Putu Indah Budi Apsari1* , Putu Khrisna Dharma Jaya2
, Putu Khrisna Dharma Jaya2 , Pande Made Alitta Cantika Putri Nadya Dewi2
, Pande Made Alitta Cantika Putri Nadya Dewi2 , Desak Putu Oki Lestari3
, Desak Putu Oki Lestari3
1Department of Parasitology Faculty of Medicine and Health Sciences, Warmadewa University, Denpasar, Indonesia,
2Medical Study Program, Faculty of Medicine and Health Sciences, Warmadewa University, Denpasar, Bali, Indonesia,
3Department of Anatomical Pathology, Faculty of Medicine and Health Sciences, Warmadewa University, Bali, Indonesia.
Corresponding Author E-mail:putuindah51@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/3042
Abstract
Malaria is a disease that still haunts Indonesia today. The high level of anti-malarial resistance and the severity of the disease cause high mortality in malaria cases. Previous research found that Moringa oleifera can eliminate the Plasmodium berghei parasite and is an immunomodulator in mice. But how moringa works at the cellular level is still unclear. This study aimed to analyse effect of Moringa oleifera treatment in lipid vacuolization of liver, number of pyknosis cell, and organ enlargement in Mus musculus infected by Plasmodium berghei. True experimental design use 40 mice were divided into 5 group: negative control, positive control treated by dehydroartemisinin piperaquine, group 1,2, and 3 treated by 25%, 50%, and 75% Moringa extract respectively. Parasite count was determined by blood smear with giemsa staining, and their organs were collected for histopathological analysis via hematoxylin-eosin staining. Lipid vacuolization, pyknotic cell of liver was observed under light microscope. Index of Liver, spleen, and kidney were examiden by organ weight per mice body weight using digital scale. All data tested by One-Way ANOVA. The results of the study stated that Moringa oleifera reduce lipid vacuolization and reduce index of the spleen and kidney organs. However, there were no significant difference effect of Moringa oleifera in liver index and pyknotic cell. As conclusion Moringa oleifera reduce lipid vacuolization, pyknotic cell and organ enlargement in mus musculus infected by Plasmodium berghei.
Keywords
Histopathology; lipid vacuolization; malaria; Moringa oleifera; Organ index; Nuclear pyknosis
Download this article as:| Copy the following to cite this article: Apsari P. I. B, Jaya P. K. D, Dewi P. M. A. C. P. N, Lestari D. P. O. Moringa oleifera Reduce Lipid Vacuolization, Pyknotic Cell and Organ Enlargement in Mus musculus Infected by Plasmodium berghei. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Apsari P. I. B, Jaya P. K. D, Dewi P. M. A. C. P. N, Lestari D. P. O. Moringa oleifera Reduce Lipid Vacuolization, Pyknotic Cell and Organ Enlargement in Mus musculus Infected by Plasmodium berghei. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/485GhGQ |
Introduction
Indonesia is one of the many countries with a tropical climate. This tropical climate is like a double-edged sword, bringing advantages and disadvantages. The benefit of a tropical environment is its year-round sunshine to the region. However, the disadvantages include higher temperatures, the absence of winter, and health issues. Health problems resulting from this climate include skin cancer due to prolonged exposure to sunlight and the spread of several diseases that thrive in tropical climates 1.
Anopheles mosquitoes are one of the insects that thrive well in tropical climates. These mosquitoes even live while spreading the diseases they carry. One such disease is malaria, caused by infection from Plasmodium sp. Malaria is a disease that attacks red blood cells, with the main issue being the number of red blood cells resulting in anemia 2.
In Indonesia, malaria has been a major problem in recent decades. Despite being endemic, malaria remains prevalent among the population. According to data from the Ministry of Health of the Republic of Indonesia, in 2021, there were approximately 94,610 active malaria cases in Indonesia 3. The highest cases are in Papua, West Papua, and East Nusa Tenggara. 4
In malaria infection, the liver, spleen, and kidneys play important roles 5–7. The liver is a site for developing one of the life cycles of Plasmodium sp. parasites 8. Additionally, the liver is also a site for the metabolism of drugs used to combat malaria infection. The spleen plays a role in eliminating infected red blood cells. This includes the immune cell’s function in eliminating parasites 6. The kidneys are affected during malaria infection due to the characteristics of Plasmodium sp., which undergo rosetting, cytoadherence, and sequestration in the blood vessel. Kidney damage can worsen malaria infection and indicate severe malaria 5,7. Previous research has shown that Moringa leaf extract has anti-malarial activity by suppressing parasitemia levels 4, but how it works in cellular morphology and reduce severe degradation feature has not been investigated yet. Furthermore, researchers aim to examine the histopathological changes in organs crucial to malaria infection. This research aimed to analyse effect or Moringa oleifera treatment in lipid vacuolization, liver cell pyknosis, and organ index (liver, spleen, and kidney) in Mus musculus infected by Plasmodium berghei.
