Manuscript accepted on :16-10-2024
Published online on: 24-10-2024
Plagiarism Check: Yes
Reviewed by: Dr. Takkella Nagamma and Dr. Deepak Kumar
Second Review by: Dr. Ahmed Falayh Hassan
Final Approval by: Dr. Anton R Keslav
Vasudevan Elumalai1* , Yasotha Mahadevan2, Shanthi Balasubramanium1
, Yasotha Mahadevan2, Shanthi Balasubramanium1 , Mary Chandrika Anton3
, Mary Chandrika Anton3 , Bikkipatti Jyothirmayi4
, Bikkipatti Jyothirmayi4 and Chaganti Sridevi5
and Chaganti Sridevi5
1Department of Biochemistry, Sree Balaji Medical College and Hospital, Chrompet, Chennai ,Tamilnadu, India.
2Department of Biochemistry, JR Medical College and Hospital, Avanampattu, Chennai, Tamil Nadu, India.
3Department of Biochemistry, Bharath Medical College and Hospital. Selaiyur, Chennai, Tamil Nadu, India.
4Department of Biochemistry, Madha Medical College and Hospital Kovur, Thandalam, Tamil Nadu, India.
5Department of Biochemistry, Prathima Relief institute of medical sciences Vangapahad, Telangana, India.
Corresponding Author E-mail: Vasukalai24@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3049
Abstract
Background: Type 2 Diabetes Mellitus (T2DM), a prevalent metabolic disease, is characterized by chronic hyperglycemia and insulin resistance. Diabetic patients frequently experience thyroid dysfunction, which has an impact on their metabolic condition. The goal of this study is to evaluate the thyroid state of T2DM patients and look into the link between insulin resistance and thyroid hormone levels. Methods:The study included 30 male participants aged 30 to 60 years who were diagnosed with T2DM using the American Diabetes Association's (ADA) criteria. Insulin resistance was determined using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). Thyroid function tests, including serum Thyroid Stimulating Hormone (TSH), Triiodothyronine (T3), and Thyroxine (T4) levels, were performed. Patients were divided into three groups based on their HOMA-IR values: low (≤2.5), moderate (2.5-4.0), and high (>4.0). Statistical analysis was used to determine the relationship between insulin resistance and thyroid hormone levels. Results: The study population's mean age was 51.9 ± 5.94 years. Our study denotes that Insulin resistance, as depicted by HOMA-IR correlates positively with TSH levels (r = 0.50). T4 correlates positively with TSH (r = 0.545) and T3 correlates well with T4 (0.598). Insulin resistance correlates positively with TSH levels (r = 0.50) Conclusion: This study confirms that insulin resistance in T2DM is associated with moderately enhanced levels of TSH, T3, and T4. The positive correlation between HOMA-IR and thyroid hormone levels suggests that insulin resistance may influence thyroid function. Regular thyroid status monitoring in T2DM patients is critical for early detection and management of thyroid dysfunction, which could improve overall metabolic control and quality of life. Further research is needed to determine the underlying mechanisms that link insulin resistance and thyroid dysfunction in T2DM.
Keywords
Diabetes Mellitus; insulin Resistance; Thyroid; TSH
Download this article as:| Copy the following to cite this article: Elumalai V, Mahadevan Y, Balasubramanium S, Anton M. C, Jyothirmayi B, Sridevi C. Evaluation of thyroid status in type 2 Diabetes Mellitus with Reference to insulin Resistance. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Elumalai V, Mahadevan Y, Balasubramanium S, Anton M. C, Jyothirmayi B, Sridevi C. Evaluation of thyroid status in type 2 Diabetes Mellitus with Reference to insulin Resistance. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3Yy9NBQ |
Introduction
Type 2 diabetes mellitus (T2DM), constituting 90-95% of all diabetes cases, is the most common form of the illness and a significant global public health issue1. Chronic hyperglycemia caused by insulin resistance and relative insulin deficiency is a characteristic of type 2 diabetes. Ageing populations, urbanization, and changes in lifestyle are major factors contributing to the rising prevalence of type 2 diabetes. According to World Health Organization (WHO) projections, there will be 700 million diabetics worldwide by 2045, up from 463 million in 2019. This will present serious challenges for healthcare systems around the world2.
