Lakshmana Rao Atmakuri1* , Jhansi Nelapati2
, Jhansi Nelapati2 , Bhaskar Vallamkonda3
, Bhaskar Vallamkonda3 , Ranadheer Reddy Challa3
, Ranadheer Reddy Challa3 , Subrahmanya Sai Malleswara Sharma Sonti4
, Subrahmanya Sai Malleswara Sharma Sonti4 and Krishna Sudarsana Bhuvanagiri5
and Krishna Sudarsana Bhuvanagiri5
1Department of Pharmaceutical Analysis, V. V. Institute of Pharmaceutical Sciences, Gudlavalleru, Andhra Pradesh, India.
2Department of Pharmaceutics, V. V. Institute of Pharmaceutical Sciences, Gudlavalleru, Andhra Pradesh, India.
3Department of Pharmaceutical Sciences, Vignan’s Foundation for Science, Technology and Research, Vadlamudi, Andhra Pradesh, India.
4Department of Pharmaceutical Analysis, Sri Vasavi Institute of Pharmaceutical Sciences, Tadepalligudem, Andhra Pradesh, India.
5Department of Pharmaceutical Analysis, Lydia College of Pharmacy, Ravulapalem, Andhra Pradesh, India.
Corresponding Author E-mail:dralrao@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/3024
Abstract
This study investigates the design, development, and evaluation of sustained-release Saxagliptin microparticles, utilizing Eudragit L-100 and Eudragit S-100 as polymers to achieve prolonged drug release. A total of eight formulations were prepared, employing varying drug-to-polymer ratios (1:1, 1:2, 1:3, and 1:4) for both core and coat materials. This approach facilitated an assessment of the concentration of coating material influenced the drug release rate. The solvent evaporation method proved effective in producing discrete, spherical microparticles characterized by good flowability and minimal stickiness. The surfactant Span 80 was optimized at a concentration of 1.0% to ensure optimal emulsion stability and microparticle formation. Results demonstrated that Eudragit S-100 based microparticles exhibited a significantly slower drug release profile compared to those formulated with Eudragit L-100. Notably, the formulation with a 1:4 Saxagliptin-to-Eudragit S-100 ratio achieved sustained release for up to 12 hours, confirming the trend of decreasing release rates with increasing concentrations of coating material. Dissolution data analysis revealed that the release kinetics best fit a zero-order model, indicating a constant drug release rate over time. Furthermore, the Peppas model provided the most suitable framework for understanding the drug release mechanism. The exponential coefficient (n) values indicated a non-Fickian release pattern, signifying a complex interplay between diffusion and erosion processes, which are critical for maintaining the sustained-release profile. Additionally, FTIR spectroscopy was employed to assess the compatibility of Saxagliptin within the optimized formulation. The results confirmed that the drug retained its chemical identity, with no evidence of chemical interactions between Saxagliptin and the excipients. This comprehensive study underscores the potential of Eudragit S-100 as an effective polymer for developing sustained-release formulations of Saxagliptin, enhancing patient compliance and therapeutic outcomes.
Keywords
Design; Eudragit L-100; Eudragit S-100; Microparticles; Saxagliptin
Download this article as:| Copy the following to cite this article: Atmakuri L. R, Nelapati J, Vallamkonda B, Challa R. R, Sonti S. S. M. S, Bhuvanagiri K. S. Design and Development of Saxagliptin Microparticles for Diabetes. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Atmakuri L. R, Nelapati J, Vallamkonda B, Challa R. R, Sonti S. S. M. S, Bhuvanagiri K. S. Design and Development of Saxagliptin Microparticles for Diabetes. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4hYdcSb |
Introduction
Type 2 diabetes is a chronic condition that affects the way the body processes blood glucose1. It is characterized by insulin resistance, where the body’s cells do not respond effectively to insulin, combined with a gradual decline in insulin production from the pancreas. This leads to elevated blood glucose levels, which can result in various health complications over time, such as heart disease, kidney damage, and neuropathy. Risk factors for developing type 2 diabetes include obesity, sedentary lifestyle, family history, and advanced age2. Management typically involves lifestyle changes, such as diet and exercise, along with medications when necessary to help regulate blood sugar levels and maintain overall health3.
