Aparna Tripathy1* , Babi Dutta2
, Babi Dutta2 , Archana Parampalli Raghavendra1
, Archana Parampalli Raghavendra1 and Sudarshan Surendran3
and Sudarshan Surendran3
1Division of Physiology, Department of Basic Medical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, India.
2Division of Biochemistry, Department of Basic Medical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, India.
3Department of Clinical Anatomy and Medical Imaging, American University of Antigua, College of Medicine, Coolidge, Antigua.
Corresponding Author E-mail:aparna.tripathy@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/3051
Abstract
Background: Cisplatin (CP) is used to treat various solid tumors but is associated with nephrotoxicity, which varies with dose and duration. Vitex Agnus castus (VAC) berries, known for their anti-inflammatory and antioxidant properties, may alleviate CP-induced renal toxicity. Objective: To investigate the gender-specific responses to cisplatin-induced nephrotoxicity and evaluate VAC extract's nephroprotective effects. Methods: Four-month-old Wistar rats (n=36) (24 male, 12 female) were used. In phase 1, gender-based differences in CP-induced nephrotoxicity were assessed. The gender group with higher nephrotoxicity was selected for phase 2 to evaluate VAC's nephroprotective properties. Animals were randomly grouped as Normal Control (6 males & 6 females), CP Control (6 males & 6 females) received CP (7 mg/kg bw) injection, VAC Control (received 165 mg/kg bw VAC for 7 days daily), and CP+VAC (CP injection followed by VAC orally for 7 days). Results: CP-treated male rats showed significantly higher plasma creatinine, urea, and BUN levels (p<0.05) than controls, while female rats showed slight increases. Male rats were chosen for phase 2, where VAC treatment post-CP injection lowered the kidney function parameters, though not significantly compared to CP controls. Histopathology revealed severe tubular damage and dilation in CP-treated kidneys compared to controls. Conclusion: Cisplatin (7 mg/kg bw) causes acute kidney injury, with male rats showing more nephrotoxicity. VAC extract reduced biochemical markers of nephrotoxicity but did not reverse CP-induced damage, suggesting potential mitigation of some CP-induced renal toxicity.
Keywords
Cisplatin; Nephrotoxicity; Vitex Agnus-castus; Wistar Rats
Download this article as:| Copy the following to cite this article: Tripathy A, Dutta B, Raghavendra A. P, Surendran S. Assessment of Nephroprotective Properties of Vitex Agnus castus Extract in Cisplatin-Treated Wistar Rats: A Pilot Study. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Tripathy A, Dutta B, Raghavendra A. P, Surendran S. Assessment of Nephroprotective Properties of Vitex Agnus castus Extract in Cisplatin-Treated Wistar Rats: A Pilot Study. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/49jXexI |
Introduction
Cisplatin (CP) is a platinum-containing drug that treats various cancers like sarcomas, lymphoma carcinomas, and germ cell tumors1. Cisplatin enters the cells via organic cationic transporters (OCT), hydrolyzes due to low ICF chloride concentration, and gets activated, which later causes havoc within the cell1,2. Activated cisplatin attaches to the DNA strands and forms covalent bonds, creating cross-links in DNA and preventing the cancer cells from dividing and growing, thus leading to the death of the cancer cell3,4. While it is an effective chemotherapy drug, its use can also adversely affect healthy cells, particularly in the kidneys, because cisplatin is eliminated via the kidneys. Kidneys have organic cationic transporters (OCT 1 & OCT 2) at the basolateral membrane of proximal convoluted tubular (PCT) cells. OCT 2 is primarily responsible for the cellular uptake of cisplatin in the kidneys5,6. Efflux of cisplatin from the PCT cells is handled by multidrug and toxin extrusion (MATE) channels, which are present on the brush border membrane of the tubular cells. It is suggested that MATE channels transport cisplatin less than OCT 2, which results in a quick entry but slow release of cisplatin in cells. Consequently, cisplatin accumulates in higher concentrations in renal tubular cells5. This accumulation manifests as an acute kidney injury or chronic kidney disease, as activated cisplatin generates reactive