Anu Altangerel1,2 , Chimedragchaa Chimedtseren2*
, Chimedragchaa Chimedtseren2* , Myadagbadam Urtnasan2
, Myadagbadam Urtnasan2 , Dejidmaa Buyantogtokh2
, Dejidmaa Buyantogtokh2 , Dagvatseren Begzsuren2
, Dagvatseren Begzsuren2 and Zulgerel Dandii3
and Zulgerel Dandii3
1International School of Mongolian Medicine, Mongolian National University of Medical Science, Ulaanbaatar, Mongolia.
2Institute of Traditional Medicine and Technology, Ulaanbaatar, Mongolia.
3School of Medicine, Mongolian National University of Medical Science, Ulaanbaatar, Mongolia.
Corresponding Author E-mail: ch.chimedragchaa@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/3048
Abstract
The study aimed to evaluate the antihypertensive effect of Marchin-13 traditional prescription (M13T) in the dexamethasone-induced model of hypertension. Quercetin, a biologically active substance contained in M13T, was identified by HPLC analysis. Forty rats were randomly assigned to five different groups. The experimental groups induced hypertension models by dexamethasone (20 mcg/kg/day). Blood pressure was assessed in the tail with a Neurobotic Systole 1.2 instrument on days 5, 8, 13, and 15. The serum levels of renin, ANGII, aldosterone, and AT1R were measured in all groups by ELISA. Our research determined that the quercetin content in M13T is 0.255 mg/g. In the dexamethasone-induced hypertension model, M13T significantly reduced SBP, DBP, and MAP on days 8, 13, and 15 (p < 0.01). Moreover, the renin, ANGII, AT1R, and aldosterone levels were significantly reduced in the captopril and M13T groups compared to the DEX group (p < 0.01). As a result of our study, M13T has an antihypertensive effect by reducing serum renin, angiotensin II, AT1R, and aldosterone levels in hypertensive rats induced by dexamethasone.
Keywords
Antihypertensive; Angiotensin; Aldosterone; Experimental; Renin
Download this article as:| Copy the following to cite this article: Altangerel A, Chimedtseren C, Urtnasan M, Buyantogtokh D, Begzsuren D, Dandii Z. Antihypertensive Effects of Quercetin-Rich Marchin-13 in a Dexamethasone-Induced Rat Model. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Altangerel A, Chimedtseren C, Urtnasan M, Buyantogtokh D, Begzsuren D, Dandii Z. Antihypertensive Effects of Quercetin-Rich Marchin-13 in a Dexamethasone-Induced Rat Model. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4iOuX6X |
Introduction
Hypertension raises the risk of stroke, kidney damage, and death, and 54% of adults worldwide have hypertension1. Key factors contributing to the pathogenesis of hypertension include the sympathetic nervous system, oxidative stress, the renin-angiotensin-aldosterone system (RAAS), and various hormonal activities2. In our country, a total of 1,398 cases of hypertension were recorded among patients who utilized traditional medicine services from 2021 to 2023. Of these cases, 65% of people with hypertension in 2021, 80% in 2022, and 90% in 2023 are hospitalized in Ulaanbaatar, and the number is expected to increase every year3.
Mongolian traditional medicine, which has a history of 5,000 years, has used many herbal prescriptions to lower blood pressure. One of them is the Marchin-13 traditional prescription (M13T). The Jigmeddanzanjamts “Uzegsdiin Baysgalan” scripture prescribes using M13T to treat “wind and blood ascending disorder,” corresponding to arterial hypertension4. M13T is a traditional prescription composed of 13 medicinal plants5. Research has indicated that Sambucus manshurica L, Carthamus tinctorius L, T. asiaticus L., Gardenia jasminoides J, Quercus robur L, Inula helenium L, Rubia cordifolia L, and Arnebia guttata Bunge exhibit anti-hypertensive, antioxidant, anti-inflammatory, antidepressant, antidiabetic, and cardioprotective effects6-13. Zingiber officinale Roscoe acts as a calcium channel blocker and regulates vascular impairment and NO synthesis14. Terminalia bellirica Roxb has anti-hypertensive effects and acts as a calcium antagonist15, while Terminalia chebula Retz exhibits anti-hypertensive and ACE-inhibitor activities16. In Traditional Chinese Medicine formula, many herbal drugs inhibit the RAS. For instance, Qingxuanjiangya decoction and Qingxin capsule can inhibit the activity of the RAS. Also, Xiaxi traditional medicine has been demonstrated to decrease AT1R17. The utilization of herbal medicines within the healthcare sector of developing countries is on the rise18,19.
