zobayed A, Sayeed M. A, Moghal M. M. R. Phytochemical Assessment and Pharmacological Evaluation of Curcuma zedoaria (Christm.) Roscoe Methanolic Extract – Preliminary Study. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4gsY665 |
Introduction
The significance of plants as reservoirs of medicinal substances has been widely recognized throughout history, especially in the context of traditional medicine in various civilizations. Usually, the bioactive components synthesized during the secondary metabolism of the plants are considered the source of their therapeutic potential.1,2 According to the World Health Organisation (WHO), a substantial number of people in countries like Bangladesh, Burma, and India rely on medicinal plants to preserve their health and treat a variety of disorders.3 Additionally, it has become more crucial due to the side effects of various modern medications and the absence of effective remedies for several emerging disorders.4 However, using phytomedicines to treat numerous complications has been demonstrated to be very safe and have minimal to no adverse effects.5
Nowadays, numerous life-threatening ailments are plaguing people worldwide. Cardiovascular diseases are not only the world’s predominant cause of mortality, but they also significantly deteriorate health and drive up the expense of medical care.6 A large proportion of these deaths take place in developing countries like Bangladesh, whose populations are severely affected.7 The underlying risk factors encompass hypertension, dyslipidemia, diabetes, obesity, sedentary behavior, advanced age, and smoking.8 Besides, owing to the noteworthy rate of chemotherapy resistance, cancer is still the second foremost reason for deaths around the world.9 Fourteen million new cases of cancer were reported in 2012; by 2035, that number is predicted to rise to nearly 24 million new cases worldwide which directs a rapid growth in the cancer burden.10 Additionally, one of the most prevalent health issues worldwide, especially in underdeveloped states, is infection with intestinal parasites. It still poses a serious threat to public health, mostly in developing countries, and wreaks havoc on vulnerable rural populations’ socioeconomic conditions.11 Therefore, the demand for more advanced therapies and/or technologies to combat these hurdles is continuously growing. Moreover, exploring medicinal plants is becoming more popular as well since using synthetic drugs improperly can lead to resistance and other complications.
Curcuma zedoaria (Christm.) Roscoe rhizome or white turmeric, a perennial herb of the Zingiberaceae family, has a long-standing traditional usage in multiple regions such as India, Bangladesh, Indonesia, China, Vietnam, Malaysia, and Japan.12 Globally, there are around 133 species in the genus Curcuma, and herbs of this genus are mostly inhabited in Australia, Brazil, and Southeastern Asia.13,14 Asians have traditionally utilized this rhizome as a carminative and to cure arthritis, digestive disorders, liver diseases, anorexia, helminthiasis, gastritis, stroke, cardiovascular complications, and even cancer.15-17 Contrary to some other species in this genus that have received continual focus, C. zedoaria was attracting more attention because of its traditional use and some newly revealed biological properties. Still, there is a dearth of research regarding the antioxidant, anthelmintic, and cardioprotective activities. Although the traditional uses are well recorded, scientific data supporting its medicinal abilities remains scarce. To bridge this knowledge gap, this study embarks on an ethnopharmacological journey, exploring the diverse pharmacological properties of this easily accessible herb. The primary objective was preserving and documenting vital cultural knowledge before it disappeared into obscurity. Additionally, we intended to evaluate the antioxidant, anthelmintic, and cardioprotective potential of C. zedoaria rhizome along with phytochemical assessment to provide scientific evidence of the herb’s traditional applications and support future research for novel therapies.
Materials and Methods
Collection and preparation of plant materials
Having collected from Bangladesh’s Tangail District, the rhizome of C. zedoaria was verified and recognized by the National Herbarium, Dhaka, Bangladesh (Accession number: DACB87211). We collected 6 kg of C. zedoaria rhizome and removed undesirable components. They were then carefully cleaned with double-distilled water, chopped into tiny pieces, and let to shed dry for a week. The dried rhizomes were further crushed into fine particles using a suitable grinder.
Preparation of methanolic extract
Crushed material (rhizome) weighing 500 g was soaked with 1500 ml of methanol (95%) in a flat-bottomed fresh glass vessel, firmly sealed, and left for 21 days with infrequent shaking and stirring. The entire mixture was filtered through a sterilized piece of white cotton material and then run through Whatman (Grade 2) filter paper.18 A sticky black concentrate obtained was designated as methanolic extract (ME) after the resulting filtrate was allowed to evaporate in normal conditions. Then the yield value (%) was calculated by Equation 1.
