Fatma Abdel-Kader Attia Mohamed1, Eman Refaat Youness2* , Marwa M. Hassan1
, Marwa M. Hassan1 and Nesma Hassan Hasanein Ashour1
and Nesma Hassan Hasanein Ashour1
1Department of Internal Medicine, Faculty of Medicine for Girls, Al-Azhar University
2Department of Medical Biochemistry, Medical Research and Clinical Studies Institute, National Research Centre, Cairo, Egypt.
Corresponing Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/3058
Abstract
Inflammatory bowel disease (IBD) is a life-long illness; accordingly, the early recognition of the illness activity is significant for the management and prevention of complications. We aimed to assess the association between Hs-CAR, markers of inflammation, and CBC parameters in inflammatory bowel disease cases and their impact on illness activity. This study involved 100 Egyptian participants, comprising 80 individuals with IBD: 40 with active illness and 40 in remission, together with a control group of 20 healthy adults. Participants were chosen from the Outpatient Clinics of the Internal Medicine Departments at Al Zahraa University Hospital and Ain-Shams University Hospital in Egypt. The results revealed that CAR displayed a highly significant variance among the groups under investigation (P-value equal 0.003). Moreover, CAR was significantly higher in active CD patients compared to CD in the remission group and ulcerative colitis (UC) cases. In UC patients, CAR had a positive correlation with erythrocyte sedimentation rate (ESR), NLR, lymphocytes, and neutrophils. In CD patients, CAR was positively correlated with ESR, NLR, platelet (PLT), and lymphocytes. The UC-Mayo score and Crohn's disease activity index (CDAI) have been positively correlated with ESR and CAR. The assessment of IBD activity by CAR is a non-invasive and easily accessible instrument. Furthermore, CAR was significantly greater in inactive IBD groups compared to IBD in remission groups. It is concluded that Hs-CAR is a promising non-invasive biomarker for early identification of IBD disease activity and severity, indicating a balance between nutritional status and inflammation.
Keywords
CAR; CDAI; CRP; IBD; Mayo
Download this article as:| Copy the following to cite this article: Mohamed F. A. K. A, Youness E. R, Hassan M. M, Ashour N. H. H. CRP/Albumin Ratio as Potential Indicator for Assessment of Disease Activity in Inflammatory Bowel Disease Patients. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Mohamed F. A. K. A, Youness E. R, Hassan M. M, Ashour N. H. H. CRP/Albumin Ratio as Potential Indicator for Assessment of Disease Activity in Inflammatory Bowel Disease Patients. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4iUjH9c |
Introduction
Inflammatory bowel disease, a chronic illness caused by the interplay of genetic and environmental factors, has become an important international health problem with a consistently rising frequency. Inflammatory bowel disease usually refers to a combination of two distinct bowel-relapsing disorders, namely ulcerative colitis and Crohn’s disease (CD). 1
Early recognition of the disease activity of inflammatory bowel disease is crucial for an efficient management of the illness. This management may prevent complications and, as a result, enhance the quality of life and prognosis. The gold standard for the assessment and diagnosis of disease activity in cases with inflammatory bowel disease remains endoscopy. As a result, additional research is required to recognize a non-invasive and cost-effective biomarker for clinic use 2, 3 .
In medical training, erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) are frequently utilized as biomarkers to evaluate IBD. Nevertheless, these biomarkers are influenced by other biological factors, as infections. In light of this, there is a requirement for new, non-invasive, and efficient biological biomarkers. The C-reactive protein/albumin ratio (CAR) is an excellent marker for illness activity in cases, as it represents the balance among nutritional status and inflammation 4.
This investigation aimed to evaluate the highly sensitive CAR (Hs-CAR), inflammatory markers, and CBC parameters (NLR, PLR, LMR, and RDW) in IBD patients, as well as their relations with the activity of the disease 5, 6.
Materials and Methods
This was a prospective cross-sectional investigation that has been conducted on 100 Egyptian participants, comprising 80 individuals with IBD: 40 with active disease and 40 in remission, together with a control group of 20 healthy adults. Cases were selected from the Outpatient Clinics of the Internal Medicine Departments at Al Zahraa University Hospital and Ain-Shams University Hospital in Egypt from September 2022 to May 2023.