Material And Methods
This research was conducted at the Biomedical Laboratory of the Faculty of Medicine and Health Sciences, Warmadewa University, and the Histology Laboratory of the Faculty of Medicine, Udayana University. This research use male BALB/c mice aged 7 to 8 weeks. BALB/c mice was selected as animal model because of it good respons in plasmodium berghei infection, and produce good immune respons bot plasmodium infection and extract treatment. All mice were infected with 0.2mL of blood containing Plasmodium berghei via intraperitoneal injection 9. There were five groups in this study: a negative control and did not receive any treatment, group 1 received therapy with 25% concentration of Moringa extract, group 2 received 50% concentration of Moringa extract, group 3 was treated with 75% concentration of Moringa extract, and positive control group was treated by Dihydro-Artemisin-Piperaquine (DHP) produced in Indonesia by PT. Mersifarma TM (6O96’15.8″S 106O78’73.9″E), with the batch number MTA-124486296. Moringa leaves were obtained from the local market in Denpasar City (08°14′17″S 115°05′02″E). The Moringa leaves were extracted with 70% ethanol for three days 4. The process was continued with evaporation to obtain 100% Moringa extract. It was then dissolved in distilled water to achieve concentrations of 25%, 50%, and 75%. Moringa extract was administered orally at 0.5 mL daily to the mice for five days. Following the 5-day administration period and an additional two days of observation, the mice were terminated, and their organs were collected 4. The organs were preserved in 10% formalin10. Parasitemia were counted by blood smear. All organ were measured by digital scale. Index organ was counted by organ weight per total body weight. Lipid vacuolization and pyknotic cell in liver section stained by Haematoxyline eosin was examined under light microscope.11
Hematoxylin-eosin staining
Initially, the organs were fixed in a neutral buffered formalin solution at 10% for approximately 24 hours. This was followed by embedding the organs in paraffin blocks and slicing the paraffin blocks into thin sections. Subsequently, the tissue sections were mounted on microscope slides, followed by rehydration and dehydration processes for staining and further analysis. Rehydration involved 3 × 10 minutes in xylene (deparaffinization), 3 × 1 minute in 100% alcohol, 1 × 1 minute in 95% alcohol, 1 × 1 minute in 70% alcohol, followed by rinsing in distilled water. Dehydration is the reverse of the rehydration process 10,12. The slides were stained with hematoxylin and eosin staining, each for 3 minutes. Samples were thoroughly rinsed with distilled water between and after staining steps to remove excess background staining 10,12.
Analysis of histological slides
The slides were analyzed using the microscope in the Biomedical Laboratory of the Faculty of Medicine and Health Sciences, Warmadewa University. The variable of lipid vacuolization was observed for the presence of lipid droplets, inflammatory cells, and ballooning 13. The data was compared by Chi-square test. Hepatic cell nuclei pyknosis was assessed based on cells and nuclei smaller than surrounding cells, intact cell membranes, and the absence of inflammatory cell infiltration around the cells14. The variable of nuclear pyknosis was assessed by counting the number of cells undergoing pyknosis in 5 fields of view 15. The last variable, organ index, was calculated by dividing the organ weight in grams by the total weight of the mice, multiplied by 100% 6. Nuclear pyknosis and organ index will generate quantitative data analyzed using SPSS version 29.0th for Mac with a confidence level of 95%. Tests to be conducted are descriptive analysis and comparison tests using One-Way ANOVA and 6,15.
Results and Discussion
As seen in Figure 1, Lipid vacuolization was present in the negative group, which did not receive therapy, with a percentage of 70%. Malaria model mice treated with 50% Moringa oleifera extract showed the lowest rate of lipid vacuolization at 6.67%. Based on the Pearson Chi-Square test, a p-value of 0.002 was obtained, indicating a significant relationship between the differences in interventions in mice infected with Plasmodium berghei and the incidence of lipid vacuolization in hepatocytes, as described in Table 1 16.