Another common endocrine disorder is thyroid dysfunction, which primarily manifests as hypothyroidism and hyperthyroidism. The thyroid gland produces two thyroid hormones: thyroxine (T4) and triiodothyronine (T3),which are essential for controlling metabolism, energy balance, and general endocrine function. Thyroid hormones are essential for many physiological functions, such as development, growth, and control of metabolism3. Because thyroid dysfunction and diabetes have similar effects on metabolism and endocrine function, the relationship between these two conditions has been the focus of much research. Insulin resistance, a defining feature of type 2 diabetes, is the reduced capacity of cells to react to insulin’s action in transferring glucose from the bloodstream into tissues. Hyperinsulinemia and compensatory beta-cell hyperactivity in the pancreas are the results of this condition4. Insulin resistance can eventually cause beta-cell malfunction and failure, culminating in hyperglycemia and type 2 diabetes mellitus. Numerous studies indicate a profound connection between thyroid disease and insulin resistance. Changes in thyroid hormone levels have been linked to insulin resistance; on the other hand, thyroid hormones affect insulin sensitivity and glucose metabolism5. Insulin sensitivity is directly correlated with thermogenesis, lipid metabolism, and basal metabolic rate—all of which are significantly influenced by thyroid hormones. Reduced insulin sensitivity has been linked to hypothyroidism, which is characterized by low thyroid hormone levels, whereas increased insulin resistance can result from hyperthyroidism, which is characterized by high thyroid hormone levels6. Type 2 diabetics are more prone than the general population to have thyroid problems. Thyroid dysfunction prevalence in T2DM patients has been found to vary widely, from 10% to 30%, with hypothyroidism being more common than hyperthyroidism, according to several studies7. Among patients with type 2 diabetes, subclinical hypothyroidism—characterized by elevated TSH levels but normal free T4 levels—is especially common8.In individuals with type 2 diabetes, thyroid dysfunction can aggravate the metabolic abnormalities linked to the disease, making glycemic control more difficult and raising the risk of cardiovascular complications9. Hypothyroidism can worsen the metabolic profile of diabetic patients by causing dyslipidemia, weight gain, and increased insulin resistance10. Conversely, hyperthyroidism can quicken metabolic processes, making it harder to maintain glycemic control and increasing glucose turnover11. Thus, it is essential to comprehend how insulin resistance and thyroid function interact in type 2 diabetes in order to optimize clinical care and enhance patient outcomes. The interaction of insulin resistance and thyroid dysfunction is governed by complex and diverse mechanisms. There are numerous possible explanations for this relationship.
The Direct Effects of Insulin on Thyroid Function
Thyroid gland function is known to be directly impacted by insulin. Thyroid cells have receptors for insulin, and insulin affects the production and release of thyroid hormones. Hyperinsulinemia in insulin-resistant states can alter thyroid hormone synthesis and secretion, potentially leading to thyroid dysfunction12.
Thyroid Hormones and Insulin Sensitivity
Thyroid hormones, especially triiodothyronine (T3) and thyroxine (T4), are crucial in glucose metabolism and insulin sensitivity. Hyperthyroidism, characterized by an excess of thyroid hormones, enhances tissue insulin sensitivity, leading to augmented glucose absorption and metabolism, potentially culminating in hypoglycemia. Hypothyroidism (low thyroid hormone levels) can result in peripheral tissues that do not react to insulin as they should, a condition known as insulin resistance. This occurs as a result of reduced glucose absorption caused by decreased glucose transporter (GLUT) function, specifically GLUT4 in muscle and adipose tissue 13.
Insulin and Thyroid Function
Thyroid function is also influenced by insulin. The thyroid axis may be impacted by variations in insulin levels in diabetes. Thyroid gland function may be hampered by hyperinsulinemia, which is commonly observed in insulin resistance and type 2 diabetes. Insulin enhances the release of thyroid hormones and supports the growth of thyroid cells. Additionally, insulin and thyroid hormones are closely related in metabolic control because they have overlapping functions in thermogenesis, protein synthesis, and lipid metabolism 14.
Thyroid-Stimulating Hormone (TSH) and Insulin Resistance
TSH levels may have an impact on insulin sensitivity. Hypothyroidism is often associated with elevated TSH levels, which are associated with greater insulin resistance. The main cause of this is TSH’s impact on lipid metabolism, which can alter adipokine levels (including adiponectin and leptin) and exacerbate insulin resistance. A reciprocal relationship between the thyroid axis and insulin regulation has also been shown, since insulin has been shown to influence TSH secretion 15.