Inclusion criteria
Patients who were previously diagnosed with IBD with a disease duration of 2–20 years, had an age of 17–50 years, and maintained on 5ASA, corticosteroids, immunomodulators, or biologics.
Exclusion criteria
Patients with the following medical conditions: Other autoimmune illnesses, chronic or acute kidney failure, heart illnesses, diabetes mellitus, hypertension, tumor, chronic or acute infections and liver cirrhosis.
The investigation involved 39 patients with UC aged 17-50 years, divided into three groups based on disease activity: 21 patients with activity (group Ia) and 18 patients with remission (group Ib); 41 cases who have cases disease aged 20-49 years, divided by the Crohn’s disease activity index (CDAI) into 19 cases with activity (group IIa) and 22 patients with remission (group IIb); and 20 healthy individuals as the control group (21-50 years) with a mean ± SD of 34.60 ± 8.05.
Interventions
Clinical Assessment
Full medical history was recorded, including personal history, age, body mass index (BMI), smoking, period of illness, and history of drugs for IBD, e.g., corticosteroids, ASA, immunomodulators, and biologics. The activity of IBD was evaluated as follows: cases have been categorized as having active illness or remission based on the CDAI scores for those who have CD and partial Mayo scores for those with US. The Mayo score is a composite measure that includes four components: stool frequency, flexible proctosigmoidoscopy, the physician’s global judgment results, and rectal hemorrhage. Patients with US exhibiting a partial Mayo score ≤ 2 have been categorized into the remission group, whereas the remaining cases have been categorized into the active disease group (Table 1). 7
Table 1: Assessment of stool frequency, rectal bleeding, findings on endoscopy, physician’s global assessment according Mayo scoring system.7
Stool frequency:
|
Rectal bleeding:
|
Findings on endoscopy:
|
Physician’s global assessment:
|
Table 2: The Crohn’s Disease Activity Index or CDAI is a research tool used to quantify the symptoms of patients with Crohn’s disease. The CDAI was developed by WR Best and colleagues from the Midwest Regional Health Center in Illinois, in 1976.8 It consists of eight factors, each summed after adjustment with a weighting factor. The components and weighting factors are the following:
| Clinical or laboratory variable | Weighting factor |
| Number of liquid or soft stools each day for seven days | x 2 |
| Abdominal pain (graded from 0-3 on severity) each day for seven days | x 5 |
| General wellbeing, subjectively assessed from 0 (well) to 4 (terrible) each day for seven days | x 7 |
| Presence of complications* | x 20 |
| Taking Lomotil or opiates for diarrhea | x 30 |
| Presence of an abdominal mass (0 as none, 2 as questionable, 5 as definite) | x 10 |
| Hematocrit of <0.47 in men and <0.42 in women | x 6 |
| Percentage deviation from standard weight | x 1 |
*One point each is added for each set of complications:
the presence of joint pains (arthralgia) or frank arthritis
inflammation of the irisor uveitis
presence of erythema nodosum, pyoderma gangrenosum, or aphthous ulcers anal fissures, fistulaeor abscesses
other fistulae
feverduring the previous week.
Remission of Crohn’s disease is defined as CDAI below 150. Moderate to severe disease was defined as CDAI score 230-400 points .Severe disease was defined as a value of greater than 450.8 Most major research studies on medications in Crohn’s disease define response as a fall of the CDAI of greater than 70 points.9 10
Endoscopy
Endoscopy was utilized for disease localization.
Laboratory Investigations
The following parameters were assessed: CBC [hemoglobin (Hb), PLR, platelet count (PLT), NLR, monocyte count, neutrophil count, lymphocyte count, and LMR), inflammatory markers (CRP and ESR), and Hs- CAR. Briefly, CBC was performed on Sysmex KX-2IN, Jaban; The Biosystem A15 autoanalyzer (Biosystems S.A., Spain) has been utilized to evaluate serum albumin utilizing the appropriate chemical principles. Anticoagulant blood was placed in an upright tube, referred to as a Westergern tube, for the erythrocyte sedimentation rate. The rate at which red blood cells descend has been determined and reported in mm/h.6 In addition, the Human Hs-CRP enzyme-linked immunosorbent assay (ELISA) reagent (Cat. No. 10603, Chemux BIOScience, South San Francisco, the United States) has been utilized in accordance with the established protocols to assess CRP.