oxygen species (ROS) and triggers an inflammatory response. The severity of the kidney damage can vary depending on the dose and duration of cisplatin treatment7. About 30% of patients treated with cisplatin suffer from nephrotoxicity. It manifests a swift decline in the excretory mechanism of the kidney, which in turn causes an accumulation of metabolites resulting from protein metabolism in the body (Urea, creatinine, and blood urea nitrogen)8–10. Cisplatin causes acute kidney injury by activating the NF-ḵB pathway in the renal tubules11, which promotes the release of cytochrome c from mitochondria, initiating intrinsic apoptotic pathway12,13 and also enabling the activation of multiple proinflammatory mediators, thus inhibiting NF-kB may facilitate the protection of kidneys from cisplatin induced injury14. The interaction of CP with female gender-specific sex hormones and the effects of estradiol in preventing the nephroprotective effects of antioxidants in CP-induced renotoxicity have been demonstrated15. Reports also suggest that G Protein-Coupled Estrogen Receptor (ER)may have a protective role in acute kidney injury caused by cisplatin16. Despite extensive research on cisplatin’s nephrotoxicity mechanism, effective safeguards remain inconclusive; hence, there is always a need to identify adequate protection against cisplatin-induced nephrotoxicity without bargaining its anticancer potential. Herbal plants like Curcuma longa, Pulsatilla dahurica17, Mucuna pruriens18, and Ginger extract19 have been used to treat various ailments of kidneys for decades. The biotic and abiotic stress helps the plants develop resistance by producing intricate phytochemicals. These compounds can synergistically target different pathways/mechanisms to boost therapeutic effects. Compounds like curcumin20 and quercetin21 have been studied for their potential to alleviate the harmful effects of cisplatin-induced kidney damage. Curcumin neutralizes reactive oxygen species, enhances antioxidant enzymes, and reduces oxidative stress. It also suppresses inflammatory pathways and the production and activation of cytokines20. Green tea extract has been found to reduce oxidative stress and inflammation while preserving renal function22. Quercetin has also shown promise in affecting oxidative stress pathways and inhibiting inflammation, suggesting its potential to mitigate cisplatin-induced nephrotoxicity21. Along with neutralizing reactive oxygen species, it enhances antioxidant enzymes, reduces oxidative stress, and suppresses cisplatin-induced inflammation. These compounds also improve renal functions by reducing BUN, Urea, and Creatinine levels in the blood. Vitex Agnus Castus (VAC) (Sanskrit: Sinduvara; Family: Verbenaceae) is a deciduous shrub native to Mediterranean Europe and Central Asia. Traditionally, the VAC berry extract is used to treat menstrual disorders, menopausal symptoms, and acne23. It contains chemical compounds like essential fatty acids (oleic acid and linolenic acid); Iridoid glycosides (aucubin and agnoside); Essential oils (limonene, pinene, and sabinene), Flavonoids (casticin and isovitexin), Diterpenes (vitexilactone, rotundifuran), Essential fatty acids (oleic acid and linolenic acid)24,48. VAC extract has antinociceptive, anti-inflammatory, antioxidant, anticancer, and antitumor properties 48,49. VAC contains essential oils & flavonoids that remarkably cause inflammation and inflammatory pain, as seen in xylene-induced ear edema48. Isovitexin, a component of VAC extract, has also shown anti-inflammatory effects by reducing ROS generation and inhibiting MAPK pathways50. Studies have demonstrated hydroethanolic extract from fruits has antitumor and anti-proliferative activities against prostate cancer and other cancer cell lines49,51, and it may have relatively low toxicity against normal cells in the living body. Casticin, a flavonoid in VAC, was found to be a potent immunomodulatory and cytotoxic compound 52. It is hypothesized that VAC could help alleviate cisplatin-induced renal toxicity because of its anti-inflammatory and antioxidant properties. This preliminary study aimed to assess the gender-specific responses to cisplatin-induced acute nephrotoxicity and evaluate the nephroprotective effects of VAC extract against cisplatin-induced toxicity.
