Abdumalik Nigmatovich Aripov1 , Lola Lazizovna Akhunzhanova1
, Lola Lazizovna Akhunzhanova1 , Abdusamad Utkirovich Nabiev1
, Abdusamad Utkirovich Nabiev1 , Orifzhon Abdumalikovich Aripov1
, Orifzhon Abdumalikovich Aripov1 and Tolmas Tolibovich Khamroev2
and Tolmas Tolibovich Khamroev2
1Department of Clinical Laboratory diagnostics of Republican Specialized Scientific and Practical Medical Center of Pediatrics of the Ministry of Health of the Republic of Uzbekistan, Tashkent City, Uzbekistan
2 The Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, Tashkent City, Uzbekistan.
Corresponding Author E-mail: tolmas4th@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/3063
Abstract
Background: Today, chronic liver diseases and pathologies that develop as their complications, such as LC or HCC, cause various socio-economic as well as medical problems, and therefore the elimination of fibrosis processes in the liver is an important urgent problem. In the event of complications such as LC and HCC, the process of LF acts as an immediate bridge. Which, in turn, is responsible for stopping changes in the liver at the stage of fibrosis. Aim: The focus of the investigations was on how the composition under investigation affected the early stages of liver fibrosis. The antifibrotic action of the substance under investigation was assessed primarily by focusing on the invariance of lowered markers or a decrease in fibrosis rates. Experimental Approach: All the studies conducted were carried out on rats, while severe chronic liver damage was caused by the introduction into the abdominal cavity according to a strict scheme of heliothrin, a substance with a hepatotoxic effect. The object of the study was a combination of heparin and yanatacin 1:1, the antifibrotic activity of which was studied in comparison with the popular drug Phosphogliv, widely used in medical practice. Key Results: In the conducted studies, it was noted that the combination of heparin and yantacin normalizes disorders caused by heliotrin in experimental animals, in particular, enzyme activity, cytokine levels, bile secretion and liver index. According to this activity, the comparative drug showed a clear advantage over phosphogliv. Conclusion: The studies show that the studied combination only prevents the transition of the fibrous process to the second or third stage, and therefore the search for drugs that can completely eliminate the stage of fibrosis in the liver has not lost its relevance.
Keywords
Bile secretion; cytokine levels; Hepalipin; heliotrin; liver index; yantacin
Download this article as:| Copy the following to cite this article: Aripov A. N, Akhunzhanova L. L, Nabiev A. U, Aripov O. A, Khamroev T. T. Evaluation of Antifibrotic Activity of a Combination of a New Phytocomposition and Proanthocyanidins in Rats.Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Aripov A. N, Akhunzhanova L. L, Nabiev A. U, Aripov O. A, Khamroev T. T. Evaluation of Antifibrotic Activity of a Combination of a New Phytocomposition and Proanthocyanidins in Rats.Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/3BX6lYY |
Introduction
Chronic liver diseases (ChLD), such as viral hepatitis, alcoholic hepatitis and non-alcoholic steatohepatitis, can occur as a result of exposure to various etiological factors that damage the liver, which, in turn, can lead to difficult-to-treat progressive liver disease liver cirrhosis (LC) 1, 2. On the other hand, LC has been one of the main causes of morbidity and mortality of people with ChLD worldwide for the past more than 20 years, and in 2019 alone, cirrhosis accounted for almost 2.5% of all deaths in the world 3, 4. Over the past forty years, the epidemiology and burden of cirrhosis of the liver have changed due to the constant decrease in the incidence of viral hepatitis and the development of promising drugs for their treatment. However, LC remains a serious problem in clinical practice due to the growing number of concomitant diseases such as obesity, alcoholic and non-alcoholic fatty liver disease, autoimmune liver diseases and some drug-related liver diseases5, 6, 7. It is worth noting that JS, in turn, can lead to hepatocellular carcinoma (HCC), which is considered the leader in prevalence and mortality among tumor diseases worldwide. It is the occurrence of LC in almost all patients with HCC that confirms these statements 1, 2, 8. Thus, LC is a global public health problem, which represents a serious burden for medical practice associated with various complications and high mortality, such as HCC, hepatic decompensation manifested by ascites, hepatic encephalopathy and varicose veins 3, 9, 10, 11, 12.