Yield (g/100 g) = (W1×100)/W2 ………………..…….. (1)
Notes: W1=Weight of the extract residue (ME) obtained after solvent elimination, W2=Weight of powder taken for extract preparation
In the study, the % of yield = (11×100)/500
= 2.2%
Phytochemical assessment
Using conventional approaches, the primary phytochemical assessment was carried out for the qualitative detection of alkaloids, flavonoids, polyphenols, tannins, saponins, carbohydrates, proteins, amino acids, terpenoids, and phytosterols. An analytical response to these qualitative investigations was the color intensity or precipitate formation.19
Antioxidant activity assay
Through slightly modified protocols, the extract’s in vitro antioxidant capacity was assessed using the DPPH free radical scavenging test.20,21 0.004% DPPH solution was utilized as the control, and the concentration levels of ME and standard ascorbic acid (AA) were 500, 400, 200, 100, 50, 25, 12.5, and 6.25 μg/ml. Two ml of 0.004% DPPH solution was added with two ml of ME and AA respectively followed by proper mixing and allowed to complete the reaction by incubating for 30 minutes. Afterward, the percentage of inhibition (IC50) of ME and the standard was calculated by measuring the solution’s absorbance at 517 nm in a UV-Vis Spectrophotometer. Concentration was determined by Equation 2, which was then plotted against the percentage of inhibition to estimate the IC50.
Concentration = {(A0-A1)/A0} ×100 …………………. (2)
Notes: A0 = Absorbance of the control, A1 = Absorbance of the ME/AA
Anthelmintic activity assay
With minor adjustments, the reported procedure was used to conduct the in vitro anthelmintic assay.22 This study employed adult earthworms (Pheretima posthuma) owing to their physiological and anatomical resemblance to the human abdominal roundworm parasite. They were split up into nine groups, each with five worms, and the first three groups (I, II, and III) were used as controls and given normal saline water. Furthermore, Albendazole (standard) and ME were administered to Groups (IV, V, VI) and Groups (VI, VIII, IX) at doses of 50, 100, and 150 mg/ml, respectively. The parasites were frequently observed for their spontaneous movement and evoked responses, immediately after incubation, which lasted for 130 minutes in total. Worm’s mortality or paralysis was detected by the inhibitions of their movement. Paralysis was defined as the worms’ inability to move after being violently shocked; whereas death time was established by their failure to move after being vigorously shaken or submerged in warm water (50°C). In this assay, the earthworm parasites’ paralysis and death were visually observed with the naked eye.
Animal selection
This study involved male Long Evans rats (age 9 weeks), weighing 160-200 gm, obtained from the International Centre for Diarrheal Disease Research, Bangladesh (ICDDRB). Before the experiment, they were adopted for seven days under standard conditions (25±2°C) and humidity with a regular 12-hour light/dark cycle and allowed free access to normal food and water.23
Cardioprotective activity test
We evaluated ME’s in vivo cardioprotective ability in cyclophosphamide (CP) induced acute cardiotoxic rats by slightly altering published protocols.24,25 However, this preliminary in vivo study aimed to compare biomarker levels of different animal groups with their normal physiological conditions without using a conventional standard. For this study, earlier approval was taken from the institutional ethical review committee that oversees animal experimentation (Ref: MBSTU/ERC/ECC/157/2024/9). In all, 20 male Long Evans rats were divided into four distinct groups of 5 animals each, and every group received the usual diet along with the following treatment orally for thirteen consecutive days. The first group, which served as the normal control, received oral saline one ml/kg body weight (BW) whereas the second group received CP 150 mg/kg BW intraperitoneally during trial days 9th through 12th. Furthermore, animals in the third and fourth groups received ME orally for eight days at doses of 150 (D1), and 300 (D2) mg/kg BW respectively. Afterward, CP (150 mg/kg BW) was administered intraperitoneally to both groups on the 9th to 12th day of the experiment. The animals were sacrificed on day 13th after a 24-hour period. Following their dissection, blood was drawn from the heart’s vein to measure the serum levels of creatine kinase-MB (CK-MB), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), troponin I, and lipid profiles such as total cholesterol (TC), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), & Triglycerides (TGs). Furthermore, their heart weights were also recorded.