Materials and Methods
Chemicals and Drugs
Cisplatin: Commercially available Cisplatin injection ( Kemoplat 50mg/50 ml vial) was obtained from FRESENIUS KABI INDIA PVT LTD, Pune, Maharashtra, India. The injection volume was calculated based on the animal’s body weight, and an undiluted CP injection (7 mg/kg body weight) was administered. The cisplatin dose for inducing acute nephrotoxicity was selected based on the previous literature 53.
Vitex Agnus Castus
Vitex Agnus castus berry extract (fine, rose-pink powder with Agnuside content >0.5%) was procured from Navchetana Kendra, New Delhi, India. The extract (200mg) was dissolved in 1 ml of deionized water, and a stock solution was prepared. During the experiment, the animals were weighed daily. The volume of the extract was calculated and administered orally based on the specified dose (165 mg/kg body weight of VAC)54
Experimental Animals
Four-month-old, 36 Wistar rats (24 male and 12 female) weighing 160–250 gms were randomly selected and grouped. These animals were kept in polypropylene cages lined with sterilized husks. They were maintained under normal conditions at a 25°C–27°C temperature range, with a 12-hour light/dark cycle and continuous access to a regular rat pellet diet and drinking water. Three animals were housed in each cage to prevent overcrowding, and they were acclimatized to the laboratory environment.
Experimental Design
The experiment was conducted in two phases; the first phase involved the assessment of the gender-based difference in cisplatin induced nephrotoxicity, where 12 male and 12 female Wistar rats were recruited and randomly divided into two groups: Normal control and Cisplatin control (n=6 per group per gender). In the Normal control groups (6 males & 6 females), animals were left untreated, and animals of the Cisplatin control group (6 males & 6 females) received a single cisplatin (7mg/Kg bw) intraperitoneal injection. These animals were observed for 7 days. A nephrotoxicity assessment was done by estimating plasma Creatinine, Urea, and Blood Urea Nitrogen (BUN) using standard kits procured from Agappe Diagnostics Ltd, Mumbai, India.
The study’s second phase assessed the nephroprotective effects of Vitex Agnus castus (VAC) extract on the gender group exhibiting higher nephrotoxicity determined in the first phase. 12 male Wistar rats were recruited for the second part of the study, and they were randomly divided into the VAC control group (n=6), which received an oral dose of 165 mg/kg body weight for 7 days. The Cisplatin + VAC group (CP+VAC) (n=6) received daily oral doses of VAC extract for 7 days following the cisplatin injection.
The body weight of all the animals was recorded, blood was drawn in EDTA tubes for biochemical analysis at the end of the experimental duration, and animals were sacrificed. Biochemical analysis was done using kidney function test kits procured from Agappe Diagnostics Ltd, Mumbai, India. Kidneys were excised, weighed, and processed for hematoxylin and eosin staining. The sections were examined for morphometrical changes in the renal tubules under a light microscope using 10x magnification. The Organosomatic index (OSI) was also calculated using the body and kidney weights.
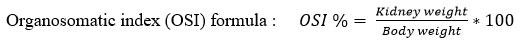
Statistical Analysis
The data was analyzed using the Statistical Package for the Social Sciences (SPSS) version 16.0, and normally distributed data are expressed as mean ± standard deviation. We used the Independent Samples Test for the first phase and One-way ANOVA with the post hoc Tukey test for the second phase. A p-value < 0.05 was considered statistically significant.
Results
Assessment of gender-specific responses to Cisplatin-induced nephrotoxicity
Cisplatin administration impaired kidney function (indicated by increased plasma concentration of creatinine, Urea, and BUN) in Wistar rats (both males and females) as compared to normal controls (shown in Graph 1, Graph 2, Graph 3). Cisplatin-treated Male rats showed significantly higher (p<0.05) plasma creatinine, urea, and BUN levels than the normal male control group rats. Female rats receiving cisplatin also showed increased creatinine, Urea, and BUN, but these changes were statistically insignificant compared to the normal female control group. When comparing cisplatin induced kidney function impairment among genders, we found higher plasma creatinine and urea levels in males, with plasma creatinine levels being statistically significantly higher only in males (p<0.05), which starkly contrasts with females who exhibited elevated levels of BUN.