It is known that cirrhosis develops after prolonged inflammation of the liver caused by virological, chemical and pharmacological factors, which, in turn, leads to the replacement of healthy liver parenchyma with fibrous tissue and regenerative nodes that cause portal hypertension. It is progressive portal hypertension, systemic inflammation and liver failure that have developed as complications of LC that determine the prognosis of the disease. It is noteworthy that the disease moves from compensated cirrhosis with an asymptomatic phase to decompensated cirrhosis with a symptomatic phase, in which complications arise that usually lead to hospitalization, a decrease in quality of life and a higher mortality rate 13, 14, 15, 16. Thus, today it may be possible to eliminate the serious problems mentioned above, preventing LC, which acts as a direct bridge in the occurrence of serious complications and death. Usually, when this serious pathology occurs, liver fibrosis (LF) first develops as a highly conservative and coordinated protective response to damaged liver tissue caused by a traumatic factor, and with LF there is an excessive accumulation of extracellular matrix proteins, including collagen, which is present in many types of ChLD. Progressive LF, in turn, leads to cirrhosis, and its serious complications lead to liver failure and portal hypertension, and often require liver transplantation. A significant increase in knowledge about the cellular and molecular mechanisms of LF in recent years has prompted researchers to develop antifibrotic drugs that prevent the recurrence of progressive LF in patients. In this regard, new antifibrotic treatments are aimed at preventing the accumulation of fibrogenic cells or deposition of extracellular matrix proteins, but although they are effective in experimental LF models, their efficacy and safety for humans remain unknown 17, 18, 19, 20.
In this regard, in our country, including scientists from the Institute of Plant Chemistry of the Academy of Sciences of the Republic of Uzbekistan and the Republican Specialized Center for Applied Medicine of Pediatric Science, promising research work is underway to summarize the latest achievements in the study of pathogenesis and diagnosis of liver fibrosis and discuss modern antifibrotic strategies. In particular, the antifibrotic activity of a pharmacological composition consisting of a pronantacyanidin-preserving yantacin obtained on the basis of the alhaga pseudalhaga plant with high antioxidant activity and hepolipin preserving lycopene was studied in research conditions22, 23, 24, 25, 26, 27. To this end, in rats, the process of liver fibrosis was caused by the introduction of the pyrrolizidine alkaloid heliothrin, which has a toxic and carcinogenic effect on most organs and tissues, into the abdominal cavity according to a strict scheme with an atraumatic needle 28, 29, 30, 31, 32. The studies mainly studied the effect of the studied composition on the early stage of liver fibrosis, and the antifibrotic effect of the studied substance was evaluated with an emphasis on a decrease in fibrosis rates or the invariance of reduced indicators.
Experimental
Materials and Methods
When evaluating the antifibrotic activity of the test substance, all studies used male laboratory rats weighing 175 ± 5 grams, and during the experiments all the studied animals were kept in 2-week quarantine 33.
In order to cause severe ChLD in the condition of the study, experimental rats first injected heliothrin through a needle causing destruction and stress into the abdominal cavity, in decreasing doses according to the scheme, starting with the lethal dose administered to the studied animal. Substance with hepatotoxic effect heliotrin to rats intraperitonialy 250 mg/kg daily 3 times a week, 150 mg/kg 2 times a day for 2 weeks, 100 mg/ kg once every 3 days for 10 for days, 30 mg/kg for 4 days is administered 1 time for 12 days. Objective, laboratory and instrumental studies of experimental animals were conducted at weekly intervals to assess chronic changes in the liver. In objective studies, the main attention was paid to such indicators as the condition of experimental animals, appearance, body weight, and the need for food and water. Through laboratory and instrumental studies, chronic changes in the liver they were confirmed by laboratory and instrumental analyses on the last day of the study21, 27. Toxic damage to the liver, obtaining the necessary materials for analysis from the studied animals was carried out in accordance with the existing requirements for conducting experiments on animals, i.e. at the same time, rats in the experimental group were decapitated under ether anesthesia after diagnosis of fibrosis and treatment with the test substance 33. All research work in this article was carried out on the basis of the permission of the Ethics Committee under the Ministry of Health of the Republic of Uzbekistan No. 1/1-1628.
It is known that substances with hepatoprotective activity are drugs that improve metabolic processes in the liver, increase resistance to pathogens and help restore their activity in various diseases. It is the products with hepatoprotective activity that improve metabolic processes in the body, as well as the liver index, slow down lipid peroxidation, have antioxidant and antihypoxic activity, improve the amount of anti-inflammatory cytokines, slow down collagen synthesis, increase collagenase activity. In this regard, a pharmacological combination consisting of hepalipin and yantacin was also chosen as the object of research in these studies. The composition of the studied combination: hepalipin content: 0.0844 g – Lipoid c 80, 0.0006 g – lycopene, 0.005 g – ecdystene, 0.01 g – glycyrrhizic acid 22, 23, 24. Alhagi a substance belonging to the group of polyphenols isolated from the peseudoalhagi plant is conventionally called yantacin. This plant is widespread in Central Asia, known as camel’s cheek, rich in polyphenols and has long been used in folk medicine as a blood purifier, with indications of a good effect of the drug in gastrointestinal diseases 25, 26. Experimental animals with chronic toxic hepatitis or early stage fibrosis were injected with Hepalipin and yantacin at a dose of 100 mg/kg, that is, a solution prepared in a ratio of 1:1, and a comparable drug Phosphogliv at a dose of 50 mg/kg, and experimental animals of the control group were given distilled water orally for 35 days. It should be noted that the studied combination and the drug Phosphogliv were administered orally from the third week of the experiment along with an injection of heliotine into the intraperitonialy.