Statistical analysis
One way analysis of variance (ANOVA) and Dunnett’s t-tests using SPSS software were employed for statistical analysis. Values are expressed as mean ± standard error of the mean (SEM). With Microsoft Excel, the logarithmic equation of the graph was utilized to determine the median inhibitory concentration (IC50) of the test samples.
Results and Discussion
Phytochemical assessment
The preliminary phytochemical analysis revealed various constituents present in the ME of C. zedoaria rhizome (Table 1). Among these, multiple tests have identified the presence of alkaloids, carbohydrates, and flavonoids.
Table 1. Phytochemical constituents of C. zedoaria ME.
|
Phytochemicals |
Name of the Test |
ME |
|
Alkaloids |
a) Mayer |
+ |
|
b) Wagner |
+ |
|
|
c) Dragendorff |
+ |
|
|
Carbohydrates |
a) Molisch |
+ |
|
b) Benedict |
+ |
|
|
c) Fehling |
+ |
|
|
Saponins |
a) Foam |
+ |
|
Terpenoids |
a) Salkowski |
+ |
|
Phytosterols |
a) Liebermann-Burchard |
+ |
|
Polyphenols |
a) Ferric Chloride |
+ |
|
Tannins |
a) Ferric Chloride |
+ |
|
Flavonoids |
a) Alkaline Reagent |
+ |
|
b) Lead Acetate |
+ |
|
|
Proteins and Amino Acids |
a) Xanthoproteic |
+ |
Notes: (+) = Present and (-) = Absent of Phytochemicals
Antioxidant activity assay
The antioxidant capacity of the ME has been expressed as a median inhibitory concentration (IC50) which represents the dose of sample needed to inhibit 50% of the DPPH free radical after a certain exposure time. The extract’s potential as an antioxidant increases with the percentage of its scavenging action. As compared to reference standard AA (IC50 20.276 μg/ml), the ME (IC50 33.132 μg/ml) exhibited a significant (p<0.01) antioxidant capability in this investigation (Table 2, Table 3).
Table 2: DPPH free radical scavenging ability (IC50 value) of AA.
|
Concentration (μg/ml) |
Absorbance of AA (Mean± SEM) |
Absorbance of Blank |
% of Inhibition |
IC50 (μg/ml) |
|
500 |
0.023± 0.00231 |
0.586 |
96.075 |
20.276 |
|
400 |
0.042± 0.00233 |
0.586 |
92.833 |
|
|
200 |
0.113 ± 0.00260 |
0.586 |
80.717 |
|
|
100 |
0.164 ± 0.00173 |
0.586 |
72.014 |
|
|
50 |
0.227 ± 0.00231 |
0.586 |
61.263 |
|
|
25 |
0.277 ± 0.00231 |
0.586 |
52.730 |
|
|
12.5 |
0.338± 0.00410 |
0.586 |
42.321 |
|
|
6.25 |
0.378± 0.00353 |
0.586 |
35.495 |
Table 3: DPPH free radical scavenging ability (IC50 value) of ME.
|
Concentration (μg/ml) |
Absorbance of ME (Mean± SEM) |
Absorbance of Blank |
% of Inhibition |
IC50 (μg/ml) |
|
500 |
0.058 ± 0.00231a*** |
0.586 |
90.102 |
33.132 |
|
400 |
0.072 ± 0.00233a*** |
0.586 |
87.713 |
|
|
200 |
0.123 ± 0.00233a* |
0.586 |
79.010 |
|
|
100 |
0.179 ± 0.00260a** |
0.586 |
69.454 |
|
|
50 |
0.233 ± 0.00260 ns |
0.586 |
60.239 |
|
|
25 |
0.303 ± 0.00393a** |
0.586 |
48.294 |
|
|
12.5 |
0.377 ± 0.00296a*** |
0.586 |
35.666 |
|
|
6.25 |
0.480 ± 0.00463a*** |
0.586 |
18.089 |
Notes: a Compared the absorbance of ME with AA; ns=Not significant; Values are expressed as Mean ± SEM (n=3). Three thresholds of P values are used. *p<0.05, **p<0.01, ***p<0.001 significant when compared with the corresponding value of the standard group.
Anthelmintic activity assay
In this experiment, the earthworms died with regular saline after more than 130 minutes, and the test was conducted up to that point. The death time of worms by ME at doses 50, 100, and 150 mg/ml were 3.732 ± 0.031, 2.950 ± 0.107, 2.041 ± 0.064 minutes respectively. Furthermore, the death times were 3.562 ± 0.040, 2.803 ± 0.137, and 2.027 ± 0.083 minutes, respectively, at the same doses of the conventional drug Albendazole (standard). The anthelmintic activity of each group that received ME was compared to the standard. Regarding the paralysis and death times, the ME showed (Fig. 1) an impressive dose-dependent activity.