The increasing prevalence of type 2 diabetes, associated with modern lifestyles, has prompted intensified research into effective management strategies4. Millions of individuals grapple with this non-insulin-dependent form of diabetes, which necessitates long-term treatment often characterized by high rates of non-adherence. To address this challenge, sustained release drug delivery systems hold significant promise for improving healthcare quality. In the context of type 2 diabetes, maintaining consistent drug levels in the bloodstream is crucial. While novel drug development for type 2 diabetes is ongoing, equal emphasis is being placed on creating appropriate delivery systems that prolong drug action and enhance patient compliance by reducing dosing frequency5.
Saxagliptin is a second-generation sulfonylurea, oral antihyperglycemic medication used to manage type 2 diabetes mellitus6. It belongs to the class of drugs known as DPP-4 inhibitors, which work by enhancing the body’s incretin levels, leading to increased insulin secretion and decreased glucagon release in response to meals7. This dual action helps lower blood sugar levels while minimizing the risk of hypoglycemia8. Saxagliptin is often prescribed alongside diet and exercise and can be used alone or in combination with other diabetes medications to achieve better glycemic control9,10.
Microparticles are small particles typically ranging from 1 to 1000 micrometers in diameter, widely used in various fields such as pharmaceuticals, biotechnology, and environmental science11. These versatile materials can be composed of natural or synthetic polymers, ceramics, or metals, and serve multiple purposes, including drug delivery, targeting, and controlled release of therapeutics12. In drug delivery systems, microparticles can encapsulate active ingredients, protecting them from degradation and facilitating their transport to specific sites within the body13. Their unique size and surface characteristics also allow for functionalization, enabling enhanced interaction with biological systems and improved efficacy in medical applications14.
This study aims to develop a microparticle-based drug delivery system for Saxagliptin, its short biological half-life of approximately 3.1 hours necessitates frequent dosing (twice daily), which can contribute to non-adherence. Currently available in conventional tablet forms (2.5-5 mg/day), controlled release formulations present a promising solution.
While a few formulation techniques and analytical methods for Saxagliptin have been reported15-21, no previous studies have focused on Saxagliptin microparticles for diabetes treatment. Therefore, this investigation seeks to design and develop novel microparticles of Saxagliptin aimed at treating diabetes22. This approach focuses on creating a controlled release formulation using a lower drug dose, thereby aiming to achieve consistent plasma drug concentrations. This may lead to enhanced patient compliance due to reduced dosing frequency, improved therapeutic efficacy, and minimized side effects resulting from a more controlled drug release profile.
Materials and Methods
Chemicals
Saxagliptin was obtained as a gift sample from Shree Icon Laboratories, Vijayawada, India. Eudragit L-100 and Eudragit S-100 were commercially sourced from Loba Chemicals, Mumbai, India. All other chemicals used were of analytical grade.
Preparation of Microparticles
This study employed the emulsion solvent evaporation technique for the preparation of microparticle formulations23. The specific compositions of each formulation are given in Table 1.
Table 1: Composition of Saxagliptin microparticles
|
Formulation code |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
|
Core: Coat ratio |
1:1 |
1:2 |
1:3 |
1:4 |
1:1 |
1:2 |
1:3 |
1:4 |
|
Saxagliptin (mg) |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
|
Eudragit S-100 (mg) |
1000 |
2000 |
3000 |
4000 |
– |
– |
– |
– |
|
Eudragit L-100 (mg) |
– |
– |
– |
– |
1000 |
2000 |
3000 |
4000 |
|
Span 80 (ml) |
Q.S |
Q.S |
Q.S |
Q.S |
Q.S |
Q.S |
Q.S |
Q.S |
|
Acetone (ml) |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
Organic Phase Preparation
A measured amount of Saxagliptin and Eudragit (at a 1:1 ratio) was dissolved in 10 mL of acetone to create a homogeneous drug-polymer solution.
Emulsion Formation
The organic solution was gradually added, in a thin stream, to 100 mL of liquid paraffin containing 1% Span 80 surfactant. This mixture was continuously stirred for 1 hour to form an emulsion.
Microparticle Collection
The resulting microparticles were separated from the emulsion through filtration and subsequently washed with petroleum ether to remove any residual organic solvents24.
Drying and Storage
Finally, the microparticles were air-dried for 12 hours and stored in a desiccator for further analysis.
Preparation of Other Ratios
For microparticles with drug-to-polymer ratios of 1:2, 1:3, and 1:4, the corresponding amounts of Eudragit L-100 or Eudragit S-100 were used, while maintaining the overall process steps outlined above. The list of prepared microparticles was tabulated in Table 2.