Statistical Analysis
The data analysis in the investigation was conducted using SPSS version 24, with Shapiro-Wilk’s test for normal distribution. Quantitative data has been represented as the mean ± standard deviation, while qualitative data has been represented as a frequency and percentage. The statistical comparisons were two-tailed, with a significance threshold of P-value less than 0.05 for significant distinctions.
Results
Table 3: Demographic data among the studied groups.
| UC (n= 39) | CD (n= 41) | Control (n= 20) | Test of sig. | p | ||||
| No. | % | No. | % | No. | % | |||
| Age (years) | ||||||||
| Range ® | 17.0 – 50.0 | 20.0 – 49.0 | 21.0 – 50.0 | F=1.664 | 0.195 | |||
| Mean ± SD. | 33.46 ± 8.68 | 36.63 ± 6.86 | 34.60 ± 8.05 | |||||
| Median (IQR) | 33.0 (27.5 –
38.0) |
37.0 (34.0 – 40.0) | 34.50 (29.0 –
39.5) |
|||||
| Sex | ||||||||
| Male | 28 | 71.8 | 22 | 53.7 | 10 | 50.0 |
Χ2=3.813 |
0.151 |
| Female | 11 | 28.2 | 19 | 46.3 | 10 | 50.0 | ||
| Smoking | ||||||||
| No | 27 | 69.2 | 36 | 87.8 | 15 | 75.0 |
Χ2=4.150 |
0.126 |
| Yes | 12 | 30.8 | 5 | 12.2 | 5 | 25.0 | ||
| BMI | H= 24.647* | <0.001
* |
||||||
| Range ® | 17.84 – 23.56 | 18.09 – 23.56 | 19.42 – 30.18 | |||||
| Mean ± SD. | 20.67 ± 1.42 | 20.14 ± 1.40 | 24.55 ± 3.49 | |||||
| Median (IQR) | 20.67 (19.54 –
21.77) |
20.24 (18.93 –
20.91) |
24.12 (21.92 –
27.30) |
|||||
| Duration (years) | U= 408.50* | <0.001
* |
||||||
| Range ® | 0.15 – 20.0 | 0.16 – 10.0 | – | |||||
| Mean ± SD. | 9.0 ± 5.29 | 4.56 ± 2.27 | – | |||||
| Median (IQR) | 10.0(4.50 –
13.0) |
5.0(3.0 – 5.0) | – | |||||
p-value not more than 0.05 is considered statistically significant, p0 value not more than 0.01 is considered highly statistically significant, SD: standard deviation, analysis carried out by : Chi-square Test &*KW:Kruskal-Wallis Test .#:One Way ANOVA Test.
Table 3 showed that, the mean age was 33.46 ± 8.68 years in UC cases, 36.63 ± 6.86 years in CD patients, and 34.60 ± 8.05 years in the control group. Insignificant variance was found between the three studied groups in age (P-value equal 0.195). There was a high predominance of male gender in the two diseased groups as there were 71.8 % males in UC and 53.7% males in CD. Moreover, men to women ratio was 1:1 in the control group. There was statistically insignificant variance between the three groups under investigation in gender (P > 0.05).
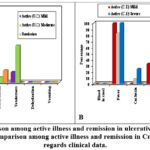 |
Figure 1: (A): Comparison among active illness and remission in ulcerative colitis cases as regards clinical data. (B): Comparison among active illness and remission in Crohn’s disease cases as regards clinical data. |
Figure 1:(A): Showed that, the study analyzed clinical data of 339 U.C patients, revealing that there were (7) active U.C patients with mild symptoms, (14) with moderate symptoms, and (18) U.C patients in remission. Significant differences were found in stool number, blood presence, fever, cachexia, tenderness, and tachycardia, but no significant difference was found in tachycardia, dehydration, and vomiting. (B): The study analyzed clinical data of 41 chronic kidney disease (CKD) patients, revealing significant variances among active and remission groups in terms of stool count, fever, tachycardia, tenderness, dehydration, and vomiting. However, no significant difference was found in cachexia and dehydration between active and remission CD patients. The findings suggest that CKD can be a significant health issue.