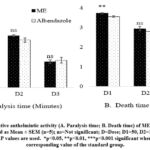 |
Figure 1: Comparative anthelmintic activity (A. Paralysis time; B. Death time) of ME & albendazole. All values are expressed as Mean ± SEM (n=5); ns=Not significant; D=Dose; D1=50, D2=100, D3=150 mg/ml. |
Cardioprotective activity test
This experiment used biomarkers to correlate outcomes from three distinct groups of animals in their normal, diseased, and test treatment conditions without using a standard drug. Since we attempted to see how the values varied with ME therapy. Therefore, we assessed the degree of protection provided by ME against CP-induced cardiotoxic rats at 150, and 300 mg/kg BW doses using the aforementioned markers (CK-MB, AST, LDH, Troponin I), lipid profile, and heart weight. CP administration resulted in a decline in body weight and an rise in heart weight, which was a measure of relative heart weight elevation, however, these issues have been resolved by the ME therapy (Table 4). Additionally, ME treatment caused a dose-dependent reduction (p<0.001) in serum biomarkers (CK-MB, AST, LDH, & Troponin I) and lipid indicators (TC, TGs, & LDL-C) that had been significantly (p<0.001) elevated during CP intoxication (Fig. 2, Fig. 3).
Table 4: Effect of ME on the body, heart, and relative heart weight.
|
Groups |
Initial body weight (gm) |
Final body weight (gm) |
Heart weight (gm) |
Relative heart weight (%) |
|
Control |
174.260 ± 4.201 |
175.768 ± 3.208 |
0.638 ± 0.035 |
0.393 ± 0.023 |
|
CP Treated |
174.732 ± 1.710 |
159.950 ± 2.874a* |
0.904 ± 0.019a*** |
0.509 ± 0.004a*** |
|
CP+ME (D1) |
179.362 ± 1.406 |
180.474 ± 2.500 b** a ns |
0.860 ± 0.016 a*** b ns |
0.477 ± 0.011 a** b ns |
|
CP+ME (D2) |
178.716 ± 2.55 |
178.244 ± 4.333b** |
0.800 ± 0.026a**b ns |
0.419 ± 0.009 a ns b** |
Notes: aCompared CP treated group & CP+ME pre-treated group with the normal control group; bCompared CP treated group with CP+ME pre-treated group; ns =Not significant; Values are expressed as Mean ± SEM (n=3). Three thresholds of P values are used. *p<0.05, **p<0.01, ***p<0.001 significant when compared with the corresponding value of the standard group.
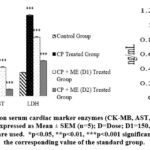 |
Figure 2: Effect of ME on serum cardiac marker enzymes (CK-MB, AST, LDH, and Troponin I, Level). All values are expressed as Mean ± SEM (n=5); D=Dose; D1=150, D2=300 mg/kg. |
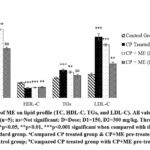 |
Figure 3: Effect of ME on lipid profile (TC, HDL-C, TGs, and LDL-C). All values are expressed as Mean ± SEM (n=5); ns=Not significant; D=Dose; D1=150, D2=300 mg/kg. Three thresholds of P values are used. |
Medicinal floras are presumed to be a promising supplier of novel compounds. Published literature has mentioned that the Curcuma herbs are found to possess a diverse range of primary and secondary metabolites. Accordingly, our phytochemical analysis has also confirmed the existence of alkaloids, carbohydrates, flavonoids, tannins, phytosterols, polyphenols, saponins, terpenoids, proteins, and amino acids in C. zedoaria ME. Meanwhile, a key sign of medicinal plants’ ability to have beneficial effects is their flavonoid content. The antioxidant activity assay of this study showed that at a dose of 500 μg/ml, ME possesses the strongest capacity to scavenge free radicals and has a 90.102% inhibition, compared to 96.075% for standard AA at the same dose (Table 2, Table 3). The outcome therefore indicates the significant (p<0.01) dose-dependent antioxidant properties of ME (Table 3), and the components, especially flavonoids and polyphenols, found in the ME may be accountable for the scavenging action. Similarly, published study revealed that ME of C. zedoaria had an intense scavenging effect that was significantly stronger in comparison to other solvent extracts.26 The hydrogen atoms from several hydroxyl groups in polyphenols’ structures may interact with the free radical DPPH resulting in an antioxidant response.27 Further exploration is necessary since it is unclear which particular compounds have the mode of action as antioxidants.