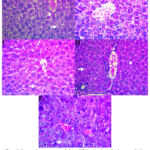 |
Figure 1: Liver section was presented above. (A) Negative Control swhoe more lipid vacuolization and pyknotic cell, (B) Moringa oleifera Extract 25%,
|
Table 1: Cross-tabulation of Lipid Vacuolization
|
Group |
Lipid vacuolization |
N |
P |
|
|
Exist (Percentage) |
None (Percentage) |
|||
|
Negative |
14 (70%) |
6 (30%) |
20 |
0.002 |
|
Extract 25% |
2 (40%) |
3 (60%) |
5 |
|
|
Extract 50% |
1 (6.67%) |
14 (93.33%) |
15 |
|
|
Extract 75% |
4 (26.7%) |
11 (73.3%) |
15 |
|
|
Positive |
1 (20%) |
4 (80%) |
5 |
|
The highest number of hepatocyte pyknotic nuclei, as seen in Table 2, was found in the 50% Moringa oleifera extract group, with an average of 1.53. Meanwhile, the lowest average of pyknotic nuclei was found in the 25% Moringa oleifera extract group, with an average of 1. Based on the One-Way ANOVA test, a p-value of 0.827 was obtained, indicating no significant difference in the number of hepatocyte nuclei undergoing pyknosis in malaria cases after treatment with Moringa oleifera extract, as in Table 2 16. When comparing each group with the others through the LSD Post Hoc Test, no significant differences were found.
Table 2: The Difference of Pyknotic Cell number between Group
|
Group |
N |
Mean |
SD |
P |
|
Negative |
20 |
1.15 |
1.1531 |
0.827 |
|
Extract 25% |
5 |
1.00 |
1.000 |
|
|
Extract 50% |
15 |
1.53 |
1.125 |
|
|
Extract 75% |
15 |
1.07 |
0.884 |
|
|
Positive |
5 |
1.20 |
0.837 |
The highest hepatic organ index was found in the positive group, reaching 8.80000 ± 3.39411. The 50% extract group yielded the lowest hepatic index result of 7.7143 ± 0.88587, as shown in Table 3. Based on the One-Way ANOVA test, a significance value of approximately 0.887 was obtained, indicating no significant difference in the hepatic organ index between groups 16.
The highest spleen organ index obtained by dividing the organ weight by the mice weight and multiplying by 100% was in the negative group, reaching 24.0000 ± 4.89898. The lowest spleen index was found in the 50% extract group, with a spleen index of 2.2286 ± 0.39036, as shown in Table 3. The mean spleen organ index in the positive group reached 4.6000 ± 0.28284. The One-Way ANOVA test indicated a significant difference in the spleen organ index with a p-value of <0.001 16.
The left kidney organ index in the negative group yielded the highest value with a mean of 24.8000 ± 3.34664, as shown in Table 3. In the 25% extract group, the left kidney index had the lowest value, with a mean of 2.0000. A comprehensive analysis of the kidney index through the One-Way ANOVA test revealed a significance value of <0.001, indicating a significant difference in the left kidney organ index between groups 16.
The right kidney organ index in the negative group also showed the highest mean value of 24.8000 ± 5.21536, as shown in Table 3. In the 25% extract group, the right kidney index had the lowest mean value of 2.0000. Based on the One-Way ANOVA test, a p-value of <0.001 was obtained, indicating a significant difference in the right kidney organ index between the untreated group, the group treated with Moringa oleifera extract, and the group treated with DHP as the standard malaria drug 16.
Table 3: The Difference of Organ Index Between Group
|
Organ |
Group |
N |
Mean (%) |
SD |
P |
|
Liver |
Negative |
5 |
8.2400 |
1.40285 |
0.887 |
|
Extract 25% |
1 |
8.0000 |
. |
||
|
Extract 50% |
7 |
7.7143 |
0.88587 |
||
|
Extract 75% |
6 |
8.0667 |
1.08566 |
||
|
Positive |
2 |
8.8000 |
3.39411 |
||
|
Spleen |
Negative |
5 |
24.0000 |
4.89898 |
<0.001 |
|
Extract 25% |
1 |
2.8000 |
. |
||
|
Extract 50% |
7 |
2.2286 |
0.39036 |
||
|
Extract 75% |
6 |
3.1333 |
1.30026 |
||
|
Positive |
2 |
4.6000 |
0.28284 |
||
|
Left Kidney |
Negative |
5 |
24.8000 |
3.34664 |
<0.001 |
|
Extract 25% |
1 |
2.0000 |
. |
||
|
Extract 50% |
7 |
2.2857 |
0.19518 |
||
|
Extract 75% |
6 |
2.6000 |
0.65727 |
||
|
Positive |
2 |
4.2000 |
0.28284 |
||
|
Right Kidney |
Negative |
5 |
24.8000 |
5.21536 |
<0.001 |
|
Extract 25% |
1 |
2.0000 |
. |
||
|
Extract 50% |
7 |
2.2857 |
0.30237 |
||
|
Extract 75% |
6 |
2.3333 |
0.30111 |
||
|
Positive |
2 |
3.8000 |
0.84853 |
Discussion
Lipid vacuolization in hepatocytes during malaria is a common manifestation of severe malaria. In malaria infection, IFN-γ levels increase as an inflammatory response 17. This cytokine can activate the expression and synthesis of nitric oxide, leading to microvascular infiltration by lipid cells in the liver, which can develop into fatty liver 17. This accumulation of lipid cells also benefits the malaria-causing parasite Plasmodium, which requires exogenous lipids to aid in its maturation, especially in the hepatic stage 18.