The results also indicate that cisplatin administration caused a significant decrease (p<0.01) in body weight in both genders compared to the normal controls (Graph 4). Histological evaluation revealed that cisplatin administration disrupts tubular epithelial cells, causes brush border loss, induces tubule dilation, and appearance of vacuoles in both genders (Figure 1).
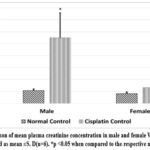 |
Graph 1: Compaison of mean plasma creatinine concentration in male and female Wistar rats. Values are represented as mean ±S. D(n=6). *p <0.05 when compared to the respective normal control.
|
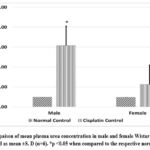 |
Graph 2: Compaison of mean plasma urea concentration in male and female Wistar rats. Values are represented as mean ±S. D (n=6). *p <0.05 when compared to the respective normal control.
|
 |
Graph 3: Compaison of mean plasma Blood Urea Nitrogen (BUN) concentration in male and female Wistar rats. Values are represented as mean ±S. D (n=6). *p <0.05 when compared to the respective normal control.
|
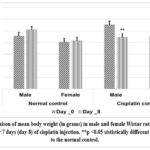 |
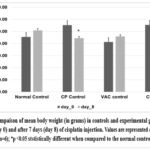
Graph 4: Compaison of mean body weight (in grams) in male and female Wistar rats on the baseline (day 0) and after 7 days (day 8) of cisplatin injection. **p <0.05 ststistically different when compared to the normal control.
|
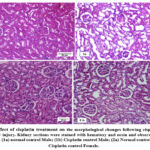 |
Figure 1: Effect of cisplatin treatment on the morphological changes following cisplatin-induced acute kindney injury.
|
Nephroprotective effect of VAC extract (165mg/kg bw) in male Wistar rats on cisplatin facilitated nephrotoxicity.
Cisplatin control and Cisplatin +VAC groups showed a lower (reduced) body weight (p<0.05) when compared to normal control, and the change in the body weight from the baseline (day 0) to day 8 was highly significant (p<0.001) (Graph 5). The Cisplatin control group had higher plasma concentrations of creatinine, urea, and BUN (p<0.05) compared to normal controls. VAC administration after cisplatin reduced the plasma concentrations of renal function indicators like creatinine, urea, and BUN compared to the Cisplatin control group. However, the reduction was not statistically significant (shown in Graph 6, Graph 7, Graph 8). On the other hand, the organosomatic index (OSI) of the kidney of the cisplatin control group (p<0.01) was higher compared to normal controls. It was also observed that the Cisplatin + VAC group (p<0.05) had significantly lower relative kidney weights than those treated with cisplatin (Graph 9).
Histopathological examination showed a well-organized and compact interstitial space, with intact epithelial lining of the proximal and distal convoluted tubules and cells with intact nuclei with eosinophilic cytoplasm in both the Normal and VAC control groups. However, the groups that received cisplatin showed disrupted interstitium and the presence of vacuoles. The proximal tubules show disrupted epithelial lining, loss of the brush border, and dilated lumen (Figure 2).
 |
Graph 5: Compaison of mean body weight (in grams) in controls and experimental groups on the baseline (day 0) and after 7 days (day 8) of cisplatin injection.
|
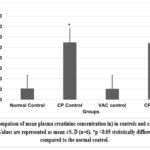 |
Graph 6: Compaison of mean plasma creatinine concentration in) in controls and experimental groups.
|
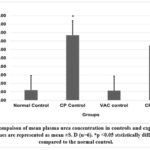 |
Graph 7: Compaison of mean plasma urea concentration in controls and experimental groups.
|
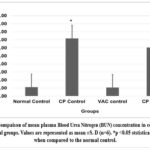 |
Graph 8: Compaison of mean plasma Blood Urea Nitrogen (BUN) concentration in controls and experimental groups.
|
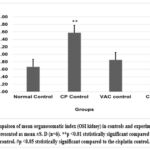 |
Graph 9: Compaison of mean organosomatic index (OSI kidney) in controls and experimental groups.
|
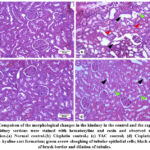 |
Figure 2: Compaison of the morphological changes in the kindney in the control and the experimental groups.