Table 4: Comparison between the studied groups as regard Highly sensitive C – reactive protein CAR).
| CAR | UC (n = 39) | CD (n = 41) | Control (n= 20) | H | p |
| Range® | 4.82 – 35.65 | 2.85 – 38.32 | 2.99 – 25.66 | 11.764
* |
0.003* |
| Mean ± SD. | 23.93 ± 8.66 | 24.48 ± 10.21 | 18.45 ± 6.69 | ||
| Median (IQR) | 24.44(21.31 –
30.67) |
25.71(19.02 –
32.18) |
21.0(16.09 –
22.73) |
The high sensitive CAR ratio was significantly greater in U.C cases compared to control subjects with mean S. D (23.938.66, 18.456.69) respectively (p=0.004). The highly sensitive CAR was significantly greater in C. D patients compared to control subjects with mean S. D (24.4810.21, 18.456.69) respectively (p=0.001). A significant variance has been observed as regard the high sensitive CAR between the studied groups (p-value equal 0.003). But insignificant variance observed as regard highly sensitive c- reactive protein / albumin ratio between U.C patients and C.D patients (p-value equal 0.627)) Table 4).
Table 5: Comparison between active disease and remission as regard Hs-CRP / Albumin ratio.
| CAR | UC | CD | Control (n= 20) | H | p | ||
| Active U.C (n= 21) | Remission U.C
(n= 18) |
Active C.D (n= 19) | Remission C.D
(n= 22) |
||||
| Range® | 9.85 –
35.65 |
4.82 – 27.08 | 9.13 –
38.32 |
2.85 –
27.12 |
2.99 –
25.66 |
57.34
0* |
<0.0
01* |
| Mean ± | 29.28 ± | 17.69 ± 7.33 | 31.89 ± | 18.08 ± | 18.45 ± | ||
| SD. | 5.63 | 6.65 | 8.24 | 6.69 | |||
| Median | 30.32 | 21.31 | 32.24 | 22.58 | 21.0 | ||
| (IQR) | (27.72 – | (9.03 – | (29.67 – | (11.63 – | (16.09 – | ||
| 32.32) | 23.26) | 39.96) | 24.97) | 22.73) | |||
The Hs-CRP / Albumin ratio were significantly greater in active U.C and active C.D groups than in remitted U.C and remitted C.D with mean S.D(29.28 5.63, 31.89 6.65, 17.69 7.33 and 18.08 8.24 ) respectively (p-value <0.001). As regard Hs-CRP / Albumin ratio has been found to be significantly greater in cases with active ulcerative colitis and Crohn’s disease compared to the control group (p-value less than 0.001).But, there was insignificant variance detected in patients in remission state of both Crohn’s disease cases and ulcerative colitis compared to the control group (p-value greater than 0.005) (Table 5).
Table 6: Correlation between laboratory parameters and the disease activity in ulcerative colitis & Crohn’s disease cases.
| MAYO (UMC) score | CDAI (CD) | |||
| rs | p | rs | P | |
| ESR | 0.766 | <0.001* | 0.748 | <0.001* |
| Hs CRP | -0.003 | 0.985 | 0.175 | 0.274 |
| Albumin | -0.737 | <0.001* | -0.758 | <0.001* |
| Hs c-reactive protein
/albumin ratio (CAR) |
0.681 | <0.001* | 0.680 | <0.001* |
| CBC | ||||
| Hb | -0.023 | 0.889 | -0.294 | 0.062 |
| RDW | -0.018 | 0.915 | -0.173 | 0.279 |
| PLT | 0.035 | 0.830 | 0.368 | 0.018* |
| Neutrophils | 0.725 | <0.001* | 0.627 | <0.001* |
| Lymphocytes | 0.261 | 0.108 | -0.205 | 0.200 |
| Monocytes | 0.026 | 0.877 | -0.172 | 0.283 |
| NLR | 0.647 | <0.001* | 0.680 | <0.001* |
| PLR | -0.781 | <0.001* | -0.723 | <0.001* |
| LMR | -0.690 | <0.001* | -0.775 | <0.001* |
CDAI: Crohn’s Disease Activity Index
The MAYO score for ulcerative colitis illustrated a significant positive correlation with ESR, CAR, neutrophils, and NLR, while a negative correlation was found with albumin, PLR, and LMR. The CDAI showed a positive correlation with ESR, CAR, platelet count, neutrophils, and NLR, but a negative correlation with albumin, platelet-lymphocyte ratio, and lymphocyte-monocyte ratio. These findings suggest that a comprehensive understanding of the disease’s pathophysiology is crucial for effective treatment. (Table 6).