Changes in the behavioral behavior of rats were detected in the “open space” method 34 in order to identify objective changes that occurred in experimental animals when a substance with a hepatotoxic effect was administered to rats through a scheme.
Experimental research methods: the diagnosis of ChLD was carried out on the basis of traditional laboratory research methods. The levels of ALT, AST, total bilirubin (TB), total protein and its fractions, alkaline phosphatase, GGT were determined. All blood serums used in these studies were taken after the use of these substances in order to study the effectiveness of substances studied before treatment in order to identify chronic toxic lesions 35, 37, 50.
Verify the blood’s cytokine levels. Among the processes contributing to the pathophysiology of hepatitis, changes indicative of liver cell damage are known to cause tissue damage in diseases that are accompanied by inflammation. Numerous pro- and anti-inflammatory cytokines carry out the interaction between these systems. Cytokines that are known to be produced when inflammation develops. The IFT method identified the following markers in blood serum: the concentration of the primary inflammatory factors that cause interleukins, namely IL-2, IL-6, IL-1beta, and IL-17 35, 36, 37,38.
The anesthesiologist was called by injecting 40 mg/kg of ethaminal sodium into the abdominal cavity of experimental animals in order to assess the ability of the liver to secrete bile, calculated on the basis of critical functions. Then, according to the generally accepted rule 39, 40, 41, 42, bile separation was observed for 4 hours by installing a special conjugate at the confluence of the rat bile duct (ductus choledochus) into the duodenum.
With the development of complications of ChLD because of pathological disorders of body weight and at the same time liver parenchyma, changes in liver mass are observed. 37, 53, 54, 55. In this regard, by measuring the body weight of rats and the weight of their liver, their liver index (LI) was calculated and then the liver was cut into pieces and stored in isotonic formalin to determine histological evaluation.
The results are presented as an average value ± standard error. For statistical analysis of the data, a one-sided ANOVA test with post-hoc testing was used, with P<0.05 as the limit of significance.
Results
Comparison of the combination of hepalipin and yantacin and the acute toxicity of Phosphogliv.
The acute toxicity of the combination consisting of hepalipin and yantacin was dissolved to a state of 10-50% aqueous emulsion and administered orally in doses from 500 mg/kg to 5000 mg/kg in male white rats. At first, they were observed for 1-2 hours, then for a day, and also for 14 days in a vivarium. At doses of 3000 and 5000 mg/kg, a decrease in relative motor activity was observed in the first minutes, followed by a return to normal after 4-5 hours. Based on the experiments conducted, it was found that the acute toxicity of the combination of hepalipin and yantacin with oral single administration in rats belongs to the LD50 -5000 group and higher, i.e. it is absolutely harmless to class IV according to GOST 34. LD50 = 800 mg/kg with oral administration of Phosphogliv, taken as a reference drug 47. In terms of acute toxicity, the studied combination is less toxic than the reference drug Phosphogliv with phage by more than 5 times.
It is worth noting that medicines used today for various pathologies have high activity, as well as low toxicity and do not have a negative effect on organs and tissues of the body, which, in turn, leads to a further increase in their value and expansion of application possibilities. In this regard, over the past thirty years, the use of plant-based medicines and food additives has increased significantly worldwide, and the demand for the search and implementation of medicines based on them continues to grow 42, 43, 44, 45, 46. In the experiments conducted, it was found that the acute toxicity of the combination of hepalipin and yantacin with oral single administration in rats exceeded LD50 -5000 mg / kg. This means that the classification of acute toxicity of biologically active substances belongs to the group of absolutely harmless, that is, to class IV according to GOST 34. LD50 = 800 mg/kg with oral administration of Phosphogliv, obtained as a reference drug and widely used today in medical practice 47, and the studied combination is clearly less toxic than the reference drug Phosphogliv in terms of acute toxicity.
Evaluation of the effect of a combination consisting of hepalipin and yantacin on the objective parameters of experimental animals with chronic toxic liver damage.
Under the action of a combination consisting of hepalipin and yantacin, a positive tendency to improve motor activity was observed in experimental animals when a substance with a hepatotoxic effect was administered to rats according to a scheme that included barking fur and refusing food and water, as well as weight loss. In particular, the combination studied in the study of the effect on the behavioral behavior of mice in the open space method 34 showed that the rats improved motor or physical and research activity and demonstrated significant activity in this indicator compared with control group and with explicit fofogliv. The combination also showed a positive trend towards normalization of lost body weight in rats (Table 1). As shown in the table, in studies it was noticed that under the influence of a combination of the studied substances hepalipin and yantacin, rats approached the limits of normal body weight, and in this regard, the comparative drug also showed significantly higher activity compared with phosphogliv. It was also noted that when exposed to the test substance, the motor activity and research activity of experimental animals increased by 1.31 and 1.33 times compared with the studied dose of Phosphogliv by 2.1 and 1.86 times, respectively, compared with the control group.
