Oleksandra Oleshchuk1 , Oresta Pinyazhko2
, Oresta Pinyazhko2 , Mykola Klantsa1
, Mykola Klantsa1 , Kateryna Posokhova1
, Kateryna Posokhova1 , Mariana Lukanyuk1
, Mariana Lukanyuk1 , Tamara Mahanova2
, Tamara Mahanova2 and Mariia Shanaida3*
and Mariia Shanaida3*
1Department of Pharmacology with Clinical Pharmacology, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
2HTA Department of State Expert Center of the Ministry of Health of Ukraine, Kyiv, Ukraine
3Department of Pharmacognosy and Medical Botany, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Corresponing Author E-mail:shanayda@tdmu.edu.ua
DOI : https://dx.doi.org/10.13005/bpj/3010
Abstract
Introduction. The article examines the role of tramadol in chronic pain (CP) treatment, focusing on its clinical effectiveness, safety profile, and market presence in Ukraine. Given the rising concerns surrounding opioid use, this manuscript seeks to provide a comprehensive assessment of the role of such medicine as Tramadol in pain management. The research aimed to the secondary and tertiary sources of clinical effectiveness, and safety of tramadol for the management of CP, and to analyze the Ukrainian market of this pharmaceutical in the sales data provided by PharmXplorer. Materials and Methods. This review thoroughly searched clinical trials, systematic reviews, and meta-analyses in various scientific databases, including PubMed, Scopus, Web of Science, Embase, ClinicalTrials.gov, and Google Scholar. It employed keywords such as "tramadol," "CP", "effectiveness," "safety," "adverse effects," "overdose," and "abuse" to identify relevant studies. Primary data from the analytical application for pharmaceutical market players - PharmXplorer were used as marketing research materials. Logical analysis, synthesis, generalization, graphic, and statistical methods were used in the research. Results and Conclusions. This study critically analyzed the clinical effectiveness of tramadol through a comprehensive evaluation of clinical trials, systematic reviews, and meta-analyses concerning its use for CP management in oncological patients, non-cancer pain management, and pediatric practice. The conducted analysis does not demonstrate the advantage of tramadol compared to other opioids in the treatment of chronic pain in cancer patients, or chronic pain of different origins, including in pediatric practice. The safety of tramadol in clinical settings does not exceed the safety of other narcotic analgesics, and in some cases is even inferior to it. 80% of tramadol drugs on the Ukrainian pharmaceutical market are produced in Ukraine. Nevertheless, tramadol is not recommended as a first-line therapy for CP management due to its limited efficacy and safety concerns.
Keywords
Chronic Pain Management; Effectiveness; Market; Safety; Tramadol
Download this article as:| Copy the following to cite this article: Oleshchuk O, Pinyazhko O, Klantsa M, Posokhova K, Lukanyuk M, Mahanova T, Shanaida M. Critical Assessment of Effectiveness and Safety of Tramadol and Evaluation of its Market in Ukraine. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Oleshchuk O, Pinyazhko O, Klantsa M, Posokhova K, Lukanyuk M, Mahanova T, Shanaida M. Critical Assessment of Effectiveness and Safety of Tramadol and Evaluation of its Market in Ukraine. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/40adQ73 |
Introduction
CP is a widespread and complicated problem that affects a significant portion of the global population, greatly impacting their quality of life and well-being. It imposes a substantial personal and economic burden, affecting more than 30% of people worldwide1,2. From ancient times, humans have sought to alleviate chronic pain (CP) using physical and chemical means. Natural opioids have been used as painkillers for centuries3. Ukraine in the period of war needed effective, safe, and affordable analgesics for managing CP.
Tramadol (АТС code N02AX02) is a centrally-acting analgesic drug with a multimodal mode of action that contributes both to its efficacy and potential toxicity. Tramadol works through a dual mechanism: it binds to the μ-opioid receptors in the brain and inhibits the reuptake of norepinephrine and serotonin, which helps modulate pain perception (Fig. 1) The widespread use of this drug for the management of CP, given the war in Ukraine, requires a thorough analysis of its clinical efficacy and safety.
Weak opioid analgesics such as codeine and its derivates and tramadol are used for severe pain control when paracetamol or nonsteroidal anti-inflammatory drugs (NSAIDs) are not sufficient. However, weak opioids are not more effective than paracetamol or NSAIDs for nociceptive pain, and they are not better tolerated than morphine4. The effectiveness of codeine and tramadol is significantly influenced by an individual’s CYP2D6 genotype, which can vary greatly among people. This genetic variability accounts for instances of both overdosing and underdosing when standard doses of these medications are administered. In contrast, the potency of morphine and buprenorphine does not appear to be affected by CYP2D6 activity.
As a weak opioid, tramadol can cause dose-dependent side effects that are similar to those of morphine. There is no evidence to suggest that weak opioids pose a lower risk of addiction than low-dose morphine when both provide comparable pain relief. Rapid metabolizers may experience respiratory depression even after short-term use of standard doses of codeine or tramadol. Additionally, tramadol carries risks of serotonin syndrome, hypoglycemia, hyponatremia, and seizures. Several studies have assessed various weak opioids for managing perioperative pain. A weak opioid, especially when combined with paracetamol, has been found to offer better pain relief than paracetamol alone, but not necessarily more than an NSAID alone. Limited research exists on the use of weak opioids for CP management. Existing trials do not show that weak opioids offer considerably more effective pain relief than paracetamol or NSAIDs. When narcotic analgesic treatment is necessary, there is no evidence indicating that tramadol poses a lower risk than morphine at its minimum effective dose. These medications often demonstrate greater variability in efficacy among patients compared to morphine, and their various pharmacokinetic interactions can complicate management. Furthermore, there is an unpredictable risk of serious overdose. Tramadol also presents additional side effects beyond its opioid properties. Therefore, careful monitoring is necessary for weak opioids, similar to what is required for morphine5.
Our research aim is to review the secondary and tertiary sources of evidence for the clinical effectiveness and safety of tramadol usage for CP management and analyze the Ukrainian market for tramadol expenditures.
Materials and methods.
This review involved a comprehensive search across multiple scientific databases, including PubMed, Scopus, Web of Science, Embase, and Google Scholar, using keywords such as “tramadol”,” pain”,” effectiveness”, “safety”, “adverse effects,” “overdose,” and “abuse.” No restrictions were applied regarding the publication year or language of the studies. In total, 43 studies were included in this review. The research uses a comprehensive approach based on the unity of theory and practice. Primary data from the analytical application for pharmaceutical market players – PharmXplorer were used as marketing research materials. International and domestic guidelines, regulated lists of medicinal products, and normative legal acts were used to analyze the regulation of the circulation and use of tramadol. The research used methods of logical analysis, synthesis, and generalization, as graphic and statistical methods. Artificial intelligence tools, such as the latest models of ChatGPT and Google Bard were utilized, to enhance the accuracy and comprehensiveness of this analysis. These tools assist in identifying relevant literature, summarizing findings, and ensuring thorough analysis. All processes were supervised and validated by researchers.
Results and Discussion.
Chemistry and history. Tramadol (2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol)6, also known as Tramal, Tramacur, or Ralivia, is a synthetic opioid of the phenylpropanolamine class. It is structurally related to natural alkaloids codeine and morphine (Fig. 1). Tramadol is unique because it is found in a combination of its stereoisomers, known as a racemate. It is produced as a racemate of its two isomers because the combination is more effective in pain management.
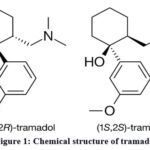 |
Figure 1: Chemical structure of tramadol
|
Tramadol acts as a weak agonist of the μ-opioid receptor and a reuptake inhibitor of serotonin and norepinephrine7. It was developed in 1962, and at the end of the 70s of the 20th century, it was approved8. Its production was launched by a pharmaceutical company Grünenthal GmbH. (Germany) under the name “Tramal”. Nowadays it is prescribed to cure patients with moderate or moderately severe pain8.
Mode of action, pharmacological characteristic.
Tramadol is a weak agonist of the mu-opioid receptors, which accounts for part of its analgesic properties. Additionally, tramadol inhibits the reuptake of serotonin and norepinephrine, enhancing descending inhibitory pain pathways (Fig. 2).
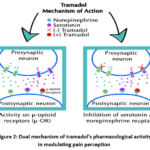 |
Figure 2: Dual mechanism of tramadol’s pharmacological activity in modulating pain perception
|
The combination of these opioid and non-opioid actions makes tramadol effective in treating moderate to severe pain. However, this multifactorial mechanism also underlies the drug’s potential for adverse effects, including seizures, serotonin syndrome, and respiratory depression. The complexity of its pharmacodynamics, particularly involving its active metabolite O-desmethyl-tramadol, further complicates its risk profile, as individual metabolic variability can influence both efficacy and toxicity9.
Clinical effectiveness and safety.