To prove the traditional indications for M13T, widely used in conventional medicine to reduce blood pressure, it is necessary to study the antihypertensive effect of several hypertension models in experimental animals. As a result of our previous experimental study, M13T increased nitric oxide and inhibited the angiotensin-converting enzyme in the hypertension model induced by L-NAME.
In the current study, we aimed to investigate the antihypertensive effect of M13T in the dexamethasone-induced hypertension model in rats. Dexamethasone (DEX), which is a type of synthetic glucocorticoid, is commonly utilized to induce hypertension in experimental animal models20. Several factors contribute to dexamethasone-induced hypertension, including changes in plasma volume, activation of the RAAS, increased sympathetic activity, and changes in vasodepressor systems21.
To scientifically validate traditional medicine’s effects, it is essential to associate them with its primary active ingredients. Therefore, our research focuses on determining the quantitative content of the biologically active substance quercetin in M13T using HPLC and investigating its anti-hypertensive effects.
Materials and Methods
Materials
The M13T (serial No: 951017) was obtained from the traditional pharmacy at ITMT in Mongolia. Quercetin standards were obtained from Sigma Aldrich in the USA. Dexamethasone (4 mg/1 mL) was acquired from A72330 – KRKA d.d. Novo Mesto (Budapest, Hungary). Rat renin, angiotensin II, AT1R, and aldosterone ELISA kits were obtained from Shanghai MLBio Co. in China. Captopril (batch No: 10722A), manufactured by Sopharma in Bulgaria, was obtained from a pharmacy located in Ulaanbaatar.
Laboratory animal
The experiment used 50 relatively healthy Wistar rats weighing 220 – 260 g, acquired from the Vivar of ITMT in Mongolia. During the experiment, animals had continuous access to food and water while the temperature was maintained at 20 ± 2°C with a regulated 12-hour light/dark cycle.
Ethics Statement
We received permission to begin the study from the Biomedical Ethics Committee of MNUMS, Mongolia (Approval No. 2021/3-06).
HPLC identification of Quercetin
Reference solution: Weigh a specific amount of quercetin and dissolve it in methanol to create a solution with a concentration of 100 μg per mL.
Sample solution: Weigh 1 gram of M13T, add 25 ml of methanol, extract using ultrasonic for 60 min, and filtrate.
Chromatographic system: The liquid chromatography setup includes a UV wavelength of 360 nm, with an Alltima C18 column (5 μm, 4.6×250 mm). The flow rate is maintained at 0.7 mL/min, and the mobile phase consists of methanol and 0.1% phosphoric acid in a 65:35 ratio22.
Procedure: Precisely inject 20 μL of the CRS and sample solutions into the chromatography apparatus and calculate the content accordingly.
Pharmacology methods
A dexamethasone-induced hypertensive model in rats was developed following the method described by Dignesh Patel23 and Sharon Leng24. Forty rats were randomly assigned to five different groups:
Group I received a vehicle for 15 days.
Group II received dexamethasone injections (20 mcg/kg/day, subcutaneous injection (s.c.)) from day 5 to 15
Group III received dexamethasone injections (20 mcg/kg/day, s.c.) from day 5 to 15 and captopril (40 mg/kg/day25, given orally) for 15 days.
Group IV received dexamethasone injections (20 mcg/kg/day, s.c.) from day 5 to 15 and M13T (90 mg/kg/day, given orally) for 15 days.
Group V received dexamethasone injections (20 mcg/kg/day, s.c.) from day 5 to 15 and M13T (180 mg/kg/day, given orally) for 15 days; each group contains eight rats.
During the experiment, rats were given daily doses of captopril (40 mg/kg20) and M13T (90 mg/kg or 180 mg/kg) via gavage between 15:00 to 16:00 for 15 consecutive days (days 1–15). Additionally, from days 5 to 15, dexamethasone was administered by s.c. Injection daily between 1500 and 1600 hours. Blood pressure was assessed in the tail with a Neurobotic Systole 1.2 instrument on days 5, 8, 13, and 15.
Blood pressure measurement method
SBP and DBP were measured between 9:00 and 11:00 am using a tail-cuff device for rats (Neurobotics, Model 124498, Moscow, Zelenograd)24. Rats were warmed on a Phlogiston platform to 28–32°C for 10–15 minutes before measuring their blood pressure to enhance circulation26. Main Arterial Pressure = DP + 1/3(SP – DP)27
Enzyme-linked immune sorbent assay (ELISA)
Blood samples were gathered from the experimental animals after 15 days. The serum levels of renin, ANGII, aldosterone, and AT1R were measured using assay kits sourced from Shanghai MLBIO Biotechnology, China. These samples were analyzed using a ChroMate-4300 microplate reader from the USA.