Table 2: List of Saxagliptin microparticles prepared
|
Polymers used |
|||
|
Eudragit S-100 |
Eudragit L-100 |
||
|
Formulation code |
Core: Coat |
Formulation code |
Core: Coat |
|
F-1 |
1:1 |
F-5 |
1:1 |
|
F-2 |
1:2 |
F-6 |
1:2 |
|
F-3 |
1:3 |
F-7 |
1:3 |
|
F-4 |
1:4 |
F-8 |
1:4 |
Characterization of Microparticles
The microparticles were assessed for their flow properties using standard methods, providing insights into their handling characteristics during production and formulation development25.
Drug Entrapment Efficiency
To quantify the microparticles, an accurate weight of 100 mg was crushed and dissolved in 100 mL of pH 6.8 phosphate buffer, and absorbance was measured at 210 nm-a standard technique for quantifying Saxagliptin.
Drug Loading and Encapsulation Efficiency Calculations
The percentage of drug loading within the microparticles (L) and encapsulation efficiency (E) were calculated using standard formulas26.
In-Vitro Release Studies
The USP XXIII apparatus was utilized at 37°C±0.5°C, with a rotation speed of 100 rpm throughout the experiment. Samples (5 mL) were withdrawn and analyzed at 210 nm.
Release Kinetics Analysis
The data were analyzed using standard models to explore the release mechanisms. By fitting the dissolution data to these models, researchers aimed to identify the dominant mechanism governing drug release from the Saxagliptin microparticles27.
Microscopic Evaluation with SEM
Scanning Electron Microscopy (SEM) was performed using a SEM-JEOL JSM 6360A model to examine the surface morphology of both loaded and unloaded microparticles at various magnifications. This imaging technique provided valuable insights into various characteristics of the microparticles28.
Compatibility Assessment Using IR Spectroscopy
FTIR analysis was conducted using the KBr pellet technique to obtain the infrared spectra. The spectra were collected in transmittance mode at a resolution of 4 cm⁻¹ and a wave number range of 380 to 4368 cm⁻¹. By comparing the spectra, researchers assessed potential interactions between the drug and excipients within the microparticles.
Microparticle Characteristics and Process Optimization
The emulsion solvent evaporation technique successfully produced discrete, spherical microparticles with good flowability and minimal stickiness, both individually and as aggregates. This technique relies on creating a stable emulsion during the initial stages to ensure isolated microparticles29.
Impact of Emulsifier Concentration
A critical factor influencing microparticle size is the concentration of the emulsifier (Span 80) used. An optimal concentration is essential for achieving the finest and most stable dispersion. In below optimal concentration, insufficient reduction in interfacial tension leads to the fusion of dispersed droplets, resulting in larger globules and, consequently, larger microparticles. In above optimal concentration, although a higher emulsifier concentration may further reduce interfacial tension, it does not significantly decrease particle size. Through optimization, a Span 80 concentration of 1.5% was identified as ideal.
Influence of Particle Size and Polymer Concentration
Microscopic analysis revealed spherical microparticles, either as discrete entities or aggregates, with particle sizes ranging from 153.23 to 189.56 μm. An increase in polymer concentration resulted in a larger mean particle size, attributed to the increased viscosity of the internal phase with higher polymer content, leading to larger emulsion droplets and ultimately larger microparticles30.
Impact of Core-to-Coat Ratio
This study aimed to investigate how the concentration of coating material (polymer) affects the release rate of Saxagliptin from the microparticles.
Results and Discussion
The microparticles were evaluated for various properties. Particle size distribution was determined by size analysis. Flowability of the microparticles was assessed. Good flow properties are desirable for efficient handling and processing during manufacturing and formulation development. The percentage of drug successfully encapsulated within the microparticles was quantified. High encapsulation efficiency indicates minimal drug loss during the preparation process, with results detailed in Table 3.