Treatment of CP in many countries is based on the recommendations of the guidelines developed by the WHO10. The guidelines recommend using analgesic therapy in three stages (III steps of WHO analgesia!), based on the degree of pain severity:
The first step is mild pain: use non–opioid analgesics – paracetamol, ibuprofen, acetylsalicylic acid (Aspirin), and indomethacin (as an alternative – naproxen, diclofenac).
The second step – moderate pain: use opioid analgesics of weak action – codeine (which is available in the National Essential Medicines List in the II section “Drugs for the pain treatment and providing palliative care” together with other opioid analgesics: morphine, hydromorphone, and oxycodone); as an alternative to codeine, dihydrocodeine, dextropropoxyphene, standardized opium, or tramadol can be used.
Third step – severe pain: use a strong opioid – morphine; methadone, hydromorphone, oxycodone, levorphanol, pethidine, buprenorphine can be used as an alternative to morphine. It is desirable to use oral forms, in the absence of the possibility of using oral forms, it is possible to use other forms – rectal, parenteral (s/c, i/m, i/v, transdermal), and others.
Tramadol as an opioid analgesic may reduce the pain of various origins (Fig. 3). However, opioids that are more powerful traditionally used in the postoperative period. Studies of the effectiveness of tramadol in chronic pain in cancer patients and in chronic pain of other origins continue.
 |
Figure 3: Indications for tramadol administration
|
Table 1 shows that 17 clinical trials of tramadol effectiveness can be found at ClinicalTrials.gov, of which 13 have been completed. These trials explore the therapeutic potential of tramadol, which is conducted in different countries for different purposes. The key points of their conducting analysis are given below. The main uses of tramadol include pain management after surgery, CP, management of postoperative conditions, and prevention of complications such as chills or inflammation. The trials include participants of all ages, from children as young as 1 year old to the elderly up to 90 years old, indicating a wide range of uses for the drug. The tests were conducted in different parts of the world such as Japan, Egypt, Tunisia, India, Croatia, and Spain. This demonstrates the global interest in tramadol research. Studies include interventional and observational studies, some of which are double-blind controlled (eg, placebo-controlled trials). Clinical stages range from early phases (Phase 1) to advanced stages of clinical trials (Phases 3 and 4), suggesting a desire to develop new forms of tramadol and assess its long-term safety. Some studies have focused on specific conditions, such as postoperative neck pain, lumbar pain, and chronic conditions, and studies of the efficacy of tramadol in drug withdrawal and its effects on platelet function. This analysis demonstrates that tramadol needs further investigation for pain management and research to refine its dosage, efficacy, safety, and use in specific settings.
 |
Table 1: Recent clinical trials investigating the therapeutic potential of tramadol (from ClinicalTrials.gov)
|
Regarding the usage of tramadol for coping chronic pain in patients due to cancer
The results of the analysis of the clinical guidelines identified that most of the international pain management guidelines agree with the WHO recommendations and believe that, indeed, the treatment of CP should be carried out in three stages. However, the guidelines also recognize that tramadol can only be an alternative to codeine in patients with moderate pain. Among them:
A National Clinical Guideline. Pharmacological Management of Cancer Pain in Adults; Health Service Executive, Royal College of Physicians, National Clinical Programme for Palliative Care, Ireland 2015»28;
Management of Cancer Pain: ESMO Clinical Practice Guidelines, 2012; European society for medical oncology29;
General Palliative Care Guidelines for the Management of Pain at the End of Life in Adult Patients, 201130;
Cancer Pain Management, 2010 British Pain Society»31;
SIGN–106. Control of pain in adults with cancer, 2008, Scottish Intercollegiate Guidelines Network»32;
Clinical Guidelines for Pharmacological Management of Cancer Pain 2020»33.
The guideline developed in Ireland28 determined that in the absence of any specific patient-dependent problems when using codeine or the codeine/paracetamol combination, it is better to continue codeine than to use tramadol or tapentadol.
The European Society of Medical Oncology’s29 states that an alternative to weak opioids can be the use of low doses of strong opioids in combination with non-opioid analgesics.
Scottish instruction32 determined that for the management of mild to moderate pain (3-6 points out of 10 on the visual analog scale), weak opioids have to be administered together with a non-narcotic analgesic; there is limited evidence data for the effectiveness of tramadol in the pharmacotherapy of these conditions.
According to the Japanese Society of Palliative Medicine, strong opioids are highly recommended for managing moderate to severe cancer pain. In contrast, weaker opioids, such as tramadol, should only be considered when strong opioids are not an option28.
An additional analysis of secondary evidence was conducted to assess the effectiveness of weak opioids for treating CP in cancer patients. A systematic review34 concluded that there is limited evidence supporting the usage of opioids for cancer pain treatment. However, the review data suggests that approximately 19 out of 20 people with moderate to severe pain who receive opioids can experience analgesia to a mild degree of pain or complete it within 14 days. This aligns with the clinical experience of opioid treatment in many cancer patients but may slightly overestimate their effectiveness as determined by WHO.
Another systematic review was devoted to the study of the analgesia by tramadol in adult cancer patients35 states that there is limited evidence from randomized controlled trials (RCTs) of poor methodological quality suggesting that tramadol leads to pain relief in some adults with cancer, and evidence for it is not used in children at all. There is very low-quality evidence that tramadol is not as effective as morphine. This review does not provide reliable data on the possible effectiveness of tramadol. Moreover, the likelihood that its analgesic effect will vary significantly is very high. Thus, the place of tramadol as the second step of analgesia (according to the WHO methodology) in the pain management of cancer patients remains uncertain.
The use of tramadol in patients for the treatment of non-cancer pain
The text below summarizes the review of international guidelines and presents the results of a meta-analysis on the efficacy and safety of tramadol in various pain-related conditions. It is also displayed in Table 2.
Table 2: The recent guidelines, systematic reviews, and meta-analysis of tramadol use for the management of non-cancer pain
|
Year |
Country/ Organization |
Type of publication |
Drugs |
Type of pain |
Severity |
Quality assessment |
Results |
|
2009 |
The Royal College of General Practitioners, Australia |
Guideline |
Tramadol mono |
Hip and knee osteoarthritis (in persons who have not responded to, or are unable to tolerate, other analgesic medications or NSAIDs, and in whom joint replacement surgery is contraindicated or delayed) |
Moderate |
High quality (Grade A Excellent evidence: body of evidence can be trusted to guide practice) |
The drug is less effective than strong opioids but has the same adverse side effects |
|
2016 |
The European Society for Clinical and economic aspects of the treatment of osteoporosis and osteoarthritis |
Guideline |
Tramadol mono |
Knee osteoarthritis (last pharmacological option) |
Moderate |
Moderate quality (Good evidence) |
Tramadol can be used only as the 3rd step (the last step before surgery) of treatment, when other methods of intervention are already ineffective |
|
2017 |
American College of Physicians |
Guideline |
Tramadol combined with other drugs |
Acute, subacute, and chronic low back pain (patients have an inadequateresponseto nonpharmacologic therap) |
Moderate
|
Low quality |
The pharmacotherapy of NSAIDs as the first line of therapy, tramadol (combined serotonin, norepinephrine, dopamine reuptake inhibitor) as the second line of therapy |
|
2017 |
Danish Health Authority |
National clinical guidelines |
Tramadol combined with other drug Tramadol mono |
Recent onset neck pain or cervical radiculopathy (after first-line paracetamol) |
Sever
|
Low quality
Very low quality |
Tramadol after careful consideration |
|
2017 |
Cochrane Group “Tramadol for neuropathic pain in adults, 2017”, Cochrane Neuromuscular Group |
Systematic review |
Tramadol mono |
Neuropathic pain |
Moderate or severe |
Low or very low-quality |
There is limited information on the use of tramadol in patients with neuropathic pain, resulting from small studies with a potential risk of bias |
|
2015 |
Germany |
Systematic review and meta-analysis |
Morphine, tramadol, oxycodone, tapentadol |
Chronic neuropathic pain (short-term studies during 4–12 weeks) |
Not specified (any type) |
Low quality |
Opioids were more effective than placebo in reducing pain intensity, but worse in tolerability. Short-term opioid therapy may be considered in some patients with chronic neuropathic pain. |
|
2023 |
Included 98 randomized controlled trials |
Systematic review and network meta-analysis |
Tramadol, paracetamol plus sustained-release tramadol, baclofen, and paracetamol plus tramadol |
Acute non-specific low back pain |
|
|
Increased adverse events had moderate to very low confidence compared with placebo.
|
The data contained in the recommendations of six international guidelines for the use of weak opioids in patients with non-oncology pain are somewhat ambiguous. All guidelines unequivocally recognize that the use of NSAIDs is primarily necessary for such patients. At the same time, the Scottish instruction36 suggests considering strong opioids (morphine, diamorphine, hydromorphone, oxycodone, fentanyl, buprenorphine, methadone) as an option for pain relief in patients with chronic back pain or osteoarthritis pain, with continued use only under the condition of pain relief.