Statistical analysis
Statistical analysis involved calculating the mean ± SD for each group’s recorded values. GraphPad Prism 8 software was used for the analyses. The Kruskal-Wallis test, a nonparametric approach, was applied for statistical comparisons, with significance defined at p<0.05.
Results
Chemical study
The retention time of the standard substance of quercetin was 6.47 minutes, and the results of preparing a standard solution with a concentration of 12.5-100 μg/ml are shown in the picture. The equation curve for the standard substance of quercetin was Y = 17987x – 53917, R2=0.9961 (Figure 1).
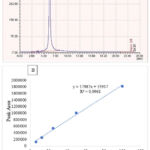 |
Figure 1: HPLC of the standard substance quercetin (A) and calibration curve (B) |
The retention time of M13T quercetin is 6.31 minutes, which agrees with the standard substance above (Figure 2). The retention time of the quercetin content of M13T compared to the standard substance was consistent, and the quercetin content was 0.255±0.007 mg/g.
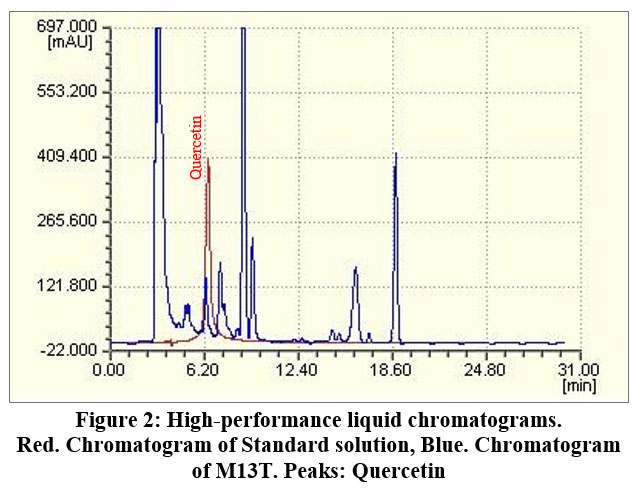 |
Figure 2: High-performance liquid chromatograms. |
Results of pharmacological research
Effect of M13T on body weight in hypertensive rats
The table shows a significant reduction in body weight across all groups induced with DEX. Additionally, the treated groups exhibited no significant differences from the DEX group. However, by day 15, body weight in the DEX group was significantly decreased compared to the control group (p < 0.01).
Table 1: Body weight of experimental rats
|
Time points |
Control (g) |
DEX (g) |
Captopril 40mg/kg (g) |
M13T 90mg/kg (g) |
M13T 180mg/kg (g) |
|
0 day |
217.1 ± 12.9 |
226.1 ± 16.0 |
220.2 ± 10.3 |
229.6 ± 15.5 |
239.8 ± 18.2 |
|
5th day |
220.3 ± 12.0 |
221.8 ± 17.3 |
219.8 ± 10.8 |
221.5 ± 17.8 |
234.2 ± 14.9 |
|
8th day |
228.8 ± 10.1 |
216.8 ± 24.6 |
218.3 ± 11.6 |
220.5 ± 23.3 |
231.6 ± 16.5 |
|
13th day |
234.3 ± 20.1 |
213.5 ± 10.8 |
216.1 ± 18.5 |
219.5 ± 24.8 |
230.7 ± 18.3 |
|
15th day |
244.0 ± 21.5 |
208.8 ± 17.4# |
217.7 ± 9.9 |
216.6 ± 22.1 |
224.0 ± 19.2 |
The data are shown as mean ± SD, n = 8. Statistical significance follows: #p < 0.01 for the DEX-group vs. control group
An antihypertensive effect of M13T on DEX-induced hypertension
We recorded the results and measured the SBP and DBP in the rat’s tail.
Table 2: The effect of the M13T on systolic blood pressure
|
Time point |
Control (mm Hg) |
DEX (mm Hg) |
Captopril 40mg/kg (mm Hg) |
M13T 90mg/kg (mm Hg) |
M13T 180mg/kg (mm Hg) |
|
5th day |
87.8 ± 18.3 |
124.2 ± 9.36# |
124.0 ± 12.2 |
89.6 ± 20.4 |
107.0 ± 23.3 |
|
8th day |
95.6 ± 16.1 |
148.6 ± 5.55## |
126.8 ± 16.9* |
106.4 ± 17.1* |
113.4 ± 18.2* |
|
13th day |
105.6 ± 24.9 |
160.6 ± 14.4## |
106.0 ± 7.58** |
110.2 ± 25.7** |
106.0 ± 17.2** |
|
15th day |
114.4 ± 18.7 |
180.2 ± 22.2## |
125.0 ± 22.1** |
120.2 ± 8.70** |
111.0 ± 6.78** |
The data are shown as mean ± SD, n = 8. Statistical significance follows #p<0.05, ##p<0.01 for the DEX group vs. control group; *p<0.05, **p<0.01 for Captopril (40 mg/kg), M13T (90 mg/kg), and (M13T 180 mg/kg) vs. DEX group.