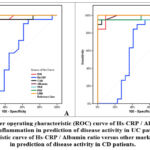 |
Figure 2: A: Receiver operating characteristic (ROC) curve of Hs-CRP / Albumin ratio versus other markers of inflammation in prediction of disease activity in UC patients. |
Figure 2 (A): show the receiver operating characteristic (ROC) analysis was performed to determine the diagnostic value of ESR, CAR, albumin, NLR, PLR and LMR in determining UC activity. It is found that ( ESR) can significantly determine UC activity in patients at (cut off <14) with sensitivity (95.24) and specificity(88.89), (CAR) at (cut off <23.53) with sensitivity (90.48) and specificity (83.33), albumin at( cut off3.6) with sensitivity(95.24) and specificity (100), (NLR) at (cut off <7.5) with sensitivity (95.24) and specificity (94.44), (PLR) at (cut off50.46) with sensitivity (100) and specificity (100) and (LMR) at (cut off 0.05) with sensitivity (100) and specificity (100).
Figure 2 (B): show the receiver operating characteristic (ROC) analysis was performed to determine the diagnostic value of ESR, CAR, albumin, NLR, PLR and LMR in determining CD activity. It is found that ( ESR) can significantly determine CD activity in patients at (cut off <17) with sensitivity (94.74) and specificity(90.91), (CAR) at (cut off <25.71) with sensitivity (94.74) and specificity (90.91), albumin at( cut off3.5) with sensitivity( 94,74) and specificity (100), (NLR) at (cut off < 2.48) with sensitivity (94.74) and specificity (86.36), (PLR) at (cut off120) with sensitivity (94.74) and specificity (90.91) and (LMR) at (cut off 0.09) with sensitivity (100) and specificity (100).
Discussion
Crohn’s disease is distinguished by transmural inflammation that might impact any part of the digestive tract in a non-continuous pattern, from the mouth to the anus. Complications, including strictures, abscesses and fistulas, may result from Crohn’s disease.11
Our investigation demonstrated that there was a high predominance of male gender in UC cases as there was 71.8 %, 53.7% males in CD patients. And 50% in control group, the mean age was (33.46 ± 8.68 years in UC cases, 36.63 ± 6.86 years in CD patients, and 34.60 ± 8.05 years in control group). There was insignificant variance was found between the studied groups as regard age and gender (p-value 0.151 and 0.195) correspondingly. In addition, there was insignificant variance reported when comparing the gender distribution and age between active and remission UC and CD groups.
These were supported by 12 who reported that there was statistically insignificant variance was found among active and remission groups in UC and CD patients as regard gender and age (p= 0.76).
The investigation observed a significant variance in body mass index (BMI) among the control and UC patients. The control group showed a significant elevation in BMI compared to both the UC and CD groups. However, insignificant variance has been observed between the UC and CD patients. The control group also demonstrated a significant elevation in BMI compared to both active and remission UC or CD groups. Insignificant variance has been detected between active and remission states in the UC and Crohn’s disease groups.
Our result was supported by 13 who reported that there was difference in CD patients as regard BMI compared to controls (P-value less than 0.001), while there was no obvious variance was observed in UC cases compared to controls (P< 0.064)
Our investigation observed that clinical symptoms in cases with UC were mild in seven patients (33.3%), moderate in fourteen patients (66.6%). In comparison, CD patients exhibited mild clinical symptoms in three patients (15.7%), moderate symptoms in twelve patients (63.15%), and severe symptoms in four patients (21.0%)
Contrary to our findings, researcher conducted a study which demonstrated that the severity of clinical symptoms in UC cases has been classified as mild in 149 cases (49.0%), moderate in 125 cases (41.1%), and severe in 26 cases (8.6%).14
Our investigation illustrated a significant variance among the groups under investigation as regard the occurrence of fever (p-value equal 0.032), and vomiting (p-value equal 0.002). However, insignificant variance has been found among the studied groups as regard tachycardia (p=0.178), tenderness (p=0.246)
Our study supported by researcher who illustrated a significant variance among the UC patients as regard occurrence of fever (P=0.002). But disagreed with our study as regard vomiting15.