Interferon-γ activates macrophages, the first line of defense against Plasmodium. On the other hand, macrophages, particularly Kupffer cells, play a crucial role in forming fatty liver 19. Increased NF-κB p65 levels will enhance the incidence of fatty liver 18. Moringa oleifera extract can inhibit the production of proinflammatory mediators such as IL-1, IL-6, TNF-α, PTGS2, and NF-κB (P50) and reduce the expression of NF-κB p65 20. It can be concluded that Moringa oleifera enhances macrophages to combat Plasmodium sp. infection while inhibiting the likelihood of fatty liver incidence 2122such as the vacuolization of lipid cells, can be minimized.
Nuclear pyknosis is a process of nuclear condensation, resulting in reduced size with denser DNA or Deoxyribonucleic Acid. Pyknotic nuclei are one of the signs of necrosis or cell death, occurring due to the loss of membrane integrity, leading to cell contents leaking and triggering an inflammatory reaction 14. Necrosis occurs due to the destruction of cells, including their organelles. The initial cell changes involve decreased ATP synthesis, possibly due to oxygen deficiency or hypoxia 23. In malaria infection, hypoxia occurs due to hemolysis-induced anemia or reduced hemoglobin levels due to the destruction of infected red blood cells 24. Decreased ATP can lead to the failure of active transport of sodium and potassium, resulting in calcium and water influx. Increased calcium levels can damage the mitochondria responsible for ATP production. Failed oxidative phosphorylation processes in mitochondria result in the accumulation of oxidative stress 14. Oxidative stress can cause cell damage and inflammation. Ultimately, a vicious cycle is formed 25. Malaria infection can induce oxidative stress through several mechanisms, including hemoglobin destruction, the body’s response to Plasmodium sp. infection, and direct production by the parasite 26. The use of antimalarial drugs can also induce oxidative stress production, which can have adverse effects on the body 27.
Moringa oleifera is effective against Plasmodium sp., the causative parasite of malaria, in several studies 28,29. Even at a dose of 500mg/kgbw, it suppresses parasitemia by 77%. Compounds present in its leaves can protect mitochondrial function from the numerous proinflammatory mediators produced during malaria infection 30. This occurs due to the increased production of anti-inflammatory factors post-Moringa therapy. The antioxidants in Moringa oleifera protect against oxidative stress 31. Preserving mitochondrial function also ensures no ATP depletion, thus thwarting the mechanisms leading to necrosis, one of which is marked by pyknotic nuclei 32. 9,33
The Moringa oleifera extract in this study did not yield significant results compared to the positive control (DHP) or the untreated negative control. The use of Moringa oleifera in malaria cases is often processed by boiling rather than extracting with 70% ethanol, as in this study 28. Using ethanol as a solvent may induce toxicity symptoms in mice, resulting in more significant damage compared to the effects of Moringa oleifera 34. However, Moringa oleifera is non-toxic to the liver 35. 3636Based on the results of this research, giving Moringa oleifera, showed effective in reducing the incidence of damage to the liver, one of which is the pyknosis nucleus37.
Dihydroartemisinin-piperaquine, or DHP, is known to increase oxidative stress on parasites, which can have adverse effects on patients 27. Studies have reported increased lipid peroxidase associated with oxidative stress and lower antioxidant levels at specific doses 26. So far, DHP has not shown hepatotoxic symptoms 38,39. To ensure research outcomes, further research is needed to determine whether DHP doses for mice have toxic effects on the liver.