The current investigation revealed that ME has substantial dose-dependent anthelmintic activity since the paralysis and death times of the parasites are mostly near to the reference drug Albendazole (Fig. 1). Reported studies claimed the significant anthelmintic activities of other Curcuma species notably C. longa, C. amada, C. caesia, and C. aromatic.28-30 But as far as we are aware, this is the first in vitro study that confirms the anthelmintic activity of C. zedoaria crude ME against earthworms (Pheretima posthuma). Acetylcholinesterase (AChE), a cholinergic enzyme predominantly found at postsynaptic neuromuscular junctions, has the principal physiological role is halting transmission at cholinergic synapses by rapid hydrolysis of the neurotransmitter acetylcholine. Desensitization of the muscle receptor caused by the blocking AChE leads to paralysis and eventually death.31,32 Cholinergic antagonists, such as monoterpenes and sesquiterpenes, impede the contraction of worm muscles and can induce the aforementioned effects.33,34 Moreover, the effect of polyphenols as AChE inhibitors was documented in some research.35 The early phytochemical assessment of C. zedoaria revealed the existence of alkaloids, polyphenols, flavonoids, and terpenoids in the ME, which can be suspected to have anthelmintic effects. More studies therefore needed to clarify which specific component affects nematodes in this way.
In this study, we attempted to ascertain the degree of protection provided by ME against CP-intoxicated rat hearts and lipid profiles at different dosages. CP is a cardiotoxic agent that can destroy myocardial cells, consequently, cardiac tissue damage can diagnosed by the release of CK-MB, AST, LDH, and Troponin I into the circulation.36 As expected, the findings (Table 4) also displayed that CP administration significantly (p<0.001) raised heart weight, lipid profiles, and serum cardiac biomarkers (CK-MB, AST, LDH, and Troponin I) (Fig. 2, Fig. 3). Myofibrillar degeneration, sarcoplasmic reticulum enlargement, myocyte disruption, cytoplasmic vacuolization, and fibrosis are possible effects of CP-induced cardiotoxicity which may be responsible for this increase in heart weight.37 However, in this study the ME-pretreated rats had significantly (p<0.01) lower relative heart weight compared to the positive control (Table 4) in a dose-dependent fashion. The elevated serum cardiac biomarker enzyme levels in CP-treated rats are possibly linked to the overproduction of reactive oxygen species (ROS) which damages the myocardial membranes through lipid peroxidation thus losing integrity and functionality.38 As stated by reported assay, acrolein, CP’s toxic metabolite, is also accountable for this complication.39 Furthermore, the higher plasma levels of TC, LDL-C, and TGs in the CP-administered group might be explained by the drug-induced boost in their biosynthesis, and suppression of usage. Especially the enzyme lipoprotein lipase (LPL), which transforms TGs into fatty acids, may have been inhibited by CP, therefore increasing blood TGs levels.40 Because of the ME therapy, the blood levels of cardiac indicators and lipid profiles that CP raised have dropped significantly (p<0.001) in a dose-dependent manner, restoring them to almost normal levels (Fig. 2, Fig. 3). Compared to our findings, reported study on hydroethanolic extract of C. zedoaria has less anti-hyperlipidemic activity.41 A similar experiment has found that the crude ethanolic extract (EE) at doses of 200, 400, and 800 mg/kg BW reduced serum CK-MB and Troponin T levels in a dose-dependent way.42 However, ME’s CK-MB enzyme-lowering ability in the current study is superior to the EE. Furthermore, the cardioprotective role of curcumin derivatives which are commonly found in the Curcuma herbs has been mentioned by reported assay.43
These suggested that C. zedoaria has conserved the structural integrity of the myocardial membranes, while concurrently hindering the cardiac indicators from excessive production and discharge in the bloodstream. Besides, it is plausible that this rhizome has lipid-lowering abilities as well because of the polyphenolic compounds it contains, which can bind to bile acids to facilitate their excretion, restrict the formation of hepatic cholesterol, and induce the LPL enzyme. 44,45 Furthermore, ME’s antioxidant potential may have contributed to its cardioprotective ability since free radicals are intrinsically linked with oxidative stress in cardiac damage. However, to assess myocardial damage and identify the precise phytoconstituents that provide C. zedoaria ME’s cardioprotective action, cardiac tissue histopathology and compound isolation are needed.