Thyroid hormones impact insulin sensitivity and glucose metabolism, while insulin regulates thyroid activity. This relationship between insulin and thyroid function involves several routes. Both hormones have an impact on energy regulation, thermogenesis, and lipid metabolism; an imbalance between them can lead to thyroid issues, insulin resistance, and metabolic disorders. Their essential functions in preserving metabolic balance are highlighted by the thyroid gland’s reciprocal interactions with insulin signaling pathways 16.
Impact on Thyroid Hormone Metabolism
Thyroid hormone metabolism in the peripheral circulation can be impacted by insulin resistance and hyperinsulinemia. Changes in the activity of deiodinases, which convert T4 to the more active T3, as well as thyroid hormone inactivation, have been linked to insulin resistance. In the context of type 2 diabetes abnormal deiodinase activity can cause imbalances in thyroid hormone levels17.
Inflammation and Oxidative Stress
Thyroid dysfunction and insulin resistance are characterized by oxidative stress and chronic inflammation. Pro-inflammatory cytokines and indicators of oxidative stress can disrupt thyroid hormone synthesis and function. Inflammatory mediators such as TNF-α and IL-6 can disrupt thyroid hormone function by interfering with the hypothalamic-pituitary-thyroid (HPT) axis18.
Adipokines and Metabolic Dysregulation
Adipokines like leptin and adiponectin, which are involved in controlling thyroid function and glucose metabolism, are secreted differently when adipose tissue malfunctions in obesity and insulin resistance. It has been demonstrated that leptin, in particular, affects thyroid hormone levels and the HPT axis19.
Thyroid function should be assessed in T2DM patients due to the high prevalence of both conditions and the possibility of a bidirectional relationship. This is especially important when insulin resistance is present. Early detection of thyroid dysfunction in diabetes patients can help with prompt intervention and stop the worsening of metabolic complications. Furthermore, comprehending how insulin resistance affects thyroid function can shed light on the pathophysiological processes underlying these two prevalent endocrine disorders20.
The goal of this study is to evaluate thyroid function in people with type 2 diabetes and look into the relationship between thyroid hormone levels and insulin resistance. The purpose of this study is to clarify the effect of insulin resistance on thyroid dysfunction in type 2 diabetes by investigating the relationship between HOMA-IR (Homeostatic Model Assessment of Insulin Resistance) and thyroid function parameters (TSH, T3, and T4)21. The findings of this study may improve metabolic outcomes and guide treatment of thyroid disorders in diabetic patients, influencing clinical practice.
AIM
This study’s main objectives are to assess thyroid function in individuals with Type 2 Diabetes Mellitus (T2DM) and look into the relationship between thyroid hormone levels and insulin resistance.
Materials and Methods
This analytical case-control study was conducted at the Mahatma Gandhi Medical College and Research Institute, Pillayarkuppam, Puducherry [MGMCRI], a tertiary health care institution under the jurisdiction of Sri Balaji Vidyapeeth University Puducherry, in collaboration with the Departments of General Medicine. Both the Institutional Research Committee and the Institutional Human Ethical Committee approved the project. After getting informed consent, a standardised health questionnaire was distributed to study participants, which included 30 Type 2 diabetes (male) in the age group 30-60 were included for the study (cases) and 30 healthy controls. It contained information on current and previous medication use, Type 2 Diabetes mellitus and hypertension, and Thyroid disorders. Subjects were chosen based on their responses to study-related questions.
Inclusion Criteria
Patients who are men between the ages of 30 and 60 and Men Healthy individuals
T2DM was identified using the American Diabetes Association’s (ADA) criteria for diagnosis22.
Duration of diabetes of at least one year.
Exclusion Criteria
Individuals suffering from diabetes, including Type 1 Diabetes Mellitus.
Patients with known thyroid disorders or those on thyroid hormone replacement therapy.
Patients with chronic kidney disease, liver disease, or other significant comorbidities.
Individuals taking drugs that may impact thyroid function (such as lithium and amiodarone).
The menstrual cycle affects hormone levels in female participants, particularly insulin sensitivity and thyroid hormones, which may have an impact on the study’s findings. Women’s levels of estrogen and progesterone affect lipid profiles, insulin action, and glucose metabolism, which can skew the findings of research attempting to determine how insulin resistance affects thyroid function.