Table 1: Comparison of the combination consisting of hepalipin and yantacin and the effect of Phosphogliv on rat body weight, as well as on motor and research activity.
| No. | Experimental groups | Doses in mg/kg | Body weight in gramm | Body weight in gramm | Motor activity | research activity |
| 1. | Control group | Dis.water | 176±2,24 | 126,72±2,02 | 8,6±0,67 | 10,2±0,89 |
| 2. | Hepalipin + Yantacin | 100+100 | 176,3±1,79 | 171,01±1,34* | 18,06±0,45* | 18,97±1,12* |
| 3. | Phosphogliv | 50 | 176,3±1,57 | 158,67±1,57* | 13,8±1,12* | 14,3±0,89* |
Note: * – reliability compared with the data of the control group-P<0.005
It is known that in chronic severe liver lesions of various etiologies, due to the inability of the liver to fully perform its functions, serious pathological disorders occur not only in a particular organ or tissue, but also in the entire body as a whole. Patients have severe neuropsychological disorders caused by the occurrence of hepatic encephalopathy due to liver failure, especially in pathologies such as LC or HCC 48, 49. The combination consisting of hepalipin and yantacin has a hepatotoxic effect, a substance that occurred in rats exposed to decreased motor activity, wool barking and refusal of food and water. There was also a positive tendency to improve reduced body weight. In particular, the combination studied in the study of the effect on the behavioral behavior of mice in the open space method 34 showed that the rats improved motor and search activity and demonstrated significant activity in this indicator compared to the control group compared with explicit fofogliv. This method is usually used to search for biologically active substances with sedative and sedative effects, as well as to assess cognitive functions. Based on the results obtained, it can be concluded that improving motor and search activity under the influence of the substance under study, in turn, also eliminates neuropsychological disorders caused by chronic liver damage.
Investigation of the effect of the sum of hepalipin and yantacin on the activity of enzymes in the blood in chronic heliothrin hepatitis.
Rats in the study showed indicators similar to those of the Intact group, but the administration of hepalipin and the sum of yantacin and flavonoids decreased the activity of liver enzymes when compared to rats in the control group (Fig. 2). Alkaline phosphatase activity, however, stayed within the typical range.
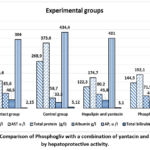 |
Figure 1. Comparison of Phosphogliv with a combination of yantacin and hepalipin by hepatoprotective activity.Click here to View Figure |
The combination of yantacin and hepalipin had a pronounced hepatoprotective activity in rat serum in pre-clinical studies.Therefore, in the chronic heliotrine hepatitis model, the combination of yantacin and hepalipin demonstrated greater hepatoprotective activity in comparison to the nasoart group, and comparable or noticeably higher hepatoprotective activity in comparison to phosphogliv.
Investigation of the effect of the amount of hepalipin and yantacin on the amount of cytokines.
Studies have shown that the studied case of BFM significantly reduces the amount of inflammatory-stimulating cytokines in the liver, which increased several times as a result of chronic toxic liver damage (Table 2). It can be concluded that the decrease in the amount of these cytokines under the action of biologically active substances is proportional to the biochemical parameters of the blood, which occurs due to the inhibitory effect of the studied biologically active substances on inflammatory processes and the reinforcing effect on regenerative ones.
Table 2: Comparative efficiency analysis of the amount of hepalipin and yantacin and Phosphogliv on cytokines under ChHH conditions.
| Research groups | Groups of healthy animals | Control group | Hepalipin + yantacin | Phosphogliv |
| Doses in mg/kg | Dis. water | Heliotrin | 200 | 50 |
| IL-17 pg/ml | 1,8±0,048 | 4,55±0,024 | 2,51±0,12* | 2,99±0,089* |
| IL-6 pg/ml | 0,31±0,012 | 3,17±0,012 | 1,27±0,048* | 1,87±0,072* |
| IL-2 pg/ml | 0,39±0,012 | 3,23±0,072 | 1,31±0,011* | 1,96±0,012* |
| IL-1beta pg/ml | 0,12±0,006 | 0,52±0,012 | 0,23±0,011* | 0,31±,0,0,8* |
Note: * – reliability comparative with the data of the control group-P<0.005
Thus, the restoration of the immunoregulatory function of cytokines can be considered as one of the important mechanisms of the protective effect of biologically active substances used in experimental chronic hepatitis. In terms of effectiveness, hepalipin, as well as the sum of yantacin and flavonoids, showed higher activity compared to yantacin and phosphogliv.
To study the effect of a combination of hepalipin and yantacin and Phosphogliv on bile secretion in experimental animals.
It was noted that the amount of hepalipin and yantacin increased bile excretion in doses up to 1.42 times or 42.1%, respectively, compared with the control group, and up to 1.2 times or 20.4% comparative with the drug phosphogliv (Table 3).