Hepatomegaly, or liver enlargement in malaria, is fundamentally related to the malaria life cycle. One of the cycles, known as the schizont, involves liver cells infected by Plasmodium sp., which can damage the liver.89 Pathological evaluation reveals necrotic lesions with Kupffer cell hyperplasia containing hemozoin, the malaria pigment 40. Previous research has shown that antimalarial treatment results in a smaller liver organ index than no treatment 6. 414142A similar mean of liver index among all groups cannot be determined whether Moringa leaf extract can maintain liver conditions resembling those of a healthy individual37.
Splenomegaly, or enlargement of the spleen in malaria, occurs due to hemozoin accumulation within macrophages and infected erythrocytes in the spleen. This enlargement is also associated with red and white pulp expansion due to increased activity during malaria infection 43. The red pulp is filled with infected erythrocytes awaiting destruction according to spleen physiology, while the white pulp contains macrophages working harder to eliminate and destroy infected erythrocytes from circulation. Plasma cell infiltration also occurs during malaria infection in the spleen 43. Spleen physiology also changes, including increased forming of new erythrocytes to replace infected ones and the maturation and differentiation of lymphocytes 44. Significant differences were observed in the spleen organ index, indicating that Moringa leaf extract can alleviate clinical symptoms of splenomegaly. The organ index results are consistent with parasitemia levels, with the 50% extract group showing the lowest parasitemia 4. Previous research has shown a smaller spleen organ index in treated groups compared to untreated ones 6. 45,464729
Kidney enlargement in malaria is associated with conditions such as glomerulonephritis, acute tubular necrosis, acute interstitial nephritis, and chronic kidney disease 48,49. Initially, Plasmodium sp. antigens adhere to the glomerulus, leading to inflammatory processes. Nephrotic syndrome symptoms such as proteinuria may also occur 49. Additionally, due to the nature of infected erythrocytes undergoing rosetting, cytoadherence, and sequestration, the kidney’s microvasculature is likely affected 50. 51This study yielded significant results regarding kidney organ index, indicating that Moringa extract successfully preserves kidney function during malaria infection. Previous research has shown similar results, with smaller kidney organ index in treated groups compared to untreated ones 6. 52
However, this study still has several limitations. Further research is needed regarding using solvents in the Moringa oleifera leaf extraction process, which will be tested on malaria-model mice. The processing methods of Moringa oleifera leaves must be compared to yield the best outcomes. Additionally, it is necessary to ascertain whether DHP, as the standard treatment in malaria model mice, has any side effects and its ideal dosage range. Consideration can also be given to using other animal models such as Rattus norvegicus or Wistar rats or hamsters. Liver and kidney biomarker examinations such as AST, ALT, Bilirubin, BUN, and creatinine need to be carried out to provide a better characteristic of organ damage. 28,33
Conclusion
Based on the research findings and discussions, it can be concluded that Moringa oleifera effective in reducing lipid vacuolization. The 50% of Moringa oleifera leaf extract had better effect compared with other group because of dose level tolerated by mice liver, higher dose may cause toxic effect. Pyknosis cell not significaltly different compared with another groups. Moringa effective in prevent organ eblargement due to reducing organ index. Further study must be conducted to investigate molecular mechanistic action of Moringa oleifera in Plasmodium infection.
Acknowledgment
We acknowledge the support from the Faculty of Medicine and Health Sciences, Warmadewa University. Also, thank you to the Faculty of Medicine and Health Sciences, Warmadewa University, for funding this research and publication. Finally, thank you to all the participants involved in this research.
Conflict of Interest
The author(s) do not have any conflict of interest
Funding Sources
This research is funded by the Faculty of Medicine and Health Sciences, Warmadewa University by grant number 1436/UNWAR/FKIK/PD-13/VIII/2023
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author on request.
Ethics Statement
This research has obtained ethical approval from the Health Research Ethics Committee of the Faculty of Medicine and Health Sciences, Universitas Warmadewa, with registration number 405/Unwar/FKIK/EC-KEPK/IV/2023 dated 03/28/2023.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Clinical Trial Registration
This trial is registered at Research Laborartory, Faculty of Medicine and Health Sciences, Warmadewa University with the registration number 010/Lab Penelitian FKIK Unwar/IV/2023.
Authors’ Contribution
Putu Indah Budi Apsari: Conceptualization, Methodology, Writing – Original Draft.
Putu Khrisna Dharma Jaya: Data Collection, Analysis, Writing – Review & Editing.
Pande Made Alitta Cantika Putri Nadya Dewi: Visualization, Project Administration.
Desak Putu Oki Lestari: Acquisition, Resources, Supervision.