Sample Collection
After a 10-12hour fast, four ml of venous blood had been taken under aseptic conditions. For the measurement of insulin and plasma glucose, two ml of blood were drawn in EDTA tubes, and two ml of blood were used to make serum.
Study Parameters
Fasting blood glucose
Fasting insulin
Glycated haemoglobin (HbA1C)
Insulin resistance was measured by HOMA – IR
T3,T4 &TSH
Clinical Evaluation
A detailed clinical history was obtained from each participant, including age, duration of diabetes, medication history, and presence of diabetic complications. A complete physical examination was performed, which involved taking blood pressure, body mass index (BMI), weight, and height measurements.
Biochemical Analysis
All participants provided fasting blood samples for the following biochemical analyses to be performed
Fasting Plasma Glucose (FPG)
Measured by means of the glucose oxidase-peroxidase technique23.
Glycated Hemoglobin (HbA1c%): High-performance liquid chromatography was used to evaluate (HPLC) 24,25.
Insulin Levels
Evaluated using the enzyme-linked immunosorbent assay (ELISA)26.
Thyroid Function Tests: Comprised of chemiluminescent immunoassay measurements of Free T3(pg/mL) (Triiodothyronine), Free T4(ng/dL) (Thyroxine), and Thyroid stimulating hormone (TSH) (mIU/L)27.
Assessment of Insulin Resistance
The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), which was computed using the following formula, was used to assess insulin resistance.
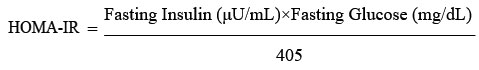
Alternatively, when glucose is measured in mmol/L:
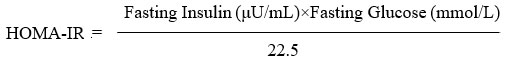
More than 2.5 on the HOMA-IR was regarded as a sign of insulin resistance28.
Statistical Analysis
The SPSS software, version 25.0, was used to analyze the data. For continuous variables, the presentation format was mean ± standard deviation (SD), while frequencies and percentages were used for categorical variables. Pearson’s correlation coefficient was used to assess the relationship between thyroid hormone levels (TSH, fT3, and fT4) and insulin resistance (HOMA-IR). Based on their HOMA-IR values, participants were divided into three groups: low (HOMA-IR < 2.5), moderate (HOMA-IR 2.5-4.0), and high (HOMA-IR > 4.0). ANOVA was used to compare the variations in thyroid hormone levels between these groups, and post hoc analysis was performed as needed. P-values less than 0.05 were deemed statistically significant.
Results
Following appropriate screening that included the below – mentioned facets, the study was undertaken. The population (case, control) pertained to Puducherry and adjoining areas.
n= 30 each for cases (Type 2 Diabetics) and control (healthy age and gender matched adults).
The observation and results depicted in the study pertain to the above-mentioned facets of inclusion criteria. Table 1 shows the mean, standard deviation, and statistical significance of the parameters (biochemical) that were included in the study. The SPSS software, version 25.0, was used to analyze the data.
Table 1: Mean, standard deviation and level of significance in case and control (males)
|
PARAMETER |
CONTROL (MEAN±SD) |
CASE (MEAN±SD) |
p-value |
|
AGE |
47.15 ± 4.27 |
51.9 ± 5.94 |
0.0061 |
|
FBG |
90.9 ± 5.29 |
153.6 ± 38.93 |
0.0000 |
|
INSULIN |
2.42 ± 0.48 |
6.05 ± 1.85 |
0.0000 |
|
HOMA IR |
9.84 ± 2.45 |
38.68 ± 27.32 |
0.0000 |
|
HBA1C |
4.08 ± 0.63 |
8.26 ± 1.95 |
0.0000 |
|
TSH |
1.64 ± 0.37 |
4.8 ± 0.77 |
0.0000 |
|
T3 |
2.04 ± 0.48 |
5.12 ± 0.65 |
0.0000 |
|
T4 |
0.64 ± 0.46 |
1.67 ± 0.42 |
0.0000 |
*pvalue ≤0.05 was deemed statistically significant
FBG– fasting plasma glucose (venous); Insulin –fasting plasma insulin (venous)
HOMA – IR- Homeostatic model of Assessment –insulin resistance; HbA1C- Glycated hemoglobin
TSH – Thyrotropin; T3- Tri-iodothyronine; T4- Thyroxine
Table 2: Comparison of glycemic control and insulin resistance between the case and control group
|
PARAMETER |
CONTROL (MEAN±SD) |
Case (MEAN±SD) |
p-value |
|
FBG |
90.9 ± 5.29 |
153.6 ± 38.93 |
0.0000 |
|
INSULIN |
2.42 ± 0.48 |
6.05 ± 1.85 |
0.0000 |
|
HOMA-IR |
9.84 ± 2.45 |
38.68 ± 27.32 |
0.0000 |
|
HbA1C |
4.08 ± 0.63 |
8.26 ± 1.95 |
0.0000 |
A significance level of P<0.05 was deemed statistically significant.