The British Medical Association offers guidelines37 for the effective use of opioids in persistent pain, noting that although opioids may be effective, other available effective interventions should be attempted. This is due to the negative consequences of opioid use: mortality, respiratory depression, hypogonadism, adrenal insufficiency, hypersensitivity to pain (hyperalgesia), addiction, and withdrawal syndrome. There is insufficient high-quality evidence to justify the recommendation for the long-term use of opioids in CP management.
According to the American guideline for the treatment of low back pain38. The American Pain Society notes that weak opioids may be an alternative to (or used in combination with) paracetamol or NSAIDs to control CP if patients do not respond to other treatments. However, this guideline recognizes that tramadol showed moderate effectiveness compared to a placebo for short-term pain relief and functional improvement in cases of chronic back pain; however, it did not demonstrate greater effectiveness than a combination of paracetamol and codeine.
The American Society of Interventional Pain Physicians39 states that to justify the need for opioids, it is necessary to have an established diagnosis and treatment that has already been carried out in various ways, including conservative or other alternative methods. Opioids can be used as second-line drugs. Recommended use of morphine and methadone. It has been determined that the benefit of tramadol in fibromyalgia is insignificant, which makes it necessary to consider other options for pain relief.
The Royal Australian College of General Practitioners (guideline for the treatment of osteoarthritis of the joints40) states that the use of weak opioids (such as codeine and tramadol) should be used cautiously, as they are less effective than strong opioids while sharing similar adverse side effects.
The guideline developed by the European Society for Clinical and Economic Aspects of the Treatment of Osteoporosis and Osteoarthritis41 postulates that tramadol can be used only as the 3rd step (the last step before surgery) of treatment when other methods of intervention are already ineffective.
The Denmark guideline42 recommended tramadol for patients with nonspecific neck pain and cervical radiculopathy only after thorough evaluation, rather than being used as a first-line treatment.
According to the guideline developed by the American College of Physicians43, for patients with chronic back pain who have had an inadequate response to non-pharmacological treatment, it is necessary to consider the pharmacotherapy of NSAIDs as the first line of therapy, and tramadol or the antidepressant duloxetine (combined serotonin, norepinephrine, dopamine reuptake inhibitor) as the second line of treatment.
The American College of Physicians and American Academy of Family Physicians suggest against clinicians treating patients with acute pain from non-low back, and musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence)44.
Twelve recommendations were made evidence-based, and nine were based on the consensus of the Danish Health Authority for non-surgical treatment of patients with recent onset neck pain or cervical radiculopathy45. The guidelines recommend various forms of supervised exercise and manual therapy, suggesting that these should be prioritized before medication for neck pain. They also endorse acupuncture for neck pain (but not for cervical radiculopathy), traction for cervical radiculopathy, and the cautious use of NSAIDs (oral or topical) and tramadol for both conditions. While these recommendations are based on low-quality evidence or expert consensus, they are consistent with similar guidelines from North America.
The use of tramadol in patients with persistent knee or hip osteoarthritis pain, which is recommended (2019) by the American College of Rheumatology/Arthritis Foundation (ACR/AF) as opposed to Veterans Affairs and Department of Defense (VA/DoD) treatment guidelines and Osteoarthritis Research Society International (OARSI) treatment guidelines who recommend against the knee or hip osteoarthritis use of opioids without exceptions46.
In cases of chronic low back pain where non-drug treatments have not been effective, patients and clinicians should consider using NSAIDs as the first treatment option47. If these are not effective, tramadol or duloxetine can be considered as the next step. Opioids should only be considered if the previously mentioned treatments have failed, and after a thorough discussion of the potential risks and benefits with the patient.
Despite a lack of robust scientific evidence to support opioids use to manage pain in fibromyalgia, 33.0 % of patients reported using them48. Tramadol was injected into 23.8% of participants, while 54.0% of patients reported using NSAIDs, despite their lack of efficacy as recommended in guidelines. Among the medications with strong recommendations, 55.6% used serotonin-norepinephrine reuptake inhibitors, 36.5% used anticonvulsants, and 22.2% used tricyclic antidepressants. Additionally, cannabinoids were reported by 17.5% of patients, with medical cannabis used by 34.9%. No significant differences were noted among these various medication classes.
The search strategy for systematic reviews in the Cochrane Database was conducted for the effectiveness of the use of weak opioids for the treatment of CP in patients with a non-oncological profile. Thus, a systematic review performed by the Cochrane Group49 stated that there is a lack of information on the use of tramadol in patients with neuropathic pain, resulting from limited studies with a potential risk of bias. Such prejudices tended to deliberately exaggerate the apparent benefits of tramadol. The evidence for the benefits of tramadol was of low or very low quality.
In a systematic review conducted in Germany50 the purpose of which was to update the data of the Cochrane systematic review on the efficacy, tolerability, and safety of the use of opioids (morphine, tramadol, oxycodone, tapentadol) in patients with chronic neuropathic pain. In short-term studies (4–12 weeks), opioids were more effective than placebo in reducing pain intensity, but worse in tolerability. Short-term opioid therapy may be considered in some patients with chronic neuropathic pain.
The aim of another systematic review carried out in Germany51 was to update the data of the Cochrane systematic review on the efficacy, tolerability, and safety of the use of opioids (oxycodone, tramadol, buprenorphine, hydromorphone, morphine, tapentadol, codeine, fentanyl, and oxymorphone) for chronic osteoarthritis pain. Opioids were more effective than placebo in reducing pain intensity, but inferior in tolerability. Short-term opioid therapy might be considered for certain patients with chronic neuropathic pain or chronic osteoarthritis pain; however, no existing guidelines advocate for opioids as a first-line treatment option.
Another systematic review, also performed in Germany52, has found that non-opioid analgesics were superior to opioids (tramadol, morphine) in their ability to improve physical function and tolerability in short-term use (4–12 weeks) for neuropathic back pain and osteoarthritis pain. The results of the review do not support the concept that patients with non-cancer pain require opioids.
The aim of another systematic review performed in Germany53, was to compare the efficacy, tolerability, and safety of the use of opioid analgesics (hydromorphone, morphine, oxymorphone, and tapentadol with oxycodone; fentanyl with morphine; buprenorphine with tramadol) and their routes of administration in patients with CP not related to cancer. In a pooled analysis, there were no significant differences among opioids regarding pain reduction (though based on low-quality evidence), improvement in physical function (very low-quality evidence), incidence of serious side effects (moderate-quality evidence), or mortality (moderate-quality evidence). Additionally, there was no statistically significant difference between transdermal and oral opioid administration in terms of pain relief, functional improvement, serious adverse events, or mortality, all of which were based on low-quality evidence. Thus, a comparison of opioids did not reveal a rational advantage of each in the treatment of patients with CP not related to cancer.
The systematic reviews54,55,56 stated that there is limited evidence that weak oral opioids may be effective analgesics for some patients with rheumatoid arthritis, osteoarthritis, and chronic back pain. Side effects are common and can negate any benefits of this class of drugs. There is insufficient evidence to conclude the feasibility of long-term use of weak opioids in this pathology.
The 2023 meta-analysis evaluated the efficacy and safety of tramadol in opioid withdrawal57. Reviewers extracted data from eight relevant clinical trials after a literature search on MEDLINE/PubMed, Cochrane databases, and clinical trial registries. No significant difference was found between tramadol and comparators (placebo, buprenorphine, clonidine, and methadone) in reducing opioid withdrawal scale score and treatment retention.
Another study58 evaluates the effectiveness of various pharmacological treatments to establish guidelines for managing acute pain following tooth extractions. The authors conducted a systematic search of databases, including Medline, EMBASE, CENTRAL, and the US Clinical Trials, focusing on RCTs involving patients who underwent dental extractions. Ten different treatments, including acetaminophen, NSAIDs, opioids, and their combinations, were compared to a placebo. After removing duplicates and extracting relevant data, a frequentist network meta-analysis was conducted to assess several outcomes at the 6-hour mark, such as pain relief, total pain relief, summed pain intensity difference, global efficacy rating, need for rescue analgesia, and adverse effects. The results indicated that treatments like oxycodone 5 mg, codeine 60 mg, and a combination of tramadol 37.5 mg with acetaminophen 325 mg did not provide better pain relief than a placebo. The global efficacy rating and the requirement for rescue analgesia were similar across all groups. Furthermore, the study found that, based on low- to very low-certainty evidence, most treatments did not cause more adverse effects than the placebo.