References
- Utami TP, Hasyim H, Kaltsum U. Faktor Risiko Pernyebab Terjadinya Malaria di Indonesia: Literature Review. Jurnal Surya Medika. 2022;7(2):96-107.
- Yahya Y, Haryanto D, Pahlevi RI, Budiyanto A. KEANEKARAGAMAN JENIS NYAMUK Anopheles DI SEMBILAN KABUPATEN (TAHAP PRE-ELIMINASI MALARIA) DI PROVINSI SUMATERA SELATAN. Vektora : Jurnal Vektor dan Reservoir Penyakit. 2020;12(1):41-52. doi:10.22435/vk.v12i1.2621
- Kemenkes RI. Malaria: Penyebab Kematian Tertinggi di Dunia. 2022. Accessed May 5, 2023. https://p2pm.kemkes.go.id/publikasi/ artikel/mengenal-malaria-penyakit-mematikan-dunia
- Jaya PKD, Apsari PIB, Dewi PMACPN, Laksemi DAAS, Sutarta IKCAW. Effects of Moringa oleifera Extract as an Immunomodulator of Lymphocyte Cells and Macrophages in BALB/c Mice Infected with Plasmodium berghei. Folia Medica Indonesiana. 2023;59(3):214-221. doi:10.20473/fmi.v59i3.45237
- Akil SNH. Parasitemia, Gangguan Fungsi Hepar Dan Ginjal Pada Mencit BALB/C Terinfeksi Plasmodium Berghei Yang Diberi Virgin Coconut Oil. Universitas Airlangga; 2020.
- Hermanto F, Anisa IN, Wahyuningsih S. Aktivitas Antiplasmodium dan Pengaruh Resveratrol terhadap Indeks Organ Mencit yang Terinfeksi Plasmodium berghei ANKA Antiplasmodium Activity and The Effect of Resveratrol on Index Organs of Mice Infected with Plasmodium berghei ANKA. Pharmaceutical Journal of Indonesia. 2022;19(01):28-39.
- Rahmah MA, Adrial, Yenita. Gambaran Histopatologi Ginjal Mencit (Mus musculus Balb/C) yang Diinfeksi dengan Plasmodium berghei. Jurnal Ilmu Kesehatan Indonesia. 2021;2(1):7-16.
- Centers for Disease Control and Prevention. Malaria Life Cycle. 2020. Accessed March 24, 2024. https://www.cdc.gov/malaria/about/ biology/index.html
- Arwati H, Bahalwan RR, Hapsari WT. Suppressive effect of goat bile in Plasmodium berghei ANKA infection in mice. Vet World. 2021;14(8):2016-2022. doi:10.14202/vetworld.2021.2016-2022
- Al Z, Wafa EI, Meyer MKR, Owen S, Salem AK. Tissue Engineering the Pinna: Comparison and Characterization of Human Decellularized Auricular Biological Scaffolds. ACS Appl Bio Mater. 2021;4:7234-7242. doi:10.1021/acsabm.1c00766
- Sri Laksemi DAA, Asri Damayanti PA, Sudarmaja IM. In-vivo antimalarial activity of Holothuria scabra simplicia in Plasmodium berghei-infected mice. Universa Medicina. 2024;43(2):195-201. doi:10.18051/UnivMed.2024.v43.195-201
- Lisowski A. Science of Tissue Processing. leica Biosystems; 2019. Accessed August 7, 2023. https://www.leicabiosystems.com/ sites/default/ files/media_document-file/2021-04/190093_Rev _A_Science_of_Tissue_Processing.pdf
- Wardani A, Wahid A, Astuti Y. Uji Aktivitas Antimalaria in vitro dari Ekstrak Etanol Batang Tanaman Ashitaba (Angelica keiskei [Miq.] Koidz). Jurnal Ilmu Kefarmasian Indonesia. 2020;18(2):202-206.
- Kumar V, Abbas AK, Aster JC. Robbins & Cotran Pathologic Basis of Disease. Tenth. Elsevier; 2021.
- Pangkahila E, Linawati NM, Sugiritama IW, SIswanto FM. Pelatihan Fisik Berlebih Meningkatkan Indeks Apoptosis pada Hepatosit Tikus (Rattus norvergicus) Wistar Jantan. Jurnal Biomedik. 2019;11(3):144-149.
- Dahlan MS. Statistik Untuk Kedokteran Dan Kesehatan. Sixth Edition. Epidemiologi Indonesia; 2014.