FBG– fasting plasma glucose (venous); Insulin –fasting plasma insulin (venous)
HOMA – IR- Homeostatic model of Assessment –insulin resistance; HbA1C- Glycated hemoglobin.
The results depicted in Tables 1 and 2 clearly show significant increase in insulin resistance and lowered glycemic control
Table 3: Comparison of serum thyroid profile between the case and control groups
|
PARAMETER |
CONTROL(MEAN±SD) |
CASE (MEAN±SD) |
p-value |
|
TSH |
1.64 ± 0.37 |
4.8 ± 0.77 |
0.0000 |
|
T3 |
2.04 ± 0.48 |
5.12 ± 0.65 |
0.0000 |
|
T4 |
0.64 ± 0.46 |
1.67 ± 0.42 |
0.0000 |
In order to facilitate Thyroid status in T2DM as related to the present study, the values of Thyroid hormones were compared. TSH, T3 and T4 were significantly elevated.
Table 4: Correlation of HOMA IR, TSH, T3, T4 in case and control groups. Group = control
| Correlations | |||||
|
|
|
HOMA_IR |
TSH |
T3 |
T4 |
|
HOMA_IR |
Pearson Correlation |
1 |
.159 |
-.051 |
-.188 |
|
Sig. (2-tailed) |
|
.503 |
.829 |
.429 |
|
|
N |
30 |
30 |
30 |
30 |
|
|
TSH |
Pearson Correlation |
.159 |
1 |
-.078 |
-.208 |
|
Sig. (2-tailed) |
.503 |
|
.745 |
.378 |
|
|
N |
30 |
30 |
30 |
30 |
|
|
T3 |
Pearson Correlation |
-.051 |
-.078 |
1 |
-.050 |
|
Sig. (2-tailed) |
.829 |
.745 |
|
.836 |
|
|
N |
30 |
30 |
30 |
30 |
|
|
T4 |
Pearson Correlation |
-.188 |
-.208 |
-.050 |
1 |
|
Sig. (2-tailed) |
.429 |
.378 |
.836 |
|
|
|
N |
30 |
30 |
30 |
30 |
|
Group = case
| Correlations | |||||
|
|
|
HOMA_IR |
TSH |
T3 |
T4 |
|
HOMA_IR |
Pearson Correlation |
1 |
.501* |
.685** |
.745** |
|
Sig. (2-tailed) |
|
.024 |
.001 |
.000 |
|
|
N |
30 |
30 |
30 |
30 |
|
|
TSH |
Pearson Correlation |
.501* |
1 |
.299 |
.545* |
|
Sig. (2-tailed) |
.024 |
|
.200 |
.013 |
|
|
N |
30 |
30 |
30 |
30 |
|
|
T3 |
Pearson Correlation |
.685** |
.299 |
1 |
.598** |
|
Sig. (2-tailed) |
.001 |
.200 |
|
.005 |
|
|
N |
30 |
30 |
30 |
30 |
|
|
T4 |
Pearson Correlation |
.745** |
.545* |
.598** |
1 |
|
Sig. (2-tailed) |
.000 |
.013 |
.005 |
|
|
|
N |
30 |
30 |
30 |
30 |
|
*. Correlation is significant at the 0.05 level (2-tailed).