The systematic review59 analyzed the effects of tramadol at daily doses of 100 mg, 200 mg, and 300 mg for knee or hip osteoarthritis. The study found that taking tramadol 300 mg daily resulted in only slight improvements in pain and function. Additionally, it was linked to a greater number of adverse events compared to the placebo. Based on these results, tramadol may not be strongly recommended for treating osteoarthritis of the knee or hip, especially in patients who are at higher risk for gastrointestinal and central nervous system side effects.
One hundred thirteen studies identified the efficacy and safety of tramadol in pain relief during diagnostic outpatient hysteroscopy. Four randomized clinical trials were deemed eligible for this review. The meta-analysis of these data suggests that tramadol is safe, effective, and gives favorable results in reducing pain during diagnostic outpatient hysteroscopy60.
Systematic review and network meta-analysis61 of various analgesic agents for acute low back pain included 98 randomized controlled trials (15 134 participants, 49% women, 69 different medicines or combinations). There was moderate to very low certainty of difference in adverse events with tramadol, paracetamol plus tramadol sustained release, baclofen and paracetamol plus tramadol compared to placebo.
The meta-analysis, which reviewed 51 studies involving a total of 101,770 patients62, revealed that the risk of seizures in individuals taking tramadol is dose-dependent and more prevalent among males, but is not influenced by naloxone administration. The rate of seizure events varied across different subgroups: in cases of tramadol poisoning, the seizure occurrence was 38%, in patients taking therapeutic doses of tramadol it was 3%, and among tramadol abusers, the rate was 37%.
A Phase III clinical trial evaluating a sustained-release formulation of tramadol (comprising 65% sustained release and 35% immediate release) demonstrated positive results in treating postherpetic neuralgia. The formulation was effective in pain management and well tolerated by patients, offering a potential option for individuals suffering from this condition63. Nausea, constipation, nasopharyngitis, somnolence, vomiting, and congestive heart failure were reported for it with no dose-dependent increase.
Tramadol misuse, an opioid prescription painkiller, is a significant public health issue worldwide. A meta-analysis conducted by Iranian researchers reviewed studies on the dissemination of non-prescribed use, regular use, and dependence on tramadol, as well as tramadol-induced poisonings and deaths in Iran64. The analysis highlighted that, despite existing control policies, tramadol use in Iran is as prevalent as the use of illegal opioids. The country has experienced a significant number of cases involving tramadol abuse, dependence, poisonings, and seizures, along with hundreds of tramadol-related deaths in recent years, indicating a serious public health concern.
A network meta-analysis of randomized controlled trials65 was conducted to assess the comparative effectiveness of various outpatient treatments for managing pain associated with non-low back musculoskeletal injuries. The findings indicated that tramadol was ineffective. The combination of opioid with acetaminophen improved intermediate pain (1 to 7 days) but did not alleviate immediate pain (≤2 hours). The use of opioid was associated with an increased risk of gastrointestinal and neurologic adverse effects, compared to placebo.
A total of 45 studies were conducted to evaluate the efficacy of various interventions for catheter-related bladder discomfort, a frequent complication of intraoperative urinary catheterization. Among the 31 drugs assessed, 11 were investigated in more than two randomized controlled trials. The medicines that generally demonstrated significantly higher efficacy than controls in the postoperative period included dexmedetomidine, gabapentin, tolterodine, tramadol, ketamine, nefopam, oxybutynin, pregabalin, and pudendal nerve block66.
Thus, the assessment of guidelines, systematic reviews, and meta-analyses of tramadol for managing non-cancer pain indicates that its clinical effectiveness is limited.
Use of opioids in children
According to WHO guidelines67 for the treatment of pain in children the II degree of pain relief, i.e. weak analgesics, is generally issued. The guidance on tramadol states the following: At the moment, there is no available evidence regarding its comparative effectiveness and safety in children. The US FDA has contraindicated the use of codeine and tramadol in children for the management of acute pain due to safety concerns68. Additional studies are needed on the use of tramadol in the pediatric population.
In a systematic review carried out by the Cochrane Group69 it was stated that it is not possible to conclude the effectiveness or harm of the use of opioids (buprenorphine, codeine, fentanyl, hydromorphone, methadone, morphine, oxycodone, and tramadol) for the treatment of CP associated with oncological diseases in children and adolescents under 17 years of age, due to the lack of data from randomized controlled trials that confirm or refute the feasibility of using opioids in these patients for the specified indication.
A review by the same group, found no evidence from randomized controlled trials to support or refute the use of opioids for CP in children and adolescents, not related to oncological diseases. It is known from randomized controlled trials in adult patients that some opioids, such as morphine and codeine, may be effective for CP. Therefore, it is not possible to conclude the effectiveness or harm of using opioids for the treatment of CP in children and adolescents, not related to cancer.
Also, it is important to recognize that involvement of community and clinical pharmacists in multidisciplinary pain treatment teams helps reduce side effects, decrease pain intensity, and lead to healthcare resource savings. Cognitive pharmaceutical services have been key to addressing the crisis, highlighting the pharmacist’s value as an active partner with physicians in improving pain management outcomes70.
Common pharmacist services include opioid consumption monitoring, take-back programs, and naloxone training/distribution71. Based on this experience abroad it will be a perspective to introduce these approaches to the reimbursement program “Affordable Medicines”.
Safety and toxicity
The toxicity of tramadol is significantly influenced by its active metabolite, O-desmethyl tramadol (M1), which is formed primarily via CYP2D6-mediated metabolism. M1 exhibits a much higher affinity for the mu-opioid receptors compared to the parent compound, contributing to both its enhanced analgesic effects and potential for opioid-related toxicity, including respiratory depression and overdose. This increased potency at the opioid receptors is a key factor in the variability of tramadol’s toxicity, particularly among patients with genetic polymorphisms, and differing CYP2D6 enzyme activity. Ultra-rapid metabolizers produce higher concentrations of M1, thereby elevating the risk of severe adverse effects, including seizures and respiratory failure. Conversely, poor metabolizers have reduced conversion to M1, which can lead to insufficient pain control but also a potentially lower risk of opioid-related adverse effects. This variability complicates dosing strategies and underscores the importance of considering pharmacogenetic testing when prescribing tramadol, particularly in populations with known CYP2D6 polymorphism diversity72.
Accordingly, it is recommended to check the genetically determined activity of cytochrome P450 isoforms when prescribing these drugs. Gene-drug pairs with at least one clinical practice guideline recommending testing or stating that testing could be considered included CYP2C19-clopidogrel, CYP2D6-codeine, CYP2D6-tramadol, CYP2B6-efavirenz, TPMT-thiopurines, and NUDT15-thiopurines73.
Additionally, the balance between tramadol’s serotonergic and noradrenergic effects, coupled with the opioid activity of M1, plays a crucial role in the drug’s complex toxicity profile74,75.
Tramadol toxicity is characterized by a broad spectrum of clinical symptoms, primarily due to its mixed mechanism of action. Common presentations include seizures, which are frequently reported even at therapeutic doses due to tramadol’s proconvulsant effects, likely mediated through inhibition of GABA receptors. Additionally, the drug’s serotonergic activity can lead to serotonin syndrome, particularly when combined with other serotonergic agents such as SSRIs or MAOIs. Clinically, serotonin syndrome manifests with a triad of cognitive–behavioral changes (agitation, confusion), autonomic hyperactivity (hyperthermia, tachycardia), and neuromuscular abnormalities (tremor, hyperreflexia, clonus). In severe cases, this condition can rapidly progress to seizures, rhabdomyolysis, and multiorgan failure, potentially resulting in fatal outcomes. Cardiovascular effects, such as tachycardia and hypertension, are also common, while severe cases may involve respiratory depression, altered mental status, and coma. Understanding the interplay between tramadol’s serotonergic properties and its opioid mechanism is crucial for anticipating and managing these toxic effects, especially in polypharmacy scenarios76,77. The variability and overlap in these clinical signs underscore the complexity of managing tramadol overdose, with the potential for life-threatening consequences depending on dose, patient susceptibility, and co-ingested substances78,79.
Seizures are a well–documented and relatively frequent complication of tramadol overdose, occurring in up to 15–20% of cases, depending on dosage and patient-specific factors. Tramadol’s proconvulsant effects are primarily attributed to its serotonergic and noradrenergic activity, which can lower the seizure threshold. This risk is particularly pronounced in individuals with a history of epilepsy, but even patients without prior seizure disorders are susceptible when exposed to high doses. Notably, seizures can occur even at therapeutic doses in certain patients, especially in those who are rapid metabolizers of the drug. The co-administration of other medications that influence neurotransmitter activity, such as antidepressants or antipsychotics, further exacerbates this risk, highlighting the need for careful monitoring and dosage control80.