|
Discussion
The present study aimed to investigate gender-based differences in cisplatin induced nephrotoxicity in Wistar rats, and we observed that both genders of Wistar rats exhibited impaired renal function parameters like plasma creatinine, urea, and BUN, which increased after injecting cisplatin6,25,26. Females only exhibited higher BUN levels, whereas males have significantly higher plasma creatinine and urea concentrations than females. These alterations may be due to the action of cisplatin, which activates the NF-kB pathway in the renal tubules, as it facilitates numerous pathways that lead to inflammation and apoptosis, resulting in acute kidney damage11,12,14,27–29. The results also show that the administration of cisplatin caused a significant decrease in body weight in both genders. This change in body weight could be attributed to cisplatin-induced anorexia, which causes a decrease in hypothalamic ghrelin secretion, which in turn leads to reduced food intake30,31. Studies have also suggested that impaired lipid metabolism pathways, such as increased lipolysis and fatty acid oxidation along with a reduction in lipogenesis30, or dysregulated muscle protein metabolism (synthesis and degradation) caused by increased atrophic gene expression32, are probable causes for cisplatin-induced weight loss.
Kidney histology shows more cellular degeneration and cytoplasmic vacuolization in males than in females. Based on the evidence, it is clear that cisplatin has nephrotoxic effects at a dose of 7 mg/kg body weight. Additionally, it was observed that male Wistar rats were more affected than their female counterparts. These results are consistent with other studies on renal damage brought on by cisplatin and its side effects20,21,33. Our findings corroborated with earlier research, which shows that the toxicities differ depending on gender, and male rats exhibit higher toxicity to cisplatin compared to female rats 25,26. The explanation behind this observation is that the uptake of cisplatin in the renal tubular cells varies in males and females, which is because there are higher levels of mRNA and protein expression of the Organic Cation Transporter 2 (OCT2) in male kidney compared to female kidney15,34.
For the second phase of our study, we selected male Wistar rats to evaluate the potential therapeutic effects of Vitex Agnus castus fruit extract; as our study indicated, male rats were more susceptible to cisplatin-induced injury.
Cisplatin treatment resulted in significant body weight loss, possibly due to increased muscle wastage, decreased intake of food, or gastrointestinal toxicity, which is reported in previous studies30–32,35,36. When VAC is administered post-Cisplatin treatment, the body weight does not change significantly compared to the Cisplatin control group. Similarly, Plasma creatinine level is markedly increased(p<0.05), which indicates kidney damage. Treatment with VAC has decreased the plasma creatinine level, but the change is insignificant. This observation may be due to the vasodilator effect of VAC, which would have reduced the plasma creatinine levels by increasing the GFR37.
Similarly, improvement in renal clearance due to the vasodilator effect of VAC has decreased the plasma urea and BUN compared to the cisplatin control group. However, the decrease is not statistically significant. Furthermore, kidney histology shows cellular degeneration, tubular dilation, and cytoplasmic vacuolization of proximal tubules and a hyaline cast, indicating severe kidney damage in the cisplatin control group. In contrast, the CP + VAC group shows lesser tubular damage, vacuolization, and no hyaline cast. The organosomatic index (OSI) of the kidney was significantly increased in the cisplatin control group, whereas a significant decrease in OSI was observed in the Cp+ VAC group. Tubular dilation and inflammation increase the kidney’s relative weight, which indicates renal damage. Previous studies also report similar observations 38,39.
It is well-documented that cisplatin damages the kidney by increasing oxidative stress. Free radical generation and intracellular antioxidant defence become unbalanced due to oxidative stress, with the latter taking precedence. The free radicals damage the cell membrane by lipid peroxidation and protein denaturation40. Flavonoids in VAC extract have antioxidant activity, so they help in decreasing the oxidative stress caused by cisplatin. VAC extract also contains phytoestrogen, which acts via estrogen receptors (ER)24,41–43, and studies also report that ER alpha receptors are present in male rats’ kidneys44,45. So, these mechanisms of action could help protect kidneys from the deleterious effects of cisplatin. Cisplatin causes acute kidney injury through vasoconstriction by activating adenosine A1 receptors in the kidney, resulting in decreased blood flow and damage to the tubular vascular endothelium, which leads to increased vascular resistance, a reduced renal blood flow, a decreased GFR, renal tubular hypoxia, and ultimately renal damage46,47. According to reports, VAC extract has vasorelaxant properties37 that could potentially counteract the negative effects of cisplatin on the kidneys, which in turn may increase GFR and facilitate the removal of Creatinine, Urea, and BUN from the blood. The current study indicates that treatment with VAC after cisplatin has lowered the renal impairment function parameters compared to the cisplatin control group, but the reduction is not statistically significant.