**. Correlation is significant at the 0.01 level (2-tailed).
Table 4 in this study denotes that Insulin resistance, as depicted by HOMA-IR correlates positively with TSH levels (r = 0.50) . T4 correlates positively with TSH (r = 0.545*) and T3 correlates well with T4 (0.598**). Insulin resistance correlates positively with TSH levels (r = 0.50)
Discussion
Furthermore, the current research has shown that slightly higher levels of TSH, free T3, and free T4 are associated with insulin resistance in type 2 diabetes, indicating subclinical secondary hyperthyroidism. However, a previous study29, which used HOMA-IR to illustrate insulin resistance, suggested hypothyroidism. According to a different study, hyperthyroidism and type 2 diabetes are related. The pathogenetic mechanism seems to be quite intricate, involving multiple factors that interact to cause insulin resistance and metabolic disorders30.Research indicates that insulin resistance is linked to both subclinical and hyperthyroidism31. The results of our study, which were gathered from Puducherry and the surrounding areas, indicate that insulin resistance positively correlates with TSH levels (r = 0.50). This finding assumes relevance given the comorbid status observed in T2DM. Furthermore, our study shows that free T3 strongly correlates with T4 (0.598**) and free T4 positively correlates with TSH (r = 0.545*). A potential correlation between insulin resistance (as assessed by HOMA-IR throughout the study) and fasting blood glucose was examined. Insulin resistance and fasting blood glucose were found to be positively correlated (r = 0.815). Moreover, there was a strong correlation (r = 0.597 and 0.629) between fasting blood glucose and the thyroid hormone levels Free T3 and Free T4, respectively. As a result, it is evident that total cholesterol in T2DM could still be utilized as a predictor of the composite study in relation to insulin resistance and subclinical hypothyroidism, given that there is a strong correlation (r = 0.685 and r = -0.745) between insulin resistance and thyroid hormone levels. Insulin resistance is a progressive condition (hyperinsulinism – hyperglycemia finally proving was for hyperglycemic beta depletion), so it is evident that insulin resistance is clearly linked to subclinical hypothyroidism. However, more research needs to be done in scenarios where HOMA-IR, a measure of insulin resistance, can increase due to elevated fasting blood glucose or fasting plasma insulin venous, or both. This would mean that the magnitude of the status involving thyroid dysfunction would change with the phase of insulin resistance. It has been observed that T2DM concurrent with thyroid dysfunction leads to the development of small events, with atherogenesis being a major concern. It is necessary to consider how therapy may affect how these endocrine diseases progress. Research suggests that yearly thyroid dysfunction screening has become essential for T2DM patients32. Subclinical hyperthyroidism and type 2 diabetes have been linked in recent years33. There aren’t many Indian studies available, though. Subclinical hyperthyroidism and Type 2 Diabetes have been linked to increased risk and mortality from cardiovascular disease. When comparing patients with subclinical hyperthyroidism to those without thyroid hyperfunction, the former group appears older and has had diabetes for a longer period of time. According to a report by Díez and Iglesias, there was a greater percentage of diet therapy and goiter treatment combined with a lower percentage of oral agent treatment. There were also lower fasting glucose levels. Age and the presence of goiter were found to be significantly correlated with subclinical hyperthyroidism in patients with type 2 diabetes, according to logistic regression analysis34. What is concerning is that our research indicates a correlation between insulin resistance and subclinical hyperthyroidism of secondary nature. Moreover, Suzuki et al. linked the hypothalamo-pituitary thyroid axis’ malfunction and the existence of thyroid hormone binding inhibitors (THBI), which inhibit the extra thyroidal conversion enzyme 5-deiodinase, which converts T4 to T3, to the aberrant thyroid hormone levels observed in diabetic patients35.Low calcium intake and vitamin D deficiency have been related to impaired fasting glucose and a higher risk of type 2 diabetes mellitus, both of which are risk factors for coronary artery disease (CAD)36 Reducing variability associated with sex variations in thyroid function, hormonal impacts, and metabolic patterns is probably the goal of the study’s decision to solely include male patients. It gives researchers a good understanding of this particular group by enabling them to concentrate on the relationships between insulin resistance and thyroid function in men. The differences in these interactions between women or between sexes37 may then be the subject of future research.However, our study found that secondary hyperthyroidism was associated with higher FT3 than FT4 levels. This could be due to a variety of deiodinase activation pathways. We carried up the investigation since few other studies have addressed the comorbid status as it is shown in Puducherry and the neighboring areas. Along with reflecting co-morbidity, we also sought to find a few simple biochemical indicators that would indicate the severity of endocrinopathies.
Conclusion
In conclusion, this study discovered that among male T2DM patients, insulin resistance and TSH levels were significantly positively correlated. Furthermore, TSH correlates positively with fT4, and fT4 correlates well with fT3, indicating that insulin resistance can impair thyroid function. These findings emphasize the importance of regular thyroid function monitoring in T2DM patients, as well as the need for integrated therapeutic approaches that address insulin resistance and thyroid dysfunction. To create efficient treatment plans for this patient group and to comprehend the mechanisms underlying this relationship, more research is required.