- Niikura M, Fukutomi T, Mineo S, Mitobe J, Kobayashi F. The Association Between Acute Fatty Liver Disease and Nitric Oxide During Malaria in Pregnancy. Malar J. 2021;20(1):1-13. doi:10.1186/s12936-021-03999-2
- Boeckmans J, Rombaut M, Demuyser T. Infections at The Nexus of Metabolic-Associated Fatty Liver Disease. Arch Toxicol. 2021;95(7):2235-2253. doi:10.1007/s00204-021-03069-1
- Xu L, Liu W, Bai F. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.708978
- Luetragoon T, Sranujit RP, Noysang C. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit The Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules. 2020;25(1):1-16. doi:10.3390/molecules25010191
- Gomes ARQ, Cunha N, Varela ELP. Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int J Mol Sci. 2022;23(11). doi:10.3390/ijms23115949
- Kaewdana K, Chaniad P, Jariyapong P, Phuwajaroanpong A, Punsawad C. Antioxidant and antimalarial properties of Sophora exigua Craib. root extract in Plasmodium berghei-infected mice. Trop Med Health. 2021;49(1). doi:10.1186/s41182-021-00314-2
- Khalid N, Azimpuran M. Necrosis. StatPearls; 2023.
- Park MK, Ko EJ, Jeon KY. Induction of Angiogenesis by Malarial Infection Through Hypoxia Dependent Manner. Korean Journal of Parasitology. 2019;57(2):117-125. doi:10.3347/kjp.2019.57.2.117
- Vona R, Pallotta L, Cappelletti M, Severi C, Matarrese P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants. 2021;10(2):1-26. doi:10.3390/antiox10020201
- Gomes ARQ, Cunha N, Varela ELP. Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int J Mol Sci. 2022;23(11). doi:10.3390/ijms23115949
- Vasquez M, Zuniga M, Rodriguez A. Oxidative Stress and Pathogenesis in Malaria. Front Cell Infect Microbiol. 2021;11. doi:10.3389/fcimb.2021.768182
- Bezerra JJL, Pinheiro AAV, Dourado D. Antimalarial Potential of Moringa oleifera Lam. (Moringaceae): A Review of the Ethnomedicinal, Pharmacological, Toxicological, and Phytochemical Evidence. Journal of Venomous Animals and Toxins Including Tropical Diseases. 2023;29:1-13. doi:10.1590/1678-9199-JVATITD-2022-0079
- Pilotos J, Ibrahim KA, Mowa CN, Opata MM. Moringa oleifera Treatment Increases Tbet Expression in CD4+ T Cells and Remediates Immune Defects of Malnutrition in Plasmodium chabaudi-Infected Mice. Malar J. 2020;19(1):1-16. doi:10.1186/s12936-020-3129-8
- Safutra MS, Jeffilano Barinda A, Arozal W. Potensi Moringa Oleifera sebagai Agen Neuroprotektif pada Kondisi Penuaan di Otak. Muhammadiyah Journal of Geriatric. 2023;4(2):138-151. doi:10.24853/mujg.4.2.138-151
- Dilworth LL, Stennett D, Omoruyi FO. Effects of Moringa oleifera Leaf Extract on Human Promyelocytic Leukemia Cells Subjected to Oxidative Stress. J Med Food. 2020;23(7):728-734. doi:10.1089/jmf.2019.0192
- Ndlovu SS, Chuturgoon AA, Ghazi T. Moringa oleifera Lam Leaf Extract Stimulates NRF2 and Attenuates ARV-Induced Toxicity in Human Liver Cells (HepG2). Plants. 2023;12(7):1-20. doi:10.3390/plants12071541
- Mulisa E, Girma B, Tesema S, Yohannes M, Zemene E, Amelo W. Evaluation of in vivo antimalarial activities of leaves of Moringa oleifera against Plasmodium berghei in mice. Jundishapur J Nat Pharm Prod. 2018;13(1). doi:10.5812/jjnpp.60426
- Ubang F, Siregar VO, Herman H. Efek Toksik Pemberian Ekstrak Etanol Daun Mekai (Albertisia papuana Becc.) Terhadap Mencit. Proceeding of Mulawarman Pharmaceuticals Conferences. 2022;16:49-57. doi:10.25026/mpc.v16i1.672
- Islamika FAN, Aryati F, Indriyanti N. Kajian Literatur Mengenai Tingkat Keamanan Tanaman Kelor (Moringa Oleifera L.) dari Hasil Uji Toksisitas Akut dan Subkronis. Proceeding of Mulawarman Pharmaceuticals Conferences. 2020;12:156-159. doi:10.25026/mpc.v12i1.419