Although we didn’t utilize a standard drug in this preliminary in vivo investigation, and as the promising activity of ME has been obtained, our further continuation of this research will employ it. Besides, due to insufficient funding, the separation of specific bioactive molecules and histopathologic analysis were not performed. Nonetheless, our future research will focus on uncovering lead compounds from this plant extract through in silico correlation with sophisticated phytochemical screening and pharmacological assessment.
Conclusion
This study was contrived to explore the biological activities of C. zedoaria ME. Our initial assessment for phytochemicals ensured a diversity of components. Subsequent in vitro and in vivo pharmacological investigations exhibited remarkable dose-dependent antioxidant, anthelmintic, and cardioprotective abilities. Since the extract was enormously effective in various treatments in this study, it might be a potential source of novel drugs to treat life-threatening illnesses, especially cancer and cardiac disorders. Therefore, more sophisticated research including compound isolation and histopathology of cardiac tissue is necessitated before coming to a definite conclusion on the findings of the present study.
Acknowledgment
The authors express their gratitude to the Ministry of Science and Technology, Bangladesh for providing financial support through the National Science and Technology (NST) fellowship in conducting this research work.
Funding Sources
This research work was funded by the Ministry of Science and Technology, Bangladesh through the National Science and Technology (NST) fellowship (Reg. No. 183_2021-22).
Conflict of Interest
The author(s) do not have any conflict of interest.
Data availability statement
The manuscript incorporates all datasets produced or examined throughout this research study. The derived data supporting the findings of this study are also available within the article as supplementary materials.
Ethical approval statement
This research involved animal experimentation, and therefore, prior approval was taken from the ethical review committee (ERC) of Mawlana Bhashani Science and Technology University, Santosh, Tangail-1902, Bangladesh (Ref: MBSTU/ERC/ECC/157/2024/9).
Informed consent statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ contribution
Fairuz Fatema Priya: Investigation, Data Collection, Writing–original draft;
Abu Zobayed: Data analysis, Writing–review and editing;
Md. Abu Sayeed: Conceptualization, Methodology
Md. Mizanur Rahman Moghal: Resources, Funding acquisition, Project administration, Supervision.
References
- Singh D. Application of novel drug delivery system in enhancing the therapeutic potential of phytoconstituents. Asian J Pharm. 2015; 9(4).
- Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016; 21(5): 559.
CrossRef - Rahman MH, Roy B, Chowdhury GM, Hasan A, Saimun MSR. Medicinal plant sources and traditional healthcare practices of forest-dependent communities in and around Chunati Wildlife Sanctuary in southeastern Bangladesh. Environ Sustainability. 2022; 5(2): 207–241.
CrossRef - Jaradat NA, Al-Masri M, Zaid AN, Hussein F, Al-Rimawi F, Mokh AA, Mokh JA, Ghonaim S. Phytochemical, antimicrobial and antioxidant preliminary screening of a traditional Palestinian medicinal plant, Ononis pubescens L. Eur J Integr Med. 2017; 14: 46–51.
CrossRef - Gharge S, Hiremath SI, Kagawad P, Jivaje K, Palled MS, Suryawanshi SS. Curcuma zedoaria Rosc (Zingiberaceae): a review on its chemical, pharmacological and biological activities. Future J Pharm Sci. 2021; 7: 1–9
CrossRef - Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022; 80(25): 2361–2371.
CrossRef - Chowdhury MZI, Haque MA, Farhana Z, Anik AM, Chowdhury AH, Haque SM, Marjana LLW, Bristi PD, Al Mamun BA, Uddin MJ, Fatema J. Prevalence of cardiovascular disease among Bangladeshi adult population: a systematic review and meta-analysis of the studies. Vasc Health Risk Manag. 2018; 14:165-181.
CrossRef - Gaziano T, Reddy KS, Paccaud F, Horton S, Chaturvedi V. Cardiovascular disease. In: Jamison DT, Breman JG, Measham A, Alleyne G, Claeson M, Evans DB, 2nd eds. Disease Control Priorities in Developing Countries. Oxford University Press, New York; 2006: 645–662.
CrossRef - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394–424.