Table 3: Comparative analysis of bile-excreting liver function in chronic heliotrine hepatitis
| No. | Experimental groups | Doses in mg/kg | The rate of bile excretion is mg / min. per 100 g of body weight | Total amount of isolated herb in 4 hours (mg/100 g) | |||
| 1 hour | 2 hour | 3 hour | 4 hour | ||||
| 1. | Intakt (fiz. erit.) | Dis.suv | 4,21 | 4,86 | 4,54 | 3,8 | 1044,0±2,24 |
| 2. | Control group | 2,28 | 2,61 | 2,48 | 2,14 | 570,7±2,24 | |
| 3. | Hepalipin + Yantacin | 100+100 | 3,65 | 4,33 | 3,85 | 3,13 | 897,6±1,12* |
| 4. | Phosphogliv | 50 | 3,01 | 3,12 | 3,33 | 2,96 | 745,2±1,12* |
Note: * – reliability comparative with the data of the control group-P<0.005
Thus, it was noted that the sum of hepalipin and yantacin increases the excretion of bile against the background of chronic liver changes compared with the control group and phosphogliv. This means that the experimental animals of the studied combination had a positive effect on the general population, body weight and the amount of enzymes in the blood, as well as on the level of cytokines in the blood, and also stabilized the liver function of bile secretion.
Evaluation of the effect of the studied substances on the liver index.
Research of the liver index showed that rats exposed to hepalipin and yantacin showed a positive tendency to both liver weight control and phosphogliv, in proportion to their body weight. From this it can be concluded that the substance under study indicates a clear predominance of the number of healthy hepatocytes over the number of damaged hepatocytes in the liver tissues of experimental animals.
Table 4: Comparative efficiency analysis of the studied combination on the liver index in chronic heliothrine hepatitis
| No | Experimental groups | Body weight in gramm | Liver weight in gramm | Liver index |
| 1. | Intact group | 175,4±1,12 | 3,55±0,048 | 2,024±0,0022 |
| Control group | 126,72±2,02 | 4,25±0,024 | 3,354±0,0011 | |
| 2. | Hepalipin + Yantacin | 171,01±1,34* | 4,76±0,036* | 2,783±0,0089* |
| 3. | Phosphogliv | 158,67±1,57* | 4,78±0,011* | 3,011±0,0044* |
Note: * – reliability comparative with the data of the control group-P<0.005
According to the results of the conducted researches, it can be concluded that the studied combination against the background of toxic hepatitis shows not only a high hepatoprotective effect, but also stimulates regenerative processes in the liver.
Morphological examination of the rat liver during the observation and treatment of ChHH.
Microscopic examination revealed intracellular lymphocytic infiltration as morphological signs of ChHH, which is significantly more intense and uneven compared to the norm.
In rats treated with heliotine for 2-3 weeks as an initial sign of fibrosis (F2), an expansion of the portal pathways of the liver was observed (Fig.2). There was also the development of pronounced fragmentation and degranulation of the roughness of the gallbladder. In turn, the microvilli of the bile capillaries are smoothed and their number is reduced, the phenomena of partial necrosis are observed in hepatocytes. In the liver, especially in the intercellular region, strands of connective tissue were formed and the development of fibrous tissue was also observed.
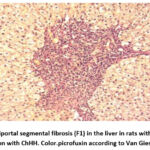 |
Figure 2: Periportal segmental fibrosis (F1) in the liver in rats with portal tract stroma dilation with ChHH. Color.picrofuxin according to Van Gieson. Inc.x200. Click here to View Figure |
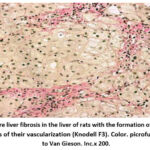 |
Figure 3: Severe liver fibrosis in the liver of rats with the formation of fibrous septa without signs of their vascularization (Knodell F3). Color. picrofuxin according to Van Gieson. Inc.x 200.Click here to View Figure |
The severity of liver fibrosis (F3) worsened, and a longer course of chronic hepatitis was noted in rats. In turn, this directly led to completed or incomplete unvascularized portal-portal or portal-central partitions in the liver parenchyma with significant changes in liver architectonics (Fig. 3).
It is worth noting that it has been noted that the combination of hepalipin and yantacin increases the resistance of the liver to the effects of a toxic substance, that is, it prevents the development of the fibrosis process occurring in the liver. The development of tertiary fibrosis in the liver of animals in the control group who did not take a combination of hepalipin and yantacin confirms this opinion.