Table 3: Evaluation data of Saxagliptin microparticles
|
Formulation |
Angle of repose |
Bulk density (g/cm3) |
Tapped density (g/cm3) |
Carr’s index |
Hausner’s ratio |
Average particle size (µm) |
%Drug content |
%Encapsulation efficiency |
|
F-1 |
26.37± 0.12 |
0.350± 0.012 |
0.408± 0.011 |
14.21± 0.022 |
1.161± 0.014 |
153.26 |
47.27 |
94.54 |
|
F-2 |
25.65± 0.10 |
0.320± 0.020 |
0.370± 0.009 |
11.89± 0.009 |
1.134± 0.017 |
164.32 |
31.56 |
94.68 |
|
F-3 |
22.76± 0.08 |
0.319± 0.005 |
0.362± 0.021 |
11.87± 0.017 |
1.130± 0.024 |
178.37 |
23.85 |
95.40 |
|
F-4 |
21.13± 0.11 |
0.276± 0.014 |
0.314± 0.013 |
12.10± 0.024 |
1.137± 0.012 |
189.56 |
19.13 |
95.65 |
|
F-5 |
28.35± 0.09 |
0.271± 0.021 |
0.316± 0.011 |
14.24± 0.019 |
1.166± 0.019 |
153.23 |
47.78 |
95.56 |
|
F-6 |
26.39± 0.08 |
0.314± 0.018 |
0.366± 0.019 |
14.20± 0.027 |
1.165± 0.011 |
168.56 |
31.93 |
95.79 |
|
F-7 |
24.17± 0.06 |
0.255±0.025 |
0.291± 0.005 |
12.37± 0.024 |
1.142± 0.014 |
177.45 |
24.08 |
96.32 |
|
F-8 |
22.86± 0.04 |
0.353±0.027 |
0.400± 0.014 |
11.75± 0.017 |
1.133± 0.027 |
188.64 |
19.17 |
95.85 |
The microparticles underwent in-vitro dissolution testing to simulate drug release within the body. The release profiles of Saxagliptin microparticles prepared with Eudragit S-100 and Eudragit L-100 are illustrated in Figure 1 and 2.
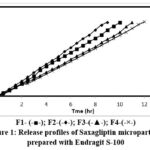 |
Figure 1: Release profiles of Saxagliptin microparticles prepared with Eudragit S-100 |
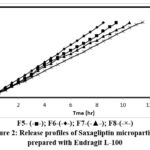 |
Figure 2: Release profiles of Saxagliptin microparticles prepared with Eudragit L-100 |
To understand the underlying mechanisms governing drug release from the microparticles, peppas plots were constructed. These plots were linear for all microparticle formulations, with details of the release kinetics summarized in Table 4.
Table 4: In-vitro dissolution kinetics parameters of Saxagliptin microparticles
|
Formulation code |
Correlation coefficient (r2) |
Release kinetics |
Diffusion exponent value (n) |
|||||
|
Zero order |
First order |
Higuchi |
Peppas |
K o (mg/hr) |
T50 (hr) |
T90 (hr) |
||
|
F1 |
0.9992 |
0.7949 |
0.9128 |
0.9998 |
0.55 |
4.6 |
8.2 |
1.0591 |
|
F2 |
0.9990 |
0.8165 |
0.9135 |
0.9993 |
0.48 |
5.1 |
9.2 |
1.1013 |
|
F3 |
0.9974 |
0.7757 |
0.9031 |
0.9994 |
0.44 |
5.9 |
10.6 |
1.0791 |
|
F4 |
0.9980 |
0.6792 |
0.9034 |
0.9988 |
0.41 |
6.2 |
11.1 |
1.1032 |
|
F5 |
0.9997 |
0.8049 |
0.9292 |
0.9992 |
0.58 |
4.3 |
7.7 |
0.9213 |
|
F6 |
0.9994 |
0.8241 |
0.9260 |
0.9973 |
0.51 |
4.8 |
8.7 |
0.9403 |
|
F7 |
0.9987 |
0.7905 |
0.9326 |
0.9983 |
0.47 |
5.2 |
9.4 |
0.9333 |
|
F8 |
0.9998 |
0.7651 |
0.9239 |
0.999 |
0.43 |
5.7 |
10.3 |
0.9438 |
For microparticles prepared using Eudragit S-100, the exponential coefficient (n) values ranged from 1.0591 to 1.1032, indicating a super case-II transport mechanism. In contrast, microparticles formulated with Eudragit L-100 exhibited n values of 0.9213 to 0.9438, suggesting an anomalous diffusion process influenced by both diffusion and other mechanisms such as erosion or swelling of the polymer matrix. The results also indicated a correlation between the concentrations of coating material applied and the release rate, with increased coating material concentration enhancing wall thickness and consequently slowing drug release. This highlights the ability to control drug release by adjusting the polymer-to-drug ratio within the microparticles. FTIR spectroscopy was employed to evaluate potential interactions between Saxagliptin and the excipients used in the optimized microparticle formulation. Scanning electron microscopy (SEM) images of the drug-loaded microparticles revealed a predominantly spherical morphology, as illustrated in Figure 3.