- Kauffmann N, Da Penha LKRL, Braga D V. Differential Effect of Antioxidants Glutathione and Vitamin C on the Hepatic Injuries Induced by Plasmodium berghei ANKA Infection. Biomed Res Int. 2021;2021. doi:10.1155/2021/9694508
- Obediah GA, Obi NC. Anti-plasmodial Effect of Moringa oleifera Seeds in Plasmodium berghei Infected Albino Rats. Biochem Pharmacol (Los Angel). 2020;09(01):1-5. doi:10.35248/2167-0501.20.9.268
- Georgewill, Adikwu, Ebong. Hepatotoxic Impact of Desloratadine/Dihydroartemisinin /Piperaquine on Healthy and Parasitized Mice. Drug Discovery. 2022;16(37). https://www.researchgate.net/publication/364274930
- Chughlay MF, Akakpo S, Odedra A. Liver Enzyme Elevations in Plasmodium falciparum Volunteer Infection Studies: Findings and Recommendations. American Journal of Tropical Medicine and Hygiene. 2020;103(1):378-393. doi:10.4269/ajtmh.19-0846
- de Menezes MN, Salles ÉM, Vieira F. IL-1α Promotes Liver Inflammation and Necrosis During Blood-Stage Plasmodium chabaudimalaria. Sci Rep. 2019;9(1):1-12. doi:10.1038/s41598-019-44125-2
- Sidiki NNA, Nadia NAC, Cedric Y. Antimalarial and Antioxidant Activities of Ethanolic Stem Bark Extract of Terminalia macroptera in Swiss Albino Mice Infected with Plasmodium berghei. J Parasitol Res. 2023;2023. doi:10.1155/2023/3350293
- Wulan JA, Laitupa AA, Prahasanti K. Risk Factors for Diabetes Mellitus Patients Against Covid-19. Qanun Medika – Medical Journal Faculty of Medicine Muhammadiyah Surabaya. 2021;5(2). doi:10.30651/jqm.v5i2.4999
- Ghosh D, Stumhofer JS. The Spleen: “Epicenter” in Malaria Infection and Immunity. J Leukoc Biol. 2021;110(4):753-769. doi:10.1002/JLB.4RI1020-713R
- McKenzie C V., Colonne CK, Yeo JH, Fraser ST. Splenomegaly: Pathophysiological Bases and Therapeutic Options. Int J Biochem Cell Biol. 2018;94:40-43. doi:10.1016/J.BIOCEL.2017.11.011
- Maslachah L, Sugihartuti R, Wahyuni RS. Hematologic Changes and Splenic Index on Malaria Mice Models Given Syzygium cumini Extract as an Adjuvant Therapy. Vet World. 2019;12(1):106-111.
- Dkhil MA, Aljawdah HMA, Abdel-Gaber R, Thagfan FA, Delic D, Al-Quraishy S. The effect of Eucalyptus camaldulensis leaf extracts from different environmental harvesting locations on Plasmodium chabaudi-induced malaria outcome. Food Science and Technology (Brazil). 2023;43. doi:10.1590/fst.006723
- Budiapsari PI, Jaya PKD, Dewi PMACPN, Laksemi DAAS, Horng JT. Effect of moringa extract on parasitemia, monocyte activation and organomegaly among Mus musculus infected by Plasmodium berghei ANKA. Narra J. 2024;4(1):e653. doi:10.52225/narra.v4i1.653
- Brown DD, Solomon S, Lerner D, Del Rio M. Malaria and Acute Kidney Injury. Pediatric Nephrology. 2020;35(4):603-608. doi:10.1007/s00467-018-4191-0
- Siagian FE. Complications of Kidney in Severe Malaria. Asian Journal of Research in Infectious Diseases. 2022;11(3):6-17. doi:10.9734/ajrid/2022/v11i3218
- Katsoulis O, Georgiadou A, Cunnington AJ. Immunopathology of Acute Kidney Injury in Severe Malaria. Front Immunol. 2021;12:1-8. doi:10.3389/fimmu.2021.651739
- Hawkes MT, Leligdowicz A, Batte A. Pathophysiology of Acute Kidney Injury in Malaria and Non-Malarial Febrile Illness: A Prospective Cohort Study. Pathogens. 2022;11(4). doi:10.3390/pathogens11040436
- Laryea MK, Sheringham Borquaye L. Antimalarial, Antioxidant, and Toxicological Evaluation of Extracts of Celtis africana, Grosseria vignei, Physalis micrantha, and Stachytarpheta angustifolia. Biochem Res Int. 2021;2021. doi:10.1155/2021/9971857