Discussion
Indeed, today, ChLD and their severe complications are the cause of significant socio-economic burdens on the healthcare system. The widespread prevalence of these diseases among the population with high work capacity is a clear confirmation of the above. Although a number of advances have been made in the early detection and treatment of chronic viral liver diseases, the number of pathologies such as liver fibrosis, cirrhosis, and hepatocellular carcinoma has increased somewhat. This, of course, is explained by the fact that, in addition to viruses, there is a sufficient number of liver damage factors, as well as the absence of drugs that completely eliminate changes in liver cells 1, 5, 8, 11. It should be noted that drugs belonging to different groups are widely used to eliminate fibrotic processes in the liver. However, the presence of a number of side effects or various inconveniences associated with prolonged use of existing drugs limits the possibility of their use. To this end, large-scale scientific research is currently being conducted on the implementation of medicinal products based on plants with high hepatoprotective activity, primarily on a natural basis. In our country, as in the rest of the world, scientific research is being conducted at the Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov on the isolation of biologically active substances from plants and the determination of their activity 22, 23, 24, 25, 26, 56. As a continuation of these scientific works, and in collaboration with the scientific department of the Republican Specialized Scientific and Practical Institute of Pediatrics, research on the forming of LF in rats and the study of the anti-fibrosis activity of a combination with a natural basis has been conducted for many years 21, 27, 31, 32. In the conducted studies, the effect of a new phytocomposition and proanthasianidine on ChLD or early stage fibrosis, induced by the introduction of the hepatotoxic agent heliotrine according to a special scheme 27, 31, was studied. The ability of the investigated substance to correct structural and functional liver disorders caused by liver fibrosis was primarily evaluated. The objective condition of the experimental animals, body weight, liver index, biliary function of the liver, and cytokine activity in blood serum were determined as evaluation indicators. Morphological indicators of the liver were also evaluated by microscopic examination of the isolated liver fragments. The effectiveness of the research results was compared to the well-known drug phosphoglyv, which is currently widely used in hepatology practice. The combination of the new phytocomposition and proanthocyanidins eliminated objective disorders in rats with chronic liver damage. It was also observed that chronic liver damage in experimental animals also improved the incretory functions of the liver, eliminating the loss of body weight associated with biolance. It has been established that it positively affects the function of bile secretion, which is one of the important functions of the liver. It was found that the indicators of the liver index, which inform about fibrotic or cirrhotic processes in the liver, also improved to a level close to normal, and at the same time, it was observed that the fibrosis process occurring in the liver tissue hindered the transition from stage F1 to stage F2. The presented results are a continuation of scientific research on the prevention of liver fibrosis, and based on the obtained results, the goal is to introduce promising anti-fibrotic drugs into practice in the future.
Conclusion
Thus, from the conducted studies, it can be concluded that the studied combination of heparin and yantacin has the ability to stop the process of liver fibrosis caused by exposure to a toxic substance at an early stage.
In addition, the combination of hepalipin and yantacin was less toxic and showed a clear advantage over the comparable drug phosphogliv in protecting the liver from toxic substances and improving regeneration.
Acknowledgment
The authors’ acknowledgements go to the Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan and the Republican Specialized Center for Applied Medicine of Pediatric Science for supporting this work.
Funding Sources
The work financial supported by the Ministry of Higher Education, Science and Innovation of Republic of Uzbekistan (project No. AL-412105140 on the topic ”Development of a new biologically active agent for the treatment of liver fibrosis in severe chronic hepatitis”).
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
All research work in this article was carried out on the basis of the permission of the Ethics Committee under the Ministry of Health of the Republic of Uzbekistan No. 1/1-1628.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
A.A.Aripov: Conceptualization, Methodology, Writing – Original Draft.
L.L.Akhunzhanova: Data Collection, Analysis, Writing – Review & Editing.
A.U.Nabiev: Visualization, Supervision, Project Administration.
O.A.Aripov: Funding Acquisition, Resources, Supervision.
T.Khamroev – Conceptualization, Methodology, Writing – Original Draft. Pharmacological and toxicological experiments, statictical Analysis
References
- Atarashi M.,. Izawa T, Mori M., Inai Y., Kuwamura M., Yamate J. Dietary Iron Overload Abrogates Chemically-Induced Liver Cirrhosis in Rats. Nutrients. 2018; 10(10):1400.
CrossRef - Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;(67):358–380.
CrossRef - Huang D.Q., Terrault N.A., Tacke F. Global epidemiology of cirrhosis — aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023; (20): 388–398
CrossRef - The Global Health Observatory. Global health estimates: Leading causes of death. (2023) WHO https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
- Allen AM., Kim WR., Moriarty JP. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016; 64(6):2165-2172.
CrossRef - Tapper EB., Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018; 18(362):2817.
CrossRef - GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018; 392(10152):1015-1035.
CrossRef - Mokdad A.A., Lopez A.D., Shahraz S. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014; (12):145.
CrossRef - Xing Wang, Bin Wu, Critical issues in the diagnosis and treatment of liver cirrhosis, Gastroenterology Report, 2019; 7(4):227–230,
CrossRef - Wang X., Lin SX., Tao J. Study of liver cirrhosis over ten consecutive years in Southern China. World J Gastroenterol. 2014; 20(37):13546-55.
CrossRef - Ginès P., Krag A., Abraldes JG. Liver cirrhosis. Lancet. 2021; 398(10308):1359-1376.
CrossRef - GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245-266.