Our study showed that highly sensitive c-reactive protein /albumin ratio concentration were significantly higher in active UC patients compared to remission UC patients (P-value less than 0.001). Similarly, highly sensitive c-reactive protein/albumin ratio levels were significantly greater in active CD patients compared to remission CD cases (P-value less than 0.001).
Our study agreed with Glapa-Nowak who demonstrated a significant variance among active and remission in both UC and CD groups as regard highly sensitive c-reactive protein /albumin ratio (p-valueless than 0.001) 16.
The results of our investigation demonstrated that the c-reactive protein/albumin ratio has been significantly elevated in cases with active Crohn’s disease and ulcerative colitis in comparison to the control group (p-value less than 0.001). And there was a significant distinction as regarding the highly sensitive CAR among the groups under investigation (p-value equal 0.003).
Our study agreed with Jeong who demonstrated a statistically significant variance between active UC patients and control group17.
Our investigation demonstrated that a significant positive association has been observed between MAYO score for ulcerative colitis (UC) and ESR (r=0.766, p-value less than 0.001), CAR (r=0.681, p-value less than 0.001), neutrophils (r=0.725, p-value less than 0.001), and NLR (r=0.647, p-value less than 0.001). But there was a negative correlation with albumin (r= -0.737, p-value less than 0.001), PLR (r= -0.781, p-value less than 0.001) and LMR (r= -0.690, p-value less than 0.001).
Chen conducted an investigation that demonstrated a positive association between the MAYO score for ulcerative colitis and ESR (r = 0.421, P-value less than 0.001), neutrophil (r = 0.344, P-value less than 0.001), CRP (r = 0.595, P-value less than 0.001), and the CRP/albumin ratio (r = 0.637, P < 0.001). However, a negative association has been observed with albumin (r = −0.692, P-value less than 0.001) and LMR (r = −0.495, P < 0.001)18.
In our study, our receiver operating characteristic analysis has been carried out to determine the diagnostic value of ESR, CAR, albumin, NLR, PLR and LMR in determining UC activity. It is found that ( ESR) can significantly determine UC activity in patients at (cut off <14) with sensitivity (945.24) and specificity(88.89), (CAR) at (cut off <23.53) with sensitivity (90.48) and specificity (83.33), albumin at( cut off ≤ 3.6) with sensitivity(95.24) and specificity (100), (NLR) at (cut off <7.5) with sensitivity (95.24) and specificity (94.44), (PLR) at (cut off≤50.46) with sensitivity (100) and specificity (100) and (LMR) at (cut off ≤0.05) with sensitivity (100) and specificity (100).
It was supported our findings as regard (ESR) cutoff value. But, they did not agree with our results as regard sensitivity (67.8%) and specificity (72.5%), as our study showed higher values (p=0.03) 19.
Conclusion
Early detection of the disease activity of IBD is of great significance for the treatment of this disease, which can effectively prevent complications and therefore improve prognosis as well as quality of life. Endoscopy continues to be the gold standard for the diagnosis and evaluation of the disease activity in IBD patients. Therefore, there is an urgent need for novel non-invasive and effective biological biomarkers for the accurate assessment of IBD activity in the clinic. Our results showed that highly sensitive c-reactive protein /albumin ratio (CAR) is strongly associated with activity of IBD disease and could be excellent noninvasive biomarker for early detection of activity and severity of disease.
In addition, The High sensitive c-reactive protein/albumin ratio (CAR) is a promising indicative biomarker of balance between inflammation and nutritional status
Recommendation
Large-scale investigation is required to assess the correlation between CAR levels and IBD activity, confirm current findings, and recommend regular monitoring and assessment of CAR parameters in IBD patients.
Acknowledgment
The author would like to thank Al-Azhar University for granting this research work. The Department of Internal medicine, Al-Azhar University, is highly appreciated for allowing the laboratory work.