CrossRef - Pilleron S, Sarfati D, Janssen‐Heijnen M, Vignat J, Ferlay J, Bray F, Soerjomataram I. Global cancer incidence in older adults, 2012 and 2035: a population‐based study. Int J Cancer. 2019; 144(1): 49–58.
CrossRef - Riaz M, Aslam N, Zainab R, Aziz-Ur-Rehman, Rasool G, Ullah MI, Daniyal M, Akram M. Prevalence, risk factors, challenges, and the currently available diagnostic tools for the determination of helminths infections in human. Eur J Inflammation. 2020; 18: 1–15.
CrossRef - Banisalam B, Sani W, Philip K, Imdadul H, Khorasani A. Comparison between in vitro and in vivo antibacterial activity of Curcuma zedoaria from Malaysia. Afr J Biotechnol. 2011; 10(55): 11676–11681.
- Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Subramaniyan V, Chidambaram K, Thangavelu L, Nordin R, Wu YS. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front Pharmacol. 2022; 13: 820806.
CrossRef - Chen W, Lu Y, Gao M, Wu J, Wang A, Shi R. Anti-angiogenesis effect of essential oil from Curcuma zedoaria in vitro and in vivo. J Ethnopharmacol. 2011; 133(1): 220–226
CrossRef - Barukial J, Sarmah JN. Ethnomedicinal plants used by the people of Golaghat district, Assam, India. Int J Med Aromat Plants. 2011; 1(3): 203–211.
- Kanase V, Khan F. An overview of medicinal value of Curcuma species. Asian J Pharm Clin Res. 2018; 11(2): 40–45.
CrossRef - Subositi D, Wahyono S. Study of the genus Curcuma in Indonesia used as traditional herbal medicines. Biodiversitas J Biol Divers. 2019; 20(5): 1356–1361.
CrossRef - Rashid MMO, Ferdous J, Banik S, Islam MR, Uddin AHMM, Robel FN. Anthelmintic activity of silver-extract nanoparticles synthesized from the combination of silver nanoparticles and M. charantia fruit extract. BMC Complementary Altern Med. 2016; 16(1): 242.
CrossRef - Shaikh JR, Patil MK. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud. 2020; 8(2): 603–608.
CrossRef - Imran H, Latif M, Yaqeen Z, Sohail T, Yaqeen SR, Yaqeen SS, Iqbal W. Prunus Domestica L.: A Domestic Source of Natural Antioxidants. Saudi J Med Pharm Sci. 2019; 5(10): 907-910.
CrossRef - Hussen EM, Endalew SA. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement Med Ther. 2023; 23(146): 1-12.
CrossRef - Ajaiyeoba EO, Onocha PA, Olarenwaju OT. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm Biol. 2001; 39(3): 217–220
CrossRef - Sohail T, Imran H, Shaukat S, Yaqeen SR, Syed S. Acute oral toxicity evaluation of ethanolic extract of di-herbal formulation (Aegle marmelos and Cymbopogon citratus) in albino mice. World J Pharm Pharm Sci. 2022; 11(7): 162-170
- Omole JG, Ayoka OA, Alabi QK, Adefisayo MA, Asafa MA, Olubunmi BO, Fadeyi BA. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J Evidence-Based Integr Med. 2018; 23, 215658721875764
CrossRef - Ali L, Nizami SA, Ramzan HS. Cardiac protective activity of ethanol extract of White Curcumin (Curcuma Zedoaria), against cyclophosphamid induced cardiovascular complications in male Sprague Dewley mice. Acta Sci Med Sci. 2021; 5(10): 82–91.
- Sumathi S, Iswariya GT, Sivaprabha B, Dharani B, Radha P, Padma PR. Comparative study of radical scavenging activity and phytochemical analysis of fresh and dry rhizomes of Curcuma zedoaria. Int J Pharm Sci Res. 2013; 4(3), 1069.
- Patra JK, Baek KH. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017; 8, 167.
CrossRef - Yuandani, Jantan I, Rohani AS, Sumantri IB. Immunomodulatory effects and mechanisms of curcuma species and their bioactive compounds: A review. Front Pharmacol. 2021; 12, 643119
CrossRef - Randeep G, Vandna K, Amandeep S. Phytochemical investigation and evaluation of anthelmintic activity of Curcuma amada and Curcuma caesia-A comparative study. Invent Rapid Ethnopharmacol. 2011; 2011(2): 1–4
- Skaria BP, Joy PP, Mathew G, Mathew S. Zingiberaceous plants in traditional medicine. Paper presented at: National seminar on role of medicinal and aromatic plants in ayurveda, unani and siddha systems of medicine; March 4–5, 2005; Haryana Agricultural University, Hissar.