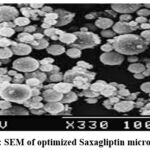 |
Figure 3: SEM of optimized Saxagliptin microparticles |
Conclusion
Formulations prepared with Eudragit S-100 displayed a slower drug release profile compared to those using Eudragit L-100, highlighting the influence of polymer selection on the release characteristics of drug formulations. Eudragit S-100 is a pH-sensitive polymer that remains insoluble in gastric conditions but dissolves in the intestinal environment, making it suitable for enteric-coated systems. In contrast, Eudragit L-100, which is soluble at lower pH levels, tends to facilitate a quicker drug release. The differences in the release profiles can be attributed to the distinct solubility characteristics of these polymers, which affect the diffusion pathways and interaction with the drug. Consequently, the choice of polymer not only influences the rate of drug release but also the overall efficacy and therapeutic outcome of the formulation.
Particularly, microparticles prepared with a 1:2 Eudragit S-100 to drug ratio exhibited controlled drug release for up to 12 hours, indicating a sustained delivery system that can be beneficial for prolonged therapeutic effects. The dissolution studies conducted revealed zero-order release kinetics, suggesting that the drug is released at a constant rate over time, independent of its concentration. This is a desirable feature for many therapeutic agents, as it allows for predictable and stable drug levels in circulation. The Peppas model further elucidated the mechanism of drug release, indicating a combination of diffusion and erosion processes, which govern how the drug is released from the polymer matrix. These findings underscore the potential of Eudragit S-100 based formulations in achieving controlled drug delivery systems that enhance patient compliance and therapeutic effectiveness.
Acknowledgement
We acknowledge V. V. Institute of Pharmaceutical Sciences, Gudlavalleru for proving necessary support for carryout the research work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Author Contributions
Lakshmana Rao Atmakuri: Conceptualization, Methodology, Original Draft.
Jhansi Nelapati: Data Collection, Analysis.
Bhaskar Vallamkonda: Visualization, Supervision.
Ranadheer Reddy Challa: Funding Acquisition, Resources.
Subrahmanya Sai Malleswara Sharma Sonti: Writing, Project Administration.
Krishna Sudarsana Bhuvanagiri: Review, Editing.
References
- Rodolfo JG, Jennifer MT, Cecilia CLW, Rozalina GMC. Advances in the management of type 2 diabetes in adults. BMJ Med. 2023; 2: e000372.
CrossRef - Galicia GU, Benito VA, Jebari S, Larrea SA, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020; 21(7): 6275.
CrossRef - Ralph AD, Curtis LT, Muhammad AG, Eugenio C. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014; 27(2): 100-112.
CrossRef - Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of currenttrends. Oman Med J. 2012; 27(4): 269-273.
CrossRef - Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010; 204(1): 1-11.
CrossRef - Dave DJ. Saxagliptin: A dipeptidyl peptidase-4 inhibitor in the treatment of type 2 diabetes mellitus. J Pharmacol Pharmacother. 2011; 2(4): 230-235.
CrossRef - Augeri DJ, Robl JA, Betebenner DA, Magnin DR, Khanna A, Robertson JG. Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005; 48 (15): 5025-5037.
CrossRef - Anderson R, Hayes J, Stephens JW. Pharmacokinetic, pharmacodynamic and clinical evaluation of Saxagliptin in type 2 diabetes. Expert Opin Drug Metab Toxicol. 2016; 12(4): 467-473.
CrossRef - Konya H, Yano Y, Matsutani S, Tsunoda T, Ikawa T, Kusunoki Y, Matsuo T, Miuchi M, Katsuno T, Hamaguchi T, Miyagawa J, Namba M. Profile of Saxagliptin in the treatment of type 2 diabetes: focus on Japanese patients. Ther Clin Risk Manag. 2014; 10: 547-558.
CrossRef - Ali S, Fonseca V. Saxagliptin overview: special focus on safety and adverse effects. Expert Opin Drug Saf. 2013; 12(1): 103-109.
CrossRef - Bale S, Khurana A, Reddy ASS, Singh M, Godugu C. Overview on therapeutic applications of microparticle drug delivery systems. Crit Rev Ther Drug Carrier Syst.2016; 33(4): 309-361.