Tramadol toxicity can result in a range of cardiovascular complications, primarily due to its combined serotonergic, adrenergic, and opioid activities. Common manifestations include tachycardia and hypertension, likely arising from the drug’s inhibition of norepinephrine reuptake, which leads to enhanced sympathetic activity. Additionally, tramadol has been associated with both bradycardia and arrhythmias, particularly in cases of overdose, where the imbalance in autonomic regulation becomes more pronounced. In severe cases, these effects can progress to cardiovascular collapse, especially in the presence of co-ingested substances or pre-existing cardiac conditions. The arrhythmogenic potential of tramadol, coupled with its dose-dependent influence on blood pressure, underscores the importance of closely monitoring cardiovascular function during toxicity management81.
Although respiratory depression is a hallmark of opioid toxicity, it is generally less pronounced with tramadol compared to classical opioids due to its weak affinity for mu–opioid receptors. However, the risk becomes significant when tramadol is combined with other CNS depressants, such as benzodiazepines, alcohol, or other opioids. The dual mechanism of tramadol, which also involves serotonin and norepinephrine reuptake inhibition, contributes less to respiratory depression but can still potentiate CNS suppression when used in polypharmacy scenarios. In cases of overdose, respiratory depression can be exacerbated by the accumulation of the active metabolite M1, especially in individuals with genetic variations that enhance this conversion. Therefore, while tramadol alone may pose a moderate risk of respiratory depression, its potential for life-threatening respiratory compromise escalates significantly in the presence of other depressants or overdose situations82,83.
The risk of tramadol toxicity is closely related to dosage. Higher doses significantly increase the likelihood of adverse effects. Clinical data indicate that doses exceeding 400 mg per day are linked to a notable increase in the incidence of toxicity, which can include seizures, serotonin syndrome, and respiratory depression. This relationship is further complicated by individual metabolic differences, particularly involving the CYP2D6 enzyme, which can lead to the variable conversion of tramadol into its more potent metabolite, M1. As the dosage increases, the accumulation of M1 also rises, resulting in stronger opioid effects and a higher potential for toxicity. Additionally, non-linear pharmacokinetics at higher doses can make adverse outcomes even more unpredictable. This underscores the importance of careful dosage titration and monitoring in clinical practice84.
The clinical manifestations of tramadol overdose can vary significantly depending on whether the exposure is acute or chronic. Acute overdose typically presents with symptoms such as seizures, altered mental status, and respiratory depression. These effects are largely attributable to the drug’s dual action on opioid receptors and the inhibition of serotonin and norepinephrine reuptake, which can lead to a rapid onset of central nervous system toxicity. In contrast, chronic overdose or prolonged use at high doses is more likely to result in dependence, tolerance, and exacerbation of side effects such as dizziness, nausea, and cognitive impairment. Chronic users may also develop withdrawal symptoms upon dose reduction, reflecting the drug’s addictive potential. Additionally, long–term exposure to high doses may increase the risk of sustained neurotoxicity and cardiovascular complications, further complicating the clinical management of such patients85.
The management of tramadol overdose requires a multifaceted approach due to the drug’s complex mechanism of action. While naloxone is commonly administered to reverse opioid effects, its efficacy in tramadol toxicity is limited, as the drug’s toxic profile extends beyond opioid receptor activation. Naloxone may mitigate respiratory depression, but it does not address the risk of seizures, which are a prominent feature of tramadol overdose. Seizure control often requires the use of benzodiazepines or other anticonvulsants, such as diazepam or lorazepam. Additionally, in cases of serotonin syndrome, which can occur due to tramadol’s serotoninergic activity, supportive care and specific interventions like cyproheptadine are necessary. Early gastrointestinal decontamination with activated charcoal can be considered if the patient presents shortly after ingestion. Given the potential for polysubstance overdose, comprehensive supportive care remains essential, including monitoring and stabilization of cardiovascular and respiratory functions86.
Several prognostic factors have been identified as key indicators of increased mortality risk in cases of tramadol overdose. High–dose ingestion remains one of the strongest predictors of fatal outcomes, as it significantly raises the likelihood of life-threatening complications such as seizures, respiratory depression, and multi-organ failure. Additionally, the co-ingestion of other psychoactive substances, including alcohol, benzodiazepines, or other opioids, exacerbates the toxic effects of tramadol and contributes to heightened mortality. Patients with a history of seizure disorders are particularly vulnerable, as tramadol lowers the seizure threshold, which can precipitate status epilepticus in severe overdose scenarios. Furthermore, delayed medical intervention and inadequate monitoring of patients‘ risks. Recognizing these factors is crucial for early risk stratification and guiding the intensity of medical intervention to prevent fatal outcomes87.
Prolonged use of tramadol is associated with the development of tolerance, where increasing doses are required to achieve the same analgesic effects, leading to a higher risk of toxicity. As tolerance builds, patients may escalate their dosage, which not only increases the potential for acute overdose but also heightens the likelihood of chronic adverse effects such as dependence and withdrawal symptoms. Chronic users are also at greater risk for developing opioid-induced hyperalgesia, where pain sensitivity is paradoxically increased, and further complicating pain management. Additionally, long–term tramadol use has been linked to neurotoxicity, manifesting as cognitive impairment, mood disturbances, and even seizures. These cumulative effects highlight the delicate balance between therapeutic use and the potential for serious toxic outcomes in patients requiring long-term tramadol therapy88.
The recent attention to tramadol toxicity is due to its overuse as a pain reliever and non-medical uses. The FDA is very concerned about the excessive and unwarranted use of opioid analgesics, which is leading to an epidemic of abuse and overdose of these drugs in the United States. In response, in 2017 the FDA team developed a comprehensive action plan to take clear steps to reduce the impact of opioid misuse and abuse. These restrictions relate to further licensing of opioid analgesics, age restrictions in children, training on prescribing opioid analgesics and pain management among physicians, strengthening post-marketing requirements, updating the risk assessment program and risk reduction strategies, etc.89
Regulation and market
In Ukraine, the results of the marketing analysis showed that tramadol has marketing authorization in Ukraine in the form of capsules and injection solutions.
According to the State Register of Medicines, as of October 1, 2024, there are 7 trade names of tramadol and 2 substances (powder) registered in Ukraine90.
Market segmentation by dosage forms revealed that the majority of the range of tramadol finished medicinal products consists of liquid forms (5% injection solution in 1 and 2 mL vials) – 71% of the market, while solid forms of tramadol (50 mg capsules) account for 24%. Ukraine is the leading country producing finished tramadol medicinal products, accounting for 80% of the market in comparison with other producing countries present in the Ukrainian pharmaceutical market. The substances are produced exclusively in India.
Tramadol is indicated for the treatment of moderate to severe pain. Injection solution can be used in children from 1 year old, while capsules are recommended for children aged 14 and older. Tramadol is listed on the 16th edition of the State Drug Formulary of Ukraine and is recommended by the Unified Clinical Protocol for Palliative Care in Chronic Pain Syndrome, approved by the Order of Ministry of Health of Ukraine No. 311 dated April 25, 2012, for the treatment of moderate CP unresponsive to non-opioid analgesics and nonsteroidal anti-inflammatory drugs (as a weak opioid, on the second step of the WHO pain ladder). But tramadol is not included on the National List of Essential Medicines purchased with budgetary funds in Ukraine91.
In other countries:
In the USA (FDA) and Canada(Government of Canada), tramadol is only approved in oral forms (tablets and capsules) for treating severe pain when alternative treatments are insufficient.
The British National Formulary (№88, September 2024 – March 2025)92 – lists tramadol in various forms (injection solution and oral forms) for the treatment of moderate and severe pain in pure form and in combination with other active ingredients.
Absence on the WHO List: Tramadol is not listed on the WHO Model List of Essential Medicines (the 23rd edition). For marketing analysis data on tramadol procurement in Ukraine from 2020 to 2024 was also analyzed using the source of PharmXplorer database (Table 3).
This analysis allowed us to identify specific patterns that could provide additional insights into the use and availability of tramadol during this study period.
Table 3: Hospital and Retail Market of Tramadol in Ukraine 2020–2024 in UAH and units
|
Type of data |
Year |
Total Packages (units) |
Total Volume (UAH) |
Total for the year (units) |
Total for the year (UAH) |
|
Retail |
2020 |
89 629,34 |
9 109 283,78 |
103 764,72 |
10 150 303,70 |
|
Hospital |
2020 |
14 135,38 |
1 041 019,92 |
||
|
Retail |
2021 |
69 041,68 |
7 154 193,96 |
75 786,54 |
7 724 112,45 |
|
Hospital |
2021 |
6 744,86 |
569 918,49 |
||
|
Retail |
2022 |
45 106,16 |
7 524 869,76 |
57 618,90 |
8 426 087,28 |
|
Hospital |
2022 |
12 512,74 |
901 217,52 |
||
|
Retail |
2023 |
38 080,70 |
7 316 536,14 |
51 402,06 |
8 853 027,88 |
|
Hospital |
2023 |
13 321,36 |
1 536 491,74 |
||
|
Retail |
2024 (6 month) |
21 941,72 |
4 725 441,92 |
26 263,16 |
5 141 943,42 |
|
Hospital |
2024 (6 month) |
4 321,44 |
416 501,50 |
A noticeable decline in the total quantity of tramadol sold annually was observed from 2020 to 2024. The overall sales volume (in UAH) exhibited fluctuations, with a significant drop from 2020 to 2021, followed by minor increases in 2022 and 2023. However, a substantial decline was noted in the first half of 2024.