Conclusion
In toto, cisplatin injection at 7mg/kg body weight leads to acute kidney injury, with male rats being more susceptible than female rats to renal damage. The presence of flavonoids and phytoestrogens in VAC extract helped to shield the kidney from damage caused by cisplatin and partially mitigated renal damage in male rats. Therefore, VAC can help in curtailing renal damage in patients undergoing cisplatin therapy and improve their overall quality of life.
Limitations of the Study
The initial findings indicate some protective effects against cisplatin-induced nephrotoxicity; however, further research is required to determine the ideal dosage and duration of VAC extract administration for maximum protection against cisplatin-induced nephrotoxicity. Studying the pharmacological profile of the extract will help identify the specific protection mechanism. Understanding these nephroprotective mechanisms will improve our knowledge of VAC’s overall efficacy and potential clinical applications.
Acknowledgement
We thank Manipal Academy of Higher Education, India, for providing the infrastructure and support required for the study.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethical Statement
This study was approved by the Institutional Animal Ethics Committee of the Manipal Academy of Higher Education (IAEC/KMC/53/2018). Experiments were conducted following CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials
Author Contributions
Aparna Tripathy, Archana Parampalli Raghavendra, Sudarshan Surendran : Study conceptualization & Study design;
Aparna Tripathy and Babi Dutta: Performed experiments & investigations.
Aparna Tripathy, Babi Dutta and Sudarshan Surendran: Statistical analysis;
Aparna Tripathy and Archana Parampalli Raghavendra : Initial draft of the manuscript;
Aparna Tripathy, Babi Dutta, Archana Parampalli Raghavendra, Sudarshan Surendran: Review, editing, and final approval of the manuscript;
Aparna Tripathy and Archana Parampalli Raghavendra : Overall project supervision.
References
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-378.
CrossRef - Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 2008;6(1):1-18.
CrossRef - Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B, Amiji MM. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017;47:71-80.
CrossRef - Mikuła-Pietrasik J, Witucka A, Pakuła M, Uruski P, Begier-Krasińska B, Niklas A, Tykarski A, Książek K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell Mol Life Sci. 2019;76(4):681-697.
CrossRef - Motohashi H, Inui KI. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 2013;15(2):581-588.
CrossRef - Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826.
CrossRef - Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol. 2021;13:303-328.
CrossRef - Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756-766.
CrossRef - Kellum JA. Acute kidney injury. Crit Care Med. 2008;36(Suppl):S141-S145.
CrossRef - Lameire N, Van Biesen W, Vanholder R. Acute kidney injury. Lancet. 2008;372(9653):1863-1865.
CrossRef - Ozkok A, Ravichandran K, Wang Q, Ljubanovic D, Edelstein CL. NF-κB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol Lett. 2016;240(1):105-113.
CrossRef - Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49-62.
CrossRef - Zhang Y, Hao J, Du Z, Li P, Hu J, Ruan M, Li S, Ma Y, Lou Q. Inhibition of hepatocyte nuclear factor 1β contributes to cisplatin nephrotoxicity via regulation of nf-κb pathway. J Cell Mol Med. 2021;25(6):2861-2871.
CrossRef - Domingo IK, Latif A, Bhavsar AP. Proinflammatory signalling PRRopels cisplatin-induced toxicity. Int J Mol Sci. 2022;23(13):7227.
CrossRef - Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, Eshraghi-Jazi F, Haghighi M, Ashrafi F. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20(1):43-47.
CrossRef - Gohar EY, Almutlaq RN, Fan C, Balkawade RS, Butt MK, Curtis LM. Does G protein-coupled estrogen receptor 1 contribute to cisplatin-induced acute kidney injury in male mice? Int J Mol Sci. 2022;23(15):8284.