CrossRef - Arpana SK, Krishna PT, Smita M, Dhole SN. A review on microparticulate drug delivery system. Bull Env Pharmacol Life Sci. 2021; 10(3): 163-177.
- Kumari S, Menu N, Geeta A, Puneet, Upendra KJ, Pankaj S. Microparticles drug delivery system a review. World J Pharm Pharm Sci.2016; 5(3): 543-566.
- Pavan Kumar B, Sarath Chandiran I, Bhavya B, Sindhuri M. Microparticulate drug delivery system; a review. Ind J Pharm Sci Res. 2011; 1(1): 19-37.
- Patil ND, Gondkar SB, Saudagar RB. Formulation and evaluation of mucoadhesive buccal patch of Saxagliptin hydrochloride. Res J Pharm Dosage Forms Technol.2016; 8(4): 237-247.
CrossRef - Rajani V, Rajendra Prasad Y, Lakshmana Rao A. In vivo evaluation of optimized formulation of Dapagliflozin and Saxagliptin bilayered tablets. Indian Drugs. 2024; 61(3): 56-60.
CrossRef - Rajani V, Rajendra Prasad Y, Lakshmana Rao A. Development and validation of a stability indicating RP-HPLC method for simultaneous estimation of Teneligliptin and Metformin. Turkish J Pharml Sci. 2020; 17(2): 141-147.
CrossRef - Rajani V, Rajendra Prasad Y, Lakshmana Rao A. Development and validation of stability indicating RP-HPLC method for simultaneous estimation of Dapagliflozin and Saxagliptin in pure and pharmaceutical dosage form. The Pharm Rev. 2019; January-February: 107-113.
- Rajani V, Rajendra Prasad Y, Lakshmana Rao A. Formulation and in vitro evaluation of Teneligliptin and Metformin bilayered tablets. J Int Pharm Sci. 2019; 6(1): 1-10.
- Sai Sruthi A, Saidatri A, Lakshmana Rao A. Development and validation of Sitagliptin and Simvastatin tablets by using RP-HPLC method. Int J Applied Pharm Sci. 2017; 4(1): 36-43.
- Raja T, Lakshmana Rao A. Validated HPTLC method for simultaneous estimation of Metformin Hydrochloride and Sitagliptin Phosphate in bulk drug and formulation. Rasayan J Chem. 2012; 5(3): 407-413.
- Rafiee MH, Rasool BKA. An overview of microparticulate drug delivery system and its extensive therapeutic applications in diabetes. Adv Pharm Bull. 2022; 12(4): 730-746.
CrossRef - Stojmenovski A, Gataric B, Vucen S, Railic M, Krstonosic V, Kukobat R, Mirjanic M, Skrbic R, Racic A. Formulation and evaluation of polysaccharide microparticles for the controlled release of Propranolol Hydrochloride. Pharmaceutics. 2024; 16(6):788.
CrossRef - Nagendra R, Charan CS, Joshi KH, Jayanthi C. Preparation and evaluation of microparticles containing Oxybutynin Chloride for controlled release. Asian J Biomed Pharm Sci. 2015; 5(41): 15-17.
CrossRef - Agnihotri SA, Aminabhavi TM. Formulation and evaluation of novel tableted chitosan microparticles for the controlled release of Clozapine. J Microencapsul. 2004; 21(7): 709-718.
CrossRef - Ravi Kumar Reddy J, Gnanaprakash K, Badrnath AV, Madhu Sudhana Chetty C. Formulation and evaluation of microparticles for Metronidazole. J Pharm Sci & Res. 2009; 1(10): 131-136.
- Silva RYP, Menezes DLB, Oliveira VDS, Converti A, Lima AAN. Microparticles in the development and improvement of pharmaceutical formulations: an analysis of in vitro and in vivo studies. Int J Mol Sci. 2023; 24(6): 5441.
CrossRef - Rathore SSS, Geetha M, Manjula BP, Joshi VG, Setty SR. Formulation of stomach-specific floating microparticles of Nizatidine and their radiographic evaluation. Braz J Pharm Sci. 2022; 58: e191009.
CrossRef - Thakur S, Sharma RB, Garg T. A review on microparticles: Preparation techniques and evaluation. J Pharm Innov. 2022; 11(4): 837-840.
- El-Kamel AH, Al-Shora DH, El-Sayed YM. Formulation and pharmacodynamic evaluation of Captopril sustained release microparticles. J Microencapsul. 2006; 23(4): 389-404.
CrossRef