Funding source
The author(s) received no financial support for the research, authorship, and/or publication of this article
Conflict of interest
The author(s) do not have any conflict of interest
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
Approval of the ethics committee at the Al-Azhar University was taken prior to the study (number no. 2022081456). Written Consents was obtained from each participant in the current study.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Authors’ Contribution
Conceptualization: Fatma Abdel-Kader Attia Mohamed and Marwa M. Hassan
Methodology: Eman Refaat Youness,
Data analysis: Marwa M. Hassan and Nesma Hassan Hasanein Ashour,
Editing and reviewing the final manuscript: Eman Refaat Youness, Marwa M. Hassan and Nesma Hassan Hasanein Ashour.
References
- Molodecky, N. A., Soon, S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G. & Kaplan, G. G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology, 2012, 142.1: 46-54. e42.
CrossRef - Bernstein CN. Assessing environmental risk factors affecting the inflammatory bowel diseases: a joint workshop of the Crohn’s & Colitis Foundations of Canada and the USA. Inflamm Bowel Dis. 2008;14(8):1139-46.
CrossRef - Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, Saruta M, Hirai F, Hata K, Hiraoka S, Esaki M, Sugimoto K, Fuji T, Watanabe K, Nakamura S, Inoue N, Itoh T, Naganuma M, Hisamatsu T, Watanabe M, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J gastroenterol. 2021; 56(6):489-526.
CrossRef - Liu, A., Lv, H., Tan, B., Shu, H., Yang, H., Li, J., & Qian, J. Accuracy of the highly sensitive C-reactive protein/albumin ratio to determine disease activity in inflammatory bowel disease. Medicine, 2021, 100.14: e25200.
CrossRef - Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse K, Hart A, Hindryckx P, Langner C, Limdi J, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F, Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11(6):649-670.
CrossRef - Cynthia E. Cherfane, Luke Gessel, Dominic Cirillo, Miriam B. Zimmerman, Steven Polyak, Monocytosis and a Low Lymphocyte to Monocyte Ratio Are Effective Biomarkers of Ulcerative Colitis Disease Activity, Inflammatory Bowel Diseases. 2015; (21) 8: 1769–1775,
CrossRef - Hyun, H. K., Zhang, H. S., Yu, J., Kang, E. A., Park, J., Park, S. J. & Cheon, J. H. Comparative effectiveness of second-line biological therapies for ulcerative colitis and Crohn’s disease in patients with prior failure of anti-tumour necrosis factor treatment. BMC gastroenterol
- Best WR, Becktel JM, Singleton JW, Kern F Jr (March 1976). “Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study”. Gastroenterology. 70 (3): 439–444.
CrossRef - Sands B, Anderson F, Bernstein C, Chey W, Feagan B, Fedorak R, Kamm M, Korzenik J, Lashner B, Onken J, Rachmilewitz D, Rutgeerts P, Wild G, Wolf D, Marsters P, Travers S, Blank M, van Deventer S. “Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876-885.
CrossRef - Hanauer S, Feagan B, Lichtenstein G, Mayer L, Schreiber S, Colombel J, Rachmilewitz D, Wolf D, Olson A, Bao W, Rutgeerts P (2002). “Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial”. Lancet. 359 (9317): 1541–9.
CrossRef - OSEI-BIMPONG, A.; MEEK, J. H.; LEWIS, S. M. ESR or CRP? A comparison of their clinical utility. Hematology, 2007, 12.4: 353-357.
CrossRef - FLYNN, Sean; EISENSTEIN, Samuel. Inflammatory bowel disease presentation and diagnosis. Surgical Clinics, 2019, 99.6: 1051-1062.
CrossRef - Liu, A., Lv, H., Tan, B., Shu, H., Yang, H., Li, J. & Qian, J. Accuracy of the highly sensitive C-reactive protein/albumin ratio to determine disease activity in inflammatory bowel disease. Medicine, 2021, 100.14: e25200.
CrossRef - Dong, J., Chen, Y., Tang, Y., Xu, F., Yu, C., Li, Y. & Dai, N. Body mass index is associated with inflammatory bowel disease: a systematic review and meta-analysis. PloS one, 2015, 10.12: e0144872.