The growth rates in terms of quantity consistently showed a negative trend, indicating a steady decrease in the number of sold packages each year. The growth rates in terms of sales volume also demonstrated a downward trend, with slight increases noted in 2022 and 2023.
Retail sales dominate over hospital sales in both quantity and volume. A significant reduction in retail sales volume and quantity was observed from 2020 to 2024 (Fig. 4, 5).
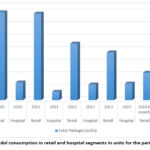 |
Figure 4: Tramadol consumption in retail and hospital segments in units for the period 2020-2024
|
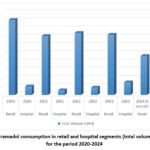 |
Figure 5: Tramadol consumption in retail and hospital segments (total volume in UAH) for the period 2020-2024
|
Hospital sales also show a decrease in quantity, though the decline is not as steep as in retail sales. The volume of hospital sales fluctuated, with a sharp drop in 2021 and a notable increase in 2023. The consistent reduction in tramadol sales may reflect changes in prescribing practices, regulatory adjustments, or shifts in market dynamics. Comparison between retail vs. hospital sales showed the dominance of retail sales and indicates that tramadol is more frequently distributed through retail channels rather than hospitals. The differing trends between retail and hospital sales could provide insights into varying demand patterns and usage contexts. Economic Impact: fluctuations in sales volume (in UAH) suggest the influence of diverse economic factors on tramadol pricing and sales. Analyzing these trends could offer a better understanding of the economic variables affecting the pharmaceutical market. This structured section maintains clarity and aligns with the style required for academic publications, providing an analytical overview based on the provided data.
At the beginning of the full-scale war, tramadol was included on the list of medicines purchased for the implementation of programs and the execution of centralized healthcare measures to provide medical care under martial law in Ukraine93, which confirms the increase in procurement volumes in the hospital sector.
It is also worth noting that tramadol is not included on the National List of Essential Medicines purchased with budgetary funds91.
Despite a significant reduction in the range of dosage forms of tramadol in Ukraine, its sales volumes indicate a stable demand for opioid analgesics, which is also associated with the war in Ukraine.
Conclusion
Critical analisis of the secondary and tertiary sources evident that tramadol has significant limitations in terms of clinical effectiveness and safety as analgesics. The findings of this review indicate that tramadol is not a preferred treatment option for chronic pain due to its limited clinical effectiveness and various safety concerns, including the risks of abuse and adverse effects. Furthermore, an examination of the Ukrainian market highlights challenges related to the accessibility, regulation, and procurement of tramadol. Despite a significant reduction in retail sales in units in 2020-2024 tramadol in Ukraine, its sales volumes and increase in the hospital segment indicate a stable demand for opioid analgesics, which is also associated with the ongoing war in Ukraine. Further research to establish clear indications, efficacy and safety of tramadol especially considering the unmet need for such pharmacological group of drugs in Ukraine.
Acknowledgment
The authors would like to express their sincere gratitude to the State Expert Center of the Ministry of Health of Ukraine and the authorities of Ternopil National Medical University for their invaluable support in preparing this paper. We also wish to thank PharmXplorer for providing the essential data needed for conducting the market research.
Funding sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
The manuscript incorporates all datasets examined throughout this research study.
Ethics Statements
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Oleksandra Oleshchuk: Conceptualization, Methodology, Writing – Original Draft, Review&Editing.
Oresta Pinyazhko: Conceptualization, Methodology, Review&Editing
Mykola Klantsa: Analysis, Writing, Visualization.
Kateryna Posokhova: Analysis, Writing.
Mariana Lukanyuk: Analysis, Writing, Visualization.
Tamara Mahanova: Analysis, Writing, Visualization.
Mariia Shanaida: Review and Editing, Supervision
Reference
- Bjørklund G, Oleshchuk O, Gontova T, Klantsa M, Lukanyuk M, Denefil O, Koshovyi O, Shanaida V,Peana M, Shanaida M. Varieties of natural analgesics in CP management: cannabinoids and other phytoconstituents. Curr Med Chem. 2024. Accessed on: Oct.01,2024. [Online]. Available:https://www.researchgate.net/publication/384660070
- Cohen SP, Vase L, Hooten WM. CP: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082-2097
- Friedman A, Nabong L. Opioids: Pharmacology, Physiology, and Clinical Implications in Pain Medicine. Phys Med Rehabil Clin N Am. 2020;31(2):289-303.
- Lassen D, Damkier P, Brøsen K. The Pharmacogenetics of Tramadol. Clin Pharmacokinet. 2015;54(8): 825-836.
- No authors listed. “Weak” opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine. Prescrire Int. 2016; 25(168): 45-50.
- KokilambigaiKS, Irina VM, Sheba Mariam KC, Adila K, Kathirvel Comprehensive overview of analytical and bioanalytical methodologies for the opioid analgesics – Tramadol and combinations. Analyt. Biochem. 2024; 692: 115579.
- Gobbi M, Moia M, Pirona L, Ceglia I, Reyes-Parada M, Scorza C, Mennini T. p-Methylthioamphetamine and 1-(m-chlorophenyl)piperazine, two non-neurotoxic 5-HT releasers in vivo, differ from neurotoxic amphetamine derivatives in their mode of action at 5-HT nerve endings in vitro. J Neurochem. 2002 Sep;82(6):1435-43.
- Leppert W. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep. 2009;61(6):978-992.
- Grond S, Sablotzki A. Clinical Pharmacology of Tramadol. Clin Pharmacokinet. 2004;43:879-923.
- World Health Organization (1996) Cancer pain relief with a guide to opioid availability, 2nd edn. Accessed on: Oct.25,2024. [Online]. Available:http://apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf.
- A Study of ULTRAM ER at Two Dose Levels in Adolescents With Pain. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT01586507?term=Tramadol&rank=1
- Tramadol and Pain Sensitization (TRAMADOL). Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT00487175?term=Tramadol&rank=3
- An Efficacy and Safety Study of Acetaminophen Plus Tramadol Hydrochloride (JNS013) in Participants With Chronic Pain. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT00736853#study-overview
- Tramadol in Penial Block Does it Improve Efficiency of Postoperative Analgesia in Circumcision. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT03260439?term=Tramadol&start=2014-01-01_2024-10-01&rank=9
- Tramadol vs.Tramadol With Paracetamol. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT03482492?term=Tramadol&start=2014-01-01_2024-10-01&rank=2
- Evaluation of the Role of Tramadol 50mg as an Analgesic During Outpatient Hysteroscopy. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT02068209?term=Tramadol&start=2014-01-01_2024-10-01&page=2&rank=20
- Evaluate Safety of Tramadol in the Management of Postoperative Pain Following Surgery. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT03395808?term=Tramadol&start=2014-01-01_2024-10-01&page=3&rank=28
- Metabolites of Tramadol in the Postoperative Surgical Patients (METRAS). Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT04004481?term=Tramadol&start=2014-01-01_2024-10-01&rank=10
- Inflammation and Postoperative Tramadol Analgesia. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT04330157?term=Tramadol&start=2014-01-01_2024-10-01&page=2&rank=19
- Evaluation of the Management of Tramadol Use Disorders. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT04550754?term=Tramadol&start=2014-01-01_2024-10-01&rank=4
- Use of Tramadol Intraligamentary Injections. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT05538052?term=Tramadol&start=2014-01-01_2024-10-01&page=2&rank=14
- The Influence of Tramadol on Platelet Function. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT05237492?term=Tramadol&start=2014-01-01_2024-10-01&rank=7
- Tramadol to Improve Its Detection in Biological Matrices in Anti-doping Controls. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT05925686?term=Tramadol&start=2014-01-01_2024-10-01&rank=8
- Tramadol and Tramadol Plus Ketamine for Shivering Prevention After Spinal Anesthesia in Lower Segment Caeserian Section. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT06134895?term=Tramadol&start=2014-01-01_2024-10-01&page=2&rank=12
- Effect of Tramadol on Postoperative Sore Throat After General Anesthesia. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT04991493?term=Tramadol&start=2014-01-01_2024-10-01&rank=3
- The Effect of Intravenous Infusion of Tramadol-ondansetron on Recovery After Caesarean Section. (TRON). Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT05879536?term=Tramadol&start=2014-01-01_2024-10-01&aggFilters=status:rec&rank=9
- Paracetamol Plus Tramadol Versus Fentanyl As Preemptive Analgesia For Enhanced Recovery After Day Case Surgeries. Clinical trials. Accessed on: Oct.01,2024. [Online]. Available:https://clinicaltrials.gov/study/NCT06561672?term=Tramadol&start=2014-01-01_2024-10-01&page=3&rank=26
- A National Clinical Guideline. Pharmacological Management of Cancer Pain in Adults. Accessed on: Oct.13,2024. [Online]. Available:https://assets.gov.ie/11598/0e15e6c055f94e9e8146d03f309f7a6b.pdf
- Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F; ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii139-vii154.