CrossRef - Pellicoro A., Ramachandran P., Iredale JP., Fallowfield JA.. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181-94.
CrossRef - Solà E., Watson H., Graupera I. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol. 2012;57(6):1199-206.
CrossRef - Fabrellas N., Moreira R., Carol M., Cervera M., de Prada G. Psychological Burden of Hepatic Encephalopathy on Patients and Caregivers. Clin Transl Gastroenterol. 2020;11(4):e00159.
CrossRef - D’Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217-31.
CrossRef - Mehal WZ., Iredale J., Friedman SL. Scraping fibrosis: expressway to the core of fibrosis. Nat Med. 2011;17(5):552-3.
CrossRef - Boulter L., Lu WY, Forbes SJ. Differentiation of progenitors in the liver: a matter of local choice. J Clin Invest. 2013;123(5):1867-73.
CrossRef - Bataller R., Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209-18.
CrossRef - Henderson NC., Arnold TD., Katamura Y., Giacomini MM., Rodriguez JD. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617-24.
CrossRef - Aripov A. N., Akhunzhanova L. L., Nabiev A. U.,. Aripov A. O, Khamroev T. T. Antifibrotic Efficacy of a New Phytocomposition of Essential Phospholipids with Glycyrrhizic Acid, Ecdysterone, Lycopene and Proanthacyanidin in Experimental Severe Chronic Hepatitis Compared with Phosphogliv. Biomed Pharmacol J 2023;16(3): 1815-1825.
CrossRef - Sagdullaev Sh. ShTursunova., N. V., Gusakova S. D. RUz Pat. UZ IAP 05701; Byull., No.12 (2018).
- Syrov V.N., Gusakova S.D., Khushbaktova Z.A. Hepatoprotective Efficacy of a New Phytocomposition of Essential Phospholipids with Glycyrrhizic Acid, Ecdysterone, and Lycopene in Experimental Chronic Hepatitis Compared to Phosphogliv. Pharm Chem J. 2023; (56): 1433–1438.
CrossRef - Syrov V.N., Gusakova S.D., Khushbaktova Z.A. Gepatozashitnaya effektivnost novoy fitokompozitsii iz essensialnyx fosfolipidovs glitsirrizinovoy kislotoy, ekdisteronom I likopinom pri eksperimentalnom xronicheskom gepatite sravnitelno s fosfoglivom. Ximikofarmasevticheskiy jurnal. 2022; 56(11): 3-8.
CrossRef - Nishanbaev S.Z., Shamyanov I.D., Bobakulov X.M., Sagdullaev Sh.Sh. Ximicheskiy sostav I biologicheskaya aktivnost metabolitov rasteniy roda alhagi (obzor). Ximiya rastitelnogo syrya. 2019; (4): 5–28.
CrossRef - Mamatkulova N.M., Alimova D.F., Nishanbaev S.Z. Neutral substances from Alhagi pseudalhagi . Chem Nat Compd. 2012; (48): 908–909.
CrossRef - Aripov A.N., Akhunjanova L.L., Khamroev T.T. Differential Analysis of Chronic Toxic Hepatitis Caused by The Introduction of Heliotrin Solution in Various Ways. Texas Journal of Medical Science, 2022; (4) 58–62.
- Casado N., Sonia Morante-Zarcero, Isabel Sierra. The concerning food safety issue of pyrrolizidine alkaloids: An overview (àíãë.) // Trends in Food Science & Technology. — 2022; (120): 123–139.
CrossRef - Dusemund B., Nowak N., Sommerfeld Ch., Lindtner O., Schäfer B. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin (àíãë.) //Food and Chemical Toxicology. 2023; (115): 63–72.
CrossRef - Ma Chuanhui, Liu Yang, Zhu Lin, Ji Hong, Song Xun. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research (àíãë.) // Food and Chemical Toxicology. — 2018; (119): 50–60.
CrossRef - Rakhimova R.B. Cholagogue activity of rutan in a model of acute toxic hepatitis induced by heliothrin. Science and innovation international scientific journal, 2020; 1(8): 755-759.
- Boboeva R.R., Mavlonov A.A., Jurayeva G.B. “Choleretic activity of rutana at therapeutic application in rats with heliotrin hepatitis” European journal of molecular & clinical medicine, 2020; (7): 5188-5193.
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. 2011; (8): 356.
- Mironov A. N. Rukovodstvo po provedeniyu doklinicheskix issledovaniy lekarstvennыx sredstv / Pod red.— M. : Grif i K, 2012; (1): 944. ISBN 978-5-.
- Reichen J., Egger B., Ohara N., Zeltner TB., Zysset T. Zimmermann A. Determinants of hepatic function in liver cirrhosis in the rat. Multivariate analysis. J Clin Invest. 1988; 82(6):2069-76.