CrossRef - Park, S. H., Kim, Y. M., Yang, S. K., Kim, S. H., Byeon, J. S., Myung, S. J. & Kim, J. H. Clinical features and natural history of ulcerative colitis in Korea. Inflammatory bowel diseases, 2007, 13.3: 278-283.
CrossRef - Sexton, K. A., Walker, J. R., Targownik, L. E., Graff, L. A., Haviva, C., Beatie, B. E. & Bernstein, C. N.. The inflammatory bowel disease symptom inventory: a patient-report scale for research and clinical application. Inflammatory bowel diseases, 2019, 25.8: 1277-1290.
CrossRef - Glapa-Nowak, A., Szczepanik, M., Banaszkiewicz, A., Kwiecień, J., Szaflarska-Popławska, A., Grzybowska-Chlebowczyk, U. & Walkowiak, JC-reactive protein/albumin ratio at diagnosis of pediatric inflammatory bowel disease: a retrospective multi-center study. Medical science monitor: international medical journal of experimental and clinical research, 2022, 28: e937842-1.
CrossRef - Jeong, Y., Jeon, S. R., Kim, H. G., Moon, J. R., Lee, T. H., Jang, J. Y. & Park, SJY. The role of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in ulcerative colitis. Intest Res. 2021;19(1):62-70.
CrossRef
- Chen YH, Wang L, Feng SY, Cai WM, Chen XF, Huang ZM. The Relationship between C-Reactive Protein/Albumin Ratio and Disease Activity in Patients with Inflammatory Bowel Disease. Gastroenterol Res Pract. 2020.
CrossRef
Abbreviations
| Abb. | Full term |
| 5-ASA | 5-aminosalicylic acid |
| ACG | American College of Gastroenterology |
| AGA | American gastroenterological association |
| ASGE | American Society for Gastrointestinal Endoscopy |
| LMR | And lymphocyte to monocyte ratio |
| pANCAs | Antineutrophil cytoplasmic antibodies |
| AZA | Azathioprine |
| CBC | Complete blood count |
| CT | Computed tomography |
| CTE | Computed tomography enterography |
| CRP | C-reactive protein |
| CD | Crohn’s disease |
| CDAI | Crohn’s disease activity index |
| CsA | Cyclosporine |
| ESR | Erythrocyte sedimentation rate |
| ECCO | European Crohn’s and Colitis Organisation |
| FC | Fecal calprotectin |
| GIT | Gastrointestinal tract |
| HBV | Hepatitis B virus |
| hs CRP | High senestive C-reactive protein |
| IBDU | IBD unclassified |
| IPAA | Ileal pouch–anal anastomosis |
| IMs | Immunomodulaors |
| IBD | Inflamtorry bowel disease |
| IFX | Infliximab |
| IOIBD | International Organization for the Study of Inflammatory bowel disease |
| kDa | Kilodalton |
| LCAP | leukocytapheresis |
| MRE | Magnetic resonance enterography |
| MRI | Magnetic resonance imaging |
| 6-MP | Mercaptopurine |
| μg/g | Microgram/gram |
| MMX | Multi Matrix systemtm |
| OmpC | Outer membrane porin |
| PC | Patency capsule |
| PLR | Platelet to lymphocyte ratio |
| POR | Post-operative recurrence |
| PSL | Prednisolone |
| RCTs | Randomized controlled trials |
| RDW | Red blood cell distribution width |
| STRIDE | Selecting Therapeutic Targets in Inflammatory Bowel Disease |
| SBCE | Small-bowel capsule endoscopy |
| BSG | The British Society of Gastroenterology guidelines |
| CAR | The highly sensitive C-reactive protein/albumin ratio |
| CRP/ALB | The highly sensitive C-reactive protein/albumin ratio |
| ECCO | The European Crohn’s and Colitis Organization |
| NLR | The neutrophil to lymphocyte ratio |
| T2T | Treat to Target |
| TNF-a | Tumor Necrosis Factor -α agents |
| UC | Ulcerative colitis |
| US | United states |
| UST | Ustekinumab |
| WBCs | White blood cells |
| WGO | World gastroenterology organisation global guidelines |