Limitation of the Study
When evaluating the findings of this study, it is important to take into account a number of limitations. Since the cross-sectional methodology precludes the demonstration of causal links, the relatively small sample size may restrict the generalizability of our findings. Furthermore, because the study only included male individuals, it cannot be applied to female T2DM patients, who can have different thyroid profiles and metabolic reactions. The link between thyroid function, insulin resistance, and type 2 diabetes requires more research with bigger, more varied populations, strict control of confounding variables, and long-term follow-up.
Acknowledgement
We gratefully acknowledge the Departments of Biochemistry, General Medicine, and Cardiology at Mahatma Gandhi Medical College and Research Institute, Puducherry, for their assistance in completing this project.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of Interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
The ethical approval to conduct this research was obtained from institutional ethics committee [IHEC] MGMCRI/2021/1653
Informed Consent Statement
Informed Consent Statement obtained from all the study participants with his own language
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ Contribution
Mr. E.Vasudevan conducted this research under the supervision of Dr. B. Shanthi. The discussion was written by Miss Yasotha,Dr. Mary Chandrika Anton, Dr. Chaganti Sridevi, and Dr.Bikkipatti Jyothirmayi.
References
- Classification and diagnosis of diabetes:standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1). doi:10.2337/dc22-s002
CrossRef - Global report on diabetes. World Health Organization. Accessed September 17, 2024. https://www.who.int/publications-detail-redirect/9789241565257.
- Brent GA. Mechanisms of thyroid hormone action. The Journal of clinical investigation. September 2012. Accessed September 17, 2024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3433956/.
- DeFronzo RA, Ferrannini E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173-194. doi:10.2337/diacare.14.3.173
CrossRef - Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Research and Clinical Practice. 2011;93. doi:10.1016/s0168-8227(11)70014-6
CrossRef - Gierach M, Gierach J, Junik R. Insulinooporność a choroby tarczycy. Endokrynologia Polska. 2014;65(1):70-76. doi:10.5603/ep.2014.0010
CrossRef - Han C, He X, Xia X. Subclinical hypothyroidism and type 2 diabetes: A systematic review and meta-analysis. PLOS ONE. 2015;10(8). doi:10.1371/journal.pone.0135233
CrossRef - Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine Reviews. 2007;29(1):76-131. doi:10.1210/er.2006-0043
CrossRef - Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. European Journal of Endocrinology. 2009;160(4):503-515. doi:10.1530/eje-08-0837
CrossRef - Pearce SHS, Brabant G, Duntas LH. 2013 ETA Guideline: Management of subclinical hypothyroidism. European Thyroid Journal. 2013;2(4):215-228. doi:10.1159/000356507
CrossRef - Evran M. Metabolic and cardiovascular aspects of subclinical hypothyroidism: Effects of L-thyroxin replacement therapy. Journal of Thyroid Disorders & Therapy. 2012;01(03). doi:10.4172/2167-7948.1000112
CrossRef - Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. Journal of Clinical Oncology. 1995;13(3):688-696. doi:10.1200/jco.1995.13.3.688
CrossRef - Dimitriadis G, Baker B, Marsh H. Effect of thyroid hormone excess on action, secretion, and metabolism of insulin in humans. American Journal of Physiology-Endocrinology and Metabolism. 1985;248(5). doi:10.1152/ajpendo.1985.248.5.e593
CrossRef - Brenta G. Why can insulin resistance be a natural consequence of thyroid dysfunction? Journal of Thyroid Research. 2011;2011:1-9. doi:10.4061/2011/152850
CrossRef - Iacobellis G, Cristina Ribaudo M, Zappaterreno A, Valeria Iannucci C, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clinical Endocrinology. 2005;62(4):487-491. doi:10.1111/j.1365-2265.2005.02247.x
CrossRef - Eggo MC, Bachrach LK, Burrow GN. Interaction of TSH, insulin and insulin-like growth factors in regulating thyroid growth and function. Growth Factors. 1990;2(2):99-109. doi:10.3109/08977199009071497
CrossRef - Sinha RA, Singh BK, Yen PM. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends in Endocrinology & Metabolism. 2014;25(10):538-545. doi:10.1016/j.tem.2014.07.001
CrossRef - Bastemir M, Akin F, Emral R, Alkis E. Impact of insulin sensitivity in relationship with prolactin and thyroid stimulating hormone. Experimental and Clinical Endocrinology & Diabetes. 2007;115(04):257-260. doi:10.1055/s-2007-960492
CrossRef - Ruiz-Núñez B, Pruimboom L, Dijck-Brouwer DAJ, Muskiet FAJ. Lifestyle and nutritional imbalances associated with western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. The Journal of Nutritional Biochemistry. 2013;24(7):1183-1201. doi:10.1016/j.jnutbio.2013.02.009
CrossRef - Anagnostis P, Efstathiadou ZA, Slavakis A, The effect of L-thyroxine substitution on lipid profile, glucose homeostasis, inflammation and coagulation in patients with subclinical hypothyroidism. International Journal of Clinical Practice. 2014;68(7):857-863. doi:10.1111/ijcp.12394
CrossRef - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. doi:10.1007/bf00280883
CrossRef - American Diabetes Association. Diagnosis and classification of diabetes mellitus. American Diabetes Association. December 16, 2013. Accessed September 17, 2024. https://diabetesjournals.org/care/ article/37/ Supplement_1/S81/37753/Diagnosis-and-Classification-of-Diabetes-Mellitus.