CrossRef - Sohn SH, Lee H, Nam JY, Kim SH, Jung HJ, Kim Y, Shin M, Hong M, Bae H. Screening of herbal medicines for the recovery of cisplatin-induced nephrotoxicity. Environ Toxicol Pharmacol. 2009;28(2):206-212.
CrossRef - Concessao P, Bairy LK, Raghavendra AP. Protective effect of Mucuna pruriens against arsenic-induced liver and kidney dysfunction and neurobehavioral alterations in rats. Vet World. 2020;13(8):1555-1566.
CrossRef - Ali DA, Abdeen AM, Ismail MF, Mostafa MA. Histological, ultrastructural and immunohistochemical studies on the protective effect of ginger extract against cisplatin-induced nephrotoxicity in male rats. Toxicol Ind Health. 2015;31(10):869-880.
CrossRef - Ueki M, Ueno M, Morishita J, Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J Biosci Bioeng. 2013;115(5):547-551.
CrossRef - Sánchez-González PD, López-Hernández FJ, Dueñas M, Prieto M, Sánchez-López E, Thomale J, Ruiz-Ortega M, López-Novoa JM, Morales AI. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem Toxicol. 2017;107:226-236.
CrossRef - Koczurkiewicz P, Klaś K, Grabowska K, Piska K, Rogowska K, Wójcik-Pszczoła K, Podolak I, Galanty A, Michalik M, Pękala E. Saponins as chemosensitizing substances that improve effectiveness and selectivity of anticancer drug-Minireview of in vitro studies. Phytother Res. 2019;33(9):2141-2151.
CrossRef - Daniele C, Coon T, Pittler J, Ernst MH. Vitex agnus castus: A Systematic Review of Adverse Events. Drug Saf. 2005;28(4):319-332.
CrossRef - Farzaei M, Niroumand M, Heydarpour F. Pharmacological and therapeutic effects of Vitex agnus-castus L.: A review. Pharmacogn Rev. 2018;12(23):103.
CrossRef - Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Gender difference in Cisplatin-induced nephrotoxicity in a rat model: greater intensity of damage in male than female. Nephrourol Mon. 2013;5(3):818-821.
CrossRef - Pezeshki Z, Maleki M, Talebi A, Nematbakhsh M. Age and gender related renal side effects of cisplatin in animal model. Asian Pac J Cancer Prev. 2017;18(6):1703-1705.
- Ghosh S, Hayden MS. New regulators of NF-κB in inflammation. Nat Rev Immunol. 2008;8(11):837-848.
CrossRef - Banoth B, Chatterjee B, Vijayaragavan B, Prasad MVR, Roy P, Basak S. Stimulus-selective crosstalk via the NF-κB signaling system reinforces innate immune response to alleviate gut infection. Elife. 2015;4.
CrossRef - Peter B, Michael K. Nuclear Factor-κB – A Pivotal Transcription Factor in Chronic Inflammatory Diseases. New England Journal of Medicine. 1997;336(15):1066-1071.
CrossRef - Garcia JM, Scherer T, Chen JA, Guillory B, Nassif A, Papusha V, Smiechowska J, Asnicar M, Buettner C, Smith RG. Inhibition of cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice. Endocrinology. 2013;154(9):3118-3129.
CrossRef - Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, Aoyama T, Sakurada T, Takabayashi H, Hattori T. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151(8):3773-3782.
CrossRef - Sakai H, Sagara A, Arakawa K, Sugiyama R, Hirosaki A, Takase K, Jo A, Sato K, Chiba Y, Yamazaki M, Matoba M, Narita M. Mechanisms of cisplatin-induced muscle atrophy. Toxicol Appl Pharmacol. 2014;278(2):190-199.
CrossRef - Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel). 2010;2(11):2490-2518.
CrossRef - Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui KI. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70(12):1823-1831.
CrossRef - Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005;212(2-3):116-123.
CrossRef - Shahid F, Farooqui Z, Khan F. Cisplatin-induced gastrointestinal toxicity: An update on possible mechanisms and on available gastroprotective strategies. Eur J Pharmacol. 2018;827:49-57.