- Martin RJ. Neuromuscular transmission in nematode parasites and antinematodal drug action. Pharmacol Ther. 1993; 58(1): 13–50
CrossRef - Veerakumari L, Chitra N. Effect of Allium sativum on the motility and acetylcholinesterase of Haemonchus contortus. Int J Sci Res. 2016; 5(1): 883–887.
CrossRef - Lei J, Leser M, Enan E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of C. aenorhabditis elegans. Biochem Pharmacol. 2010; 79(7): 1062–1071.
CrossRef - Chen HW, He XH, Yuan R, Wei BJ, Chen Z, Dong JX, Wang J. Sesquiterpenes and a monoterpenoid with acetylcholinesterase (AchE) inhibitory activity from Valeriana officinalis var. latiofolia in vitro and in vivo. Fitoterapia. 2016; 110: 142–149.
CrossRef - Jabir NR, Khan FR, Tabrez S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci Ther. 2018; 24(9): 753–762.
CrossRef - Shanmugarajan TS, Arunsundar M, Somasundaram I, Krishnakumar E, Sivaraman D, Ravichandiran V. Cardioprotective effect of Ficus hispida Linn. on cyclophosphamide provoked oxidative myocardial injury in a rat model. Int J Pharmacol. 2008; 1: 1–10.
- Baky NAA, Al-Rasheed NM, Al-Rasheed NM, Zaghloul IY, Radwan MA. Alpha-lipoic acid and amlodipine ameliorate myocardial infarction induced by isoproterenol in rats. Int J Acad Res. 2009; 1(1): 68–77.
- Chakraborty P, Sk UH, Murmu N, Das JK, Pal S, Bhattacharya S. Modulation of cyclophosphamide-induced cellular toxicity by diphenyl-methyl selenocyanate. In vivo, an enzymatic study. J Cancer Mol. 2009; 4: 183–189
- Senthilkumar S, Devaki T, Manohar BM, Babu MS. Effect of squalene on cyclophosphamide induced toxicity. Clin Chim Acta. 2006; 364: 335–342. doi: 10.1016/j.cca.2005.07.032
CrossRef - Swamy A, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy A. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013; 45(1): 44–48
CrossRef - Srividya AR, Dhanabal SP, Yadav AK, Kumar SMN, Vishnuvarthan VJ. Phytopreventive anti-hyperlipidemic activity of Curcuma zedoaria. Bulletin Pharm Res. 2012; 2: 22–25.
- Amrullah A, Fachrial E. Cardiac protection activity of ethanol extract of White Curcumin (Curcuma Zedoaria), against cyclophosphamid induced cardiovascular complications in male rat. J Pharm Res Int. 2021; 33(41A): 248–256.
CrossRef - Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012; 49(5): 306–315
- Paul S, Das S, Tanvir EM. Protective effects of ethanolic peel and pulp extracts of Citrus macroptera fruit against isoproterenol-induced myocardial infarction in rats. Biomed Pharmacother. 2017; 94: 256–264.
CrossRef - Moreno DA, Ripoll C, Ilic N, Poulev A, Aubin C, Raskin I. Inhibition of lipid metabolic enzymes using Mangifera indica extracts. J Food Agric Environ. 2006; 4(1): 21.
Abbreviations
|
AA |
ascorbic acid |
|
AChE |
acetylcholinesterase |
|
ANOVA |
analysis of variance |
|
AST |
aspartate aminotransferase |
|
BW |
body weight |
|
CK-MB |
creatine kinase-myocardial band |
|
CP |
cyclophosphamide |
|
DPPH |
2,2-diphenyl-1-picrylhydrazyl |
|
HDL-C |
high density lipoprotein cholesterol |
|
IC50 |
inhibitory concentration 50% |
|
LDH |
lactate dehydrogenase |
|
LDL-C |
low density lipoprotein cholesterol |
|
LPL |
lipoprotein lipase |
|
ME |
methanolic extract |
|
ns |
not significant |
|
SEM |
standard error of the mean |
|
SPSS |
statistical package for the social sciences |
|
TC |
total cholesterol |
|
TGs |
triglycerides |