CrossRef - Rozga J., Foss A., Alumets J., Ahren B., Jeppsson B., Bengmark S. Liver cirrhosis in rats: Regeneration and assessment of the role of phenobarbital, Journal of Surgical Research, 1991; 51 (4): 329-335,
CrossRef - Kadir FA., Othman F., Abdulla MA., Hussan F., Hassandarvish P. Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J Pharmacol. 2011;43(1):64-8.
CrossRef - Mokrenko Ye.V., Vyazmin A.Ya., Shabanov P.D., Suslikova M.I., Mokrenko M.Ye., Gubina M.I. Sitokinovy profil krovi krys pri eksperimentalnom parodontite i deystvii immunomodulyatorov // Sovremennûe problemû nauki i obrazovaniya. – 2020; (6): 30432
- Nikolaev S.M., Sambueva Z.G., Mikhailova T.M., Fedorov A.V. The effect of natural xanthones and their modified derivatives on the choleretic reaction in white rats. Byulleten VSNTS so RAMN, 2012; 4 (1): 218-220.
- Xu HS, Rosenlof LK, Jones RS. Bile secretion and liver regeneration in partially hepatectomized rats. Ann Surg. 1993; 218 (2):176-82.
CrossRef - Xu HS, Pilcher JA, Jones RS. Physiologic study of bile salt and lipid secretion in rats after liver transplantation. Ann Surg. 1993; 217(4):404-12.
CrossRef - Jacqueline E., Crinquette J., Bout D., Barrois J. and Vernes A. Trichinella spiralis in rats : in vivo effects of the bile and in vitro. Ann. Parasitol. Hum. Comp., 1981; 56 (4): 395-400.
CrossRef - Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014; 10(4):177.
CrossRef - Nissen N. Practitioners of Western herbal medicine and their practice in the UK: beginning to sketch the profession. Complement. Ther. Clin. Pract. 2010; (16): 181–186.
CrossRef - Beshna EA, Alahrish RA. Pharmacists’ knowledge and attitudes toward Pharmacogenomics in Zawia, Libya. Medical and Pharmaceutical Journal. 2023; 2(3):158-66.
CrossRef - Schneider PJ. The Impact of Technology on Safe Medicines Use and Pharmacy Practice in the US. Front. Pharmacol. 2018; (9):1361.
CrossRef - Bushmina ON, Dolgareva SA, Konoplya AI, Loktionov AL, Pharmacological correction of metabolic disorders in experimental acute pancreatitis on the background of chronic alcohol intoxication. Research Results in Pharmacology 2018; 4(4): 9–19.
CrossRef - Lapo Ye.I., Isaeva N.V., Paxomova R.A., Kochetova L.V., Rodikov M.V. Sovremennye prinsipy lecheniya pechenochnoy ensefalopatii // Sovremennye problemy nauki i obrazovaniya. – 2016; (6): 25572;
- Lapo Ye.I., Isaeva N.V., Paxomova R.A., Kochetova L.V., Rodikov M.V. Pechenochnaya ensefalopatiya pri mexanicheskoy jeltuxe, osobennosti patogeneza, kliniki i diagnostiki i lecheniya // Sovremennye problemy nauki i obrazovaniya. – 2016; (5): 25425;
- Smolina S.P., Petrova M.M., Sharobaro V.I., Fedorov G.N. The clinical course of hepatic encephalopathy in the patients with alcohol liver cirrhosis during the use of antioxidants. Vestnik novyx meditsinskix texnologiy – 2013; (1): 1-4.
- Smolina S. P., Petrova M. M., Sharobaro V. I., Fedorov G. N. Clinical and biochemical comparisons of patients with alcoholic liver cirrhosis complicated by hepatic encephalopathy. Vestnik Smolenskoy gosudarstvennoy meditsinskoy akademii. 2012; (1): 46-48.
- Kucheryavy Yu.A., Stukova N.Yu., Axtaeva M.L. Xronicheskiy gepatit, sirroz pecheni i ge-patotsellyulyarnaya karsinoma – zvenya odnoy sepi // Klinicheskie perspektivy gastroenterologii, gepatologii. – 2012;(5): 3-11.
- Ahmed Z., Ren J., Gonzalez A., Ahmed U., Walayat S., Martin DK., Moole H., Yong S., Koppe S., Dhillon S. Universal Index for Cirrhosis (UIC index): The development and validation of a novel index to predict advanced liver disease. Hepat Med. 2018; 24(10):133-138.
CrossRef - Bulatova I. A., Shevlyukova T. P. Evaluation of the diagnostic signifi cance of calculated indices for determining the class of liver cirrhosis. Experimental and Clinical Gastroenterology. 2022; 203(7): 31–37.
CrossRef - Wolf DC. Evaluation of the Size, Shape, and Consistency of the Liver. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. (94): 421.
- Rakhimova, S.K., Kurbanov, U.K., Mezhlumyan, L.G. Proteins from the Aerial Part of Delphinium leptocarpum and Their Biological Activity. Chem Nat Compd 2023;(59): 750–753.
CrossRef