- General Palliative Care Guidelines for the Management of Pain at the End of Life in Adult Patients. Accessed on: Oct.20,2024. [Online]. Available:https://www.rqia.org.uk/rqia/files/e0/e0a81c25-acb8-4982-9970-1ed62e9f2015.pdf
- Cancer Pain Management, 2010 British Pain Society. Accessed on: Oct.20,2024. [Online]. Available:https://www.britishpainsociety.org/static/uploads/resources/files/book_cancer_pain.pdf
- SIGN–106. Control of pain in adults with cancer. Accessed on: Oct.20,2024. [Online]. Available: https://www.palliativedrugs.org/download/081114_SIGN106_quick_ref_guide.pdf
- Yomiya K. For the Clinical Use-Clinical Guidelines for Pharmacological Management of Cancer Pain 2020. Gan To Kagaku Ryoho. 2022;49(2):119-124.
- Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain – an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7(7):CD012592. Published 2017 Jul 6.
- Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5(5):CD012508. Published 2017 May 16.
- Healthcare Improvement Scotland. Sign 136: Management of Chronic Pain. 2013. Scottish Intercollegiate Guidelines Network. Accessed on: Oct.03,2024. [Online]. Available:http://www.ckp.scot.nhs.uk/Published/PathwayViewer.aspx?id=609 .
- Chronic pain: supporting safer prescribing of analgesics. Accessed on: Oct.13,2024. [Online]. Available:https://www.bma.org.uk/media/2100/analgesics-chronic-pain.pdf
- Clinical Guideline for the Evaluation and Management of Low Back Pain. Accessed on: Oct.13,2024. [Online]. Available:https://mipropiolio.wordpress.com/wp-content/uploads/2015/10/american-pain-society-clinical-guideline-for-the-evaluation-and-management-of-low-back-pain.pdf
- Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, Brown KR, Bruel BM, Bryce DA, Burks PA, Burton AW, Calodney AK, Caraway DL, Cash KA, Christo PJ, Damron KS, Datta S, Deer TR, Diwan S, Eriator I, Falco FJ, Fellows B, Geffert S, Gharibo CG, Glaser SE, Grider JS, Hameed H, Hameed M, Hansen H, Harned ME, Hayek SM, Helm S 2nd, Hirsch JA, Janata JW, Kaye AD, Kaye AM, Kloth DS, Koyyalagunta D, Lee M, Malla Y, Manchikanti KN, McManus CD, Pampati V, Parr AT, Pasupuleti R, Patel VB, Sehgal N, Silverman SM, Singh V, Smith HS, Snook LT, Solanki DR, Tracy DH, Vallejo R, Wargo BW; American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2–guidance. Pain Physician. 2012;15(3 Suppl):S67-S116
- Guideline for the non-surgical management of hip and knee osteoarthritis. Accessed on: Oct.13,2024. [Online]. Available: https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Joint%20replacement/Guideline-for-the-non-surgical-management-of-hip-and-knee-osteoarthritis.pdf
- Bruyère O, Cooper C, Pelletier JP, Maheu E, Rannou F, Branco J, Luisa Brandi M, Kanis JA, Altman RD, Hochberg MC, Martel-Pelletier J, Reginster JY. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3-S11.
- Kjaer P, Kongsted A, Hartvigsen J, Isenberg-Jørgensen A, Schiøttz-Christensen B, Søborg B, Krog C, Møller CM, Halling CMB, Lauridsen HH, Hansen IR, Nørregaard J, Jørgensen KJ, Hansen LV, Jakobsen M, Jensen MB, Melbye M, Duel P, Christensen SW, Povlsen TM. National clinical guidelines for non-surgical treatment of patients with recent onset neck pain or cervical radiculopathy. Eur Spine J. 2017;26(9):2242-2257.
- Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians; Denberg TD, Barry MJ, Boyd C, Chow RD, Fitterman N, Harris RP, Humphrey LL, Vijan S. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514-530.
- Qaseem A, McLean RM, O’Gurek D, Batur P, Lin K, Kansagara DL. Nonpharmacologic and Pharmacologic Management of Acute Pain From Non-Low Back, Musculoskeletal Injuries in Adults: A Clinical Guideline From the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748.
- Kjaer P, Kongsted A, Hartvigsen J, Isenberg-Jørgensen A, Schiøttz-Christensen B, Søborg B, Krog C, Møller CM, Halling CMB, Lauridsen HH, Hansen IR, Nørregaard J, Jørgensen KJ, Hansen LV, Jakobsen M, Jensen MB, Melbye M, Duel P, Christensen SW, Povlsen TM. National clinical guidelines for non-surgical treatment of patients with recent onset neck pain or cervical radiculopathy. Eur Spine J., 2017 Sep; 26(9):2242-2257.
- Sabha M, Hochberg MC. Non-surgical management of hip and knee osteoarthritis; comparison of ACR/AF and OARSI 2019 and VA/DoD 2020 guidelines. Osteoarthr Cartil Open, 2021 Dec;4(1): 100232. doi:10.1016/j.ocarto.2021.100232
- Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians; Denberg TD, Barry MJ, Boyd C, Chow RD, Fitterman N, Harris RP, Humphrey LL, Vijan S. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514-530.
- De Clifford-Faugère G, Nguena Nguefack HL, Godbout-Parent M, Diallo MA, Guénette L, Pagé MG, Choinière M, Beaudoin S, Boulanger A, Pinard AM, Lussier D, De Grandpré P, Deslauriers S, Lacasse A. Pain Medications Used by Persons Living With Fibromyalgia: A Comparison Between the Profile of a Quebec Sample and Clinical Practice Guidelines. Can J Pain. 2023;7(2):2252037. Published 2023 Aug 25.
- Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD003726. Published 2017 Jun 15.
- Sommer C, Welsch P, Klose P, Schaefert R, Petzke F, Häuser W. Opioide bei chronischem neuropathischem Schmerz. Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit in randomisierten, placebokontrollierten Studien über mindestens 4 Wochen [Opioids in chronic neuropathic pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration]. 2015;29(1):35-46.
- Schaefert R, Welsch P, Klose P, Sommer C, Petzke F, Häuser W. Opioide bei chronischem Arthroseschmerz. Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit in randomisierten, placebokontrollierten Studien über mindestens 4 Wochen [Opioids in chronic osteoarthritis pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration]. Schmerz. 2015;29(1):47-59.
- Welsch P, Sommer C, Schiltenwolf M, Häuser W. Opioide bei chronischen nicht-tumorbedingten Schmerzen – sind sie Nichtopioidanalgetika überlegen? Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit in randomisierten Direktvergleichen von Opioiden und Nichtopioidanalgetika über mindestens 4 Wochen [Opioids in chronic noncancer pain-are opioids superior to nonopioid analgesics? A systematic review and meta-analysis of efficacy, tolerability and safety in randomized head-to-head comparisons of opioids versus nonopioid analgesics of at least four week’s duration]. Schmerz. 2015;29(1):85-95.
- Lauche R, Klose P, Radbruch L, Welsch P, Häuser W. Opioide bei chronischen nicht-tumorbedingten Schmerzen – gibt es Unterschiede? Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit in randomisierten Direktvergleichen von Opioiden über mindestens 4 Wochen [Opioids in chronic noncancer pain-are opioids different? A systematic review and meta-analysis of efficacy, tolerability and safety in randomized head-to-head comparisons of opioids of at least four week’s duration]. Schmerz. 2015;29(1):73-84.
- Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;2013(8):CD004959. Published 2013 Aug 27.
- Whittle SL, Richards BL, Husni E, Buchbinder R. Opioid therapy for treating rheumatoid arthritis pain. Cochrane Database Syst Rev. 2011;(11):CD003113. Published 2011 Nov 9.
- Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522. Published 2006 Jul 19.
- Padhan M, Maiti R, Mohapatra D, Mishra BR. Efficacy and safety of tramadol in the treatment of opioid withdrawal: A meta-analysis of randomized controlled trials. Addict Behav. 2023 Dec;147:107815. doi: 10.1016/j.addbeh.2023.107815. doi:10.1016/j.addbeh.2023.107815
- Miroshnychenko A, Ibrahim S, Azab M, Roldan Y, Martinez JPD, Tamilselvan D, He L, Little JW, Urquhart O, Tampi M, Polk DE, Moore PA, Hersh EV, Claytor B, Carrasco-Labra A, Brignardello-Petersen R. Acute Postoperative Pain Due to Dental Extraction in the Adult Population: A Systematic Review and Network Meta-analysis. J Dent Res. 2023;102(4):391-401.