CrossRef - Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. Journal of Clinical Pathology. 1969;22(2):158-161. doi:10.1136/jcp.22.2.158
CrossRef - Weykamp CW, Penders TJ, Siebelder CW, Muskiet FA, van der Slik W. Interference of carbamylated and acetylated hemoglobins in assays of glycohemoglobin by HPLC, electrophoresis, affinity chromatography, and enzyme immunoassay. Clinical Chemistry. 1993;39(1):138-142. doi:10.1093/clinchem/39.1.138
CrossRef - Bachorik PS, Ross JW. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: Executive summary. The National Cholesterol Education Program Working Group on lipoprotein measurement. Clinical Chemistry. 1995;41(10):1414-1420. doi:10.1093/clinchem/41.10.1414
CrossRef - Temple R, Clark PMS, Hales CN. Measurement of insulin secretion in type 2 diabetes: Problems and pitfalls. Diabetic Medicine. 1992;9(6):503-512. doi:10.1111/j.1464-5491.1992.tb01830.x
CrossRef - Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyrotropin (TSH) assays. Clinical Chemistry. 1996;42(1):140-145. doi:10.1093/clinchem/42.1.140
CrossRef - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. doi:10.1007/bf00280883
CrossRef - Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. Journal of Cardiovascular Risk. 1996;3(2):213-219. doi:10.1097/00043798-199604000-00014
CrossRef - Taskinen M-R. Quantitative and qualitative lipoprotein abnormalities in diabetes mellitus. Diabetes. 1992;41(Supplement_2):12-17. doi:10.2337/diab.41.2.s12
CrossRef - Abate N. Abnormal cholesterol distribution among lipoprotein fractions in normolipidemic patients with MILD NIDDM. Atherosclerosis. 1995;118(1):111-122. doi:10.1016/0021-9150(95)05597-p
CrossRef - Lambadiari V, Mitrou P, Maratou E. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine. 2010;39(1):28-32. doi:10.1007/s12020-010-9408-3
CrossRef - Tan KC, Shiu SW, Kung. AW. Effect of thyroid dysfunction on high-density lipoprotein subfraction metabolism: Roles of hepatic lipase and cholesteryl ester transfer protein1. The Journal of Clinical Endocrinology & Metabolism. 1998;83(8):2921-2924. doi:10.1210/jcem.83.8.4938
CrossRef - Díez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Experimental and Clinical Endocrinology & Diabetes. 2011;119(04):201-207. doi:10.1055/s-0031-1271691
CrossRef - Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). The American Journal of Cardiology. 1992;70(7):733-737. doi:10.1016/0002-9149(92)90550-i
CrossRef - Vasudevan E, Anton MC, Shanthi B, Sridevi C, Sumathi K, Nivethini N. Significance of serum ferritin and Vitamin-D level in coronary artery disease patients. Biomedical and Pharmacology Journal. 2023;16(1):365-369. doi:10.13005/bpj/2618
CrossRef - Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: Two closely associated disorders. Endocrine reviews. June 1, 2019. Accessed October 9, 2024. https://www.ncbi.nlm.nih.gov/pmc/ articles/ PMC6507635/.