CrossRef - Thaçi S, Krasniqi B, Dërmaku-Sopjani M, Rifati-Nixha A, Abazi S, Sopjani M. Vasorelaxant effects of the Vitex Agnus-castus extract. Evid Based Complement Alternat Med. 2022;2022:7708781.
CrossRef - Saad SY, Najjar TA, Al-Sohaibani MO. The effect of rebamipide on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2000;42(1):81-86.
CrossRef - Jana S, Mitra P, Dutta A, Khatun A, Kumar Das T, Pradhan S, Kumar Nandi D, Roy S. Early diagnostic biomarkers for acute kidney injury using cisplatin-induced nephrotoxicity in rat model. Curr Res Toxicol. 2023;5(100135):100135.
CrossRef - Ramadan LA, El-Habit OH, Arafa H, Sayed-Ahmed MM. Effect of cremophor-el on cisplatin-induced organ toxicity in normal rat. J Egyptian Nat Cancer Inst. 2001;13(2):139-145.
- Jarry H, Spengler B, Porzel A, Schmidt J, Wuttke W, Christoffel V. Evidence for Estrogen Receptor β-Selective Activity of Vitex agnus-castus and Isolated Flavones. Planta Med. 2003;69(10):945-947.
CrossRef - Zahid H, Rizwani GH, Ishaqe S. Phytopharmacological Review on Vitex agnus-castus: A Potential Medicinal Plant. Chin Herb Med. 2016;8(1):24-29.
CrossRef - Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014;2014:615917.
CrossRef - Mäkelä S, Strauss L, Kuiper G, Valve E, Salmi S, Santti R, Gustafsson JÅ. Differential expression of estrogen receptors α and β in adult rat accessory sex glands and lower urinary tract. Mol Cell Endocrinol. 2000;164(1-2):109-116.
CrossRef - Ma HY, Chen S, Du Y. Estrogen and estrogen receptors in kidney diseases. Ren Fail. 2021;43(1):619-642.
CrossRef - Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303-1353.
CrossRef - Motwani SS, Kaur SS, Kitchlu A. Cisplatin nephrotoxicity: Novel insights into mechanisms and preventative strategies. Semin Nephrol. 2022;42(6):151341.
CrossRef - Ramezani M, Amin GH, Jalili E. Antinociceptive and anti-inflammatory effects of hydroalcohol extract of Vitex agnus castus fruit. World Acad Sci Eng Technol. 2010;64:619-621.
- Aslantürk ÖS, Çelik A. Antioxidant activity and anticancer effect of Vitex agnus-castus L.(Verbenaceae) seed extracts on MCF-7 breast cancer cells. Caryologia. 2013;66:257-267.
CrossRef - Lv H, Yu Z, Zheng Y, Wang L, Qin X, Cheng G, Ci X. Isovitexin exerts anti-inflammatory and antioxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int J Biol Sci. 2016;12(1):72-86.
CrossRef - Sahib HB, Al-Zubaidy AA, Hussein SM, Dahham SS, Al-Suede FS, Shah AM. The Anti-proliferative Activity of Vitex agnus-castus Leaves Methanol Extract against Breast and Prostate Cancer Cell Line. International Journal of Medical Research and Review. 2015;3(2):159-166.
- Mesaik MA, Azizuddin, Murad S, Khan KM, Tareen RB, Ahmed A, Atta-ur-Rahman, Choudhary MI. Isolation and immunomodulatory properties of a flavonoid, casticin from Vitex agnus-castus: CASTICIN FROMVITEX AGNUS-CASTUS. Phytother Res. 2009;23(11):1516-1520.
CrossRef - Nematbakhsh M, Ashrafi F, Nasri H, Talebi A, Pezeshki Z, Eshraghi F, Haghighi M. A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. J Res Med Sci. 2013;18(5):370-373.
- Ibrahim AY, El-Newary SA, Youness ER, Ibrahim AM, El Kashak WA. Protective and therapeutic effect of Vitex agnus-castus against prostate cancer in rat. Journal of Applied Pharmaceutical Science. 2017;7(12):133-143.