- Zhang X, Li X, Xiong Y, Wang Y, Wei J, Zeng C, Sha T, Lei G. Efficacy and Safety of Tramadol for Knee or Hip Osteoarthritis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Arthritis Care Res (Hoboken). 2023;75(1):158-165.
- Mattar OM, Abdalla AR, Shehata MSA, Ali AS, Sinokrot M, Abdelazeim BA, Taher A, Samy A, Mahmoud M, Abbas AM. Efficacy and safety of tramadol in pain relief during diagnostic outpatient hysteroscopy: systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2019;111(3):547-552.
- Wewege MA, Bagg MK, Jones MD, Ferraro MC, Cashin AG, Rizzo RR, Leake HB, Hagstrom AD, Sharma S, McLachlan AJ, Maher CG, Day R, Wand BM, O’Connell NE, Nikolakopolou A, Schabrun S, Gustin SM, McAuley JH. Comparative effectiveness and safety of analgesic medicines for adults with acute non-specific low back pain: systematic review and network meta-analysis. BMJ. 2023;380:e072962. Published 2023 Mar 22.
- Nakhaee S, Amirabadizadeh A, Brent J, Miri-Moghaddam E, Foadoddini M, Farrokhfall K, Hosseini M, Abdollahi M, Mehrpour O. Tramadol and the occurrence of seizures: a systematic review and meta-analysis. Crit Rev Toxicol. 2019;49(8):710-723.
- Huerta MÁ, Garcia MM, García-Parra B, Serrano-Afonso A, Paniagua N. Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials. Int J Mol Sci. 2023;24(16):12987. Published 2023 Aug 20.
- Rostam-Abadi Y, Gholami J, Amin-Esmaeili M, Safarcherati A, Mojtabai R, Ghadirzadeh MR, Rahimi H, Rahimi-Movaghar A. Tramadol use and public health consequences in Iran: a systematic review and meta-analysis. Addiction. 2020;115(12):2213-2242.
- Busse JW, Sadeghirad B, Oparin Y, Chen E, Goshua A, May C, Hong PJ, Agarwal A, Chang Y, Ross SA, Emary P, Florez ID, Noor ST, Yao W, Lok A, Ali SH, Craigie S, Couban R, Morgan RL, Culig K, Brar S, Akbari-Kelachayeh K, Pozdnyakov A, Shergill Y, Sivananthan L, Zihayat B, Das A, Guyatt GH. Management of Acute Pain From Non-Low Back, Musculoskeletal Injuries : A Systematic Review and Network Meta-analysis of Randomized Trials. Ann Intern Med. 2020;173(9):730-738.
- Li S, Li P, Wang R, Li H. Different interventions for preventing postoperative catheter-related bladder discomfort: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78(6):897-906.
- WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses. Geneva: World Health Organization; 2012.
- Carrasco-Labra A, Polk DE, Urquhart O, Aghaloo T, Claytor JW Jr, Dhar V, Dionne RA, Espinoza L, Gordon SM, Hersh EV, Law AS, Li BS, Schwartz PJ, Suda KJ, Turturro MA, Wright ML, Dawson T, Miroshnychenko A, Pahlke S, Pilcher L, Shirey M, Tampi M, Moore PA. Evidence-based clinical practice guideline for the pharmacologic management of acute dental pain in children: A report from the American Dental Association Science and Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania. J Am Dent Assoc. 2023;154(9):814-825.e2.
- Wiffen PJ, Cooper TE, Anderson AK, Gray AL, Grégoire MC, Ljungman G, Zernikow B. Opioids for cancer-related pain in children and adolescents. Cochrane Database Syst Rev. 2017;7(7):CD012564. Published 2017 Jul 19.
- Hadi MA, Alldred DP, Briggs M, Munyombwe T, Closs SJ. Effectiveness of pharmacist-led medication review in CP management: systematic review and meta-analysis. Clin J Pain. 2014 Nov;30(11):1006-14.
- Cochran G, Hruschak V, DeFosse B, Hohmeier KC. Prescription opioid abuse: pharmacists’ perspective and response. Integr Pharm Res Pract. 2016 Aug 25;5:65-73.
- Gan SH, Ismail R, Wan Adnan WA, Zulmi W. Impact of CYP2D6 genetic polymorphism on tramadol pharmacokinetics and pharmacodynamics. Mol Diagn Ther. 2007;11(3):171-181.
- Hertz DL, Bousman CA, McLeod HL, Monte AA, Voora D, Orlando LA, Crutchley RD, Brown B, Teeple W, Rogers S, Patel JN. Recommendations for pharmacogenetic testing in clinical practice guidelines in the US. Am J Health Syst Pharm. 2024;81(16):672-683.
- Cagnardi P, Ferraresi C, Zonca A, Pecile A, Ravasio G, Zani DD, Villa R. Clinical pharmacokinetics of tramadol and main metabolites in horses undergoing orchiectomy. Vet Q. 2014;34(3):143-151.
- Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics. 2014;24(7):374-380. doi:10.1097/FPC.0000000000000057.
- Park SH, Wackernah RC, Stimmel GL. Serotonin syndrome: is it a reason to avoid the use of tramadol with antidepressants?. J Pharm Pract. 2014;27(1):71-78.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med., 2005 Mar 17;352(11):1112-1120.
- Ryan NM, Isbister GK. Tramadol overdose causes seizures and respiratory depression but serotonin toxicity appears unlikely. Clin Toxicol (Phila). 2015 Jul; 53(6):545-550.
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992 Jan; 260(1):275-285.
- Nakhaee S, Hoyte C, Dart RC, Askari M, Lamarine RJ, Mehrpour O. A review on tramadol toxicity: mechanism of action, clinical presentation, and treatment. Forensic Toxicol. 2021;39:293-310.
- Wei J, Wood MJ, Dubreuil M, Tomasson G, LaRochelle MR, Zeng C, Lu N, Lin J, Choi HK, Lei G, Zhang Y. Association of tramadol with risk of myocardial infarction among patients with osteoarthritis. Osteoarthritis Cartilage. 2020;28(2):137-145.
- Knisely JS, Campbell ED, Dawson KS, Schnoll SH. Tramadol post-marketing surveillance in health care professionals. Drug Alcohol Depend. 2002 Sep 1;68(1):15-22.
- Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am J Med. 2018 Nov;131(11):1382.e1-1382.e6.
- Nakhaee S, Mehrpour O. Tramadol poisoning-associated mortality. J Affect Disord. 2019;255:S0165-0327(19)30454-9.
- Roussin A, Soeiro T, Fouque C, Jouanjus E, Frauger E, Fouilhé N, Mallaret M, Micallef J, Lapeyre-Mestre M; French Addictovigilance Network (FAN). Increase of high-risk tramadol use and harmful consequences in France from 2013 to 2018: Evidence from the triangulation of addictovigilance data. Br J Clin Pharmacol. 2022;88(8):3789-3802.
- Manouchehri A, Nekoukar Z, Malakian A, Zakariaei Z. Tramadol poisoning and its management and complications: a scoping review. Ann Med Surg (Lond). 2023;85(8):3982-3989. Published 2023 Jul 7.
- Babahajian A, Khomand P, Manouchehri F, Fakhimi R, Ahsan B, Amjadian M, Yousefinejad V. Seizure Prevalence and Its Related Factors in Tramadol Intoxication; a Brief Report. Arch Acad Emerg Med. 2019;7(1):e28. Published 2019 May 7.
- Shigematsu-Locatelli M, Kawano T, Koyama T, Iwata H, Nishigaki A, Aoyama B, Tateiwa H, Kitaoka N, Yokoyama M. Therapeutic experience with tramadol for opioid dependence in a patient with chronic low back pain: a case report. JA Clin Rep. 2019;5(1):68. Published 2019 Oct 30.
- S. Food and Drug Administration. Opioid Medications. Accessed on: Oct.02,2024. [Online].Available: https://www.fda.gov/drugs/information-drug-class/opioid-medications.
- State Register of Pharmaceuticals of Ukraine. Accessed on: Oct.13,2024. [Online]. Available:http://www.drlz.com.ua/ibp/ddsite.nsf/all/shlist?opendocument
- National EML List Accessed on: Oct.13,2024. [Online]. Available:https://zakon.rada.gov.ua/laws/show/333-2009-%D0%BF#n15
- The British National Formulary. Accessed on: Oct.13,2024. [Online]. Available:https://www.medicinescomplete.com/#/
- Resolution of the cabinet of ministers of Ukraine. Accessed on: Oct.13,2024. [Online]. Available:https://zakon.rada.gov.ua/laws/show/291-2022-%D0%BF#n12