Table 2 indicates that in the DEX group, the SBP of rats increased by 1.5 times on days 5, 8, 13, and 15 than the control group (p<0.01). In contrast, the captopril group in SBP decreased by 14.6% (p<0.05) starting on day 8, 33.9% on day 13, and 30.6% on day 15 (p<0.01). M13T group receiving a dosage of 90 mg/kg experienced a reduction in SBP of 28.3% (p < 0.05) on day 8, 31.3% on day 13, and 33.2% on day 15 (p < 0.01). The group receiving 180 mg/kg of M13T showed a SBP decrease of 23.6% on day 8, 33.9% on day 13, and 38.3% on day 15 (p < 0.01).
Table 3: The effect of M13T on diastolic blood pressure
|
Time point |
Control (mm Hg) |
DEX (mm Hg) |
Captopril 40mg/kg (mm Hg) |
M13T 90mg/kg (mm Hg) |
M13T 180mg/kg (mm Hg) |
|
5th day |
64.2 ± 12.3 |
102.0 ± 17.0# |
99.4 ± 20.8 |
64.4 ± 17.4* |
68.8 ± 20.0* |
|
8th day |
73.6 ± 19.0 |
120.0 ± 14.9## |
94.6 ± 25.7# |
74.6 ± 22.4** |
90.4 ± 12.7** |
|
13th day |
74.4 ± 18.2 |
120.6 ± 13.8## |
75.6 ± 4.61** |
86.0 ± 21.8** |
83.0 ± 19.5** |
|
15th day |
73.6 ± 14.4 |
141.0 ± 21.2## |
100.0 ± 16.2** |
92.0 ± 19.6** |
75.6 ± 16.5** |
The data are presented as mean ± SD, n = 8. Statistical significance follows: #p<0.05, ##p<0.01 for the DEX group vs. control group; *p<0.05, **p<0.01 for Captopril 40 mg/kg, M13T 90 mg/kg, and M13T 180 mg/kg vs. DEX-group.
Table 3 indicates that DBP was significantly augmented in the DEX group than in the control group (p<0.01). The Captopril and M13T-treated groups showed a considerably lower SBP than the DEX group (p<0.01).
Table 4: The effect of M13T on mean arterial pressure
|
Time point |
Control (mm Hg) |
DEX (mm Hg) |
Captopril 40mg/kg (mm Hg) |
M13T 90mg/kg (mm Hg) |
M13T 180mg/kg (mm Hg) |
|
5th day |
72.0 ± 13.7 |
109.4 ± 14.0# |
107.6 ± 17.6 |
72.8 ± 17.7* |
81.5 ± 20.7* |
|
8th day |
80.9 ± 17.8 |
129.5 ± 11.5## |
105.3 ± 22.1* |
85.2 ± 20.3** |
98.0 ± 14.4* |
|
13th day |
84.8 ± 20.0 |
133.9 ± 13.8## |
85.7 ± 3.45** |
94.0 ± 22.5** |
90.6 ± 18.6** |
|
15th day |
87.2 ± 14.8 |
154.0 ± 20.8## |
108.3 ± 17.6** |
101.4 ± 12.5** |
87.4 ± 12.8** |
The data are presented as mean ± SD, n = 8. Statistical significance follows: #p < 0.05, ##p < 0.01 for the DEX group vs. control group; *p < 0.05, **p < 0.01 for Captopril 40 mg/kg, M13T 90 mg/kg, and M13T 180 mg/kg vs. DEX group.
In the DEX-induced arterial hypertension, the MAP of rats in the DEX group was significantly higher than that of the control group, approximately 1.5 times greater on days 5, 8, 13, and 15 (p < 0.01). In the captopril group, the MAP decreased by 35.9% on day 13 and 29.6% on day 15 (p < 0.01). Furthermore, in the M13T 90 mg/kg group, the MAP was reduced by 33.5% on day 5, 34.2% on day 8, and 29.7% on days 13 and 15 (p < 0.01). In the M13T 180 mg/kg group, the MAP decreased by 25.6% on day 5, 24.3% on day 8, 32.3% on day 13, and 43.2% on day 15 (p < 0.01) (Table 4).
Effect of M13T on renin, angiotensin II, AT1R, and aldosterone in hypertensive rats
We measured the serum levels of renin, angiotensin II, angiotensin 1 receptor (AT1R), and aldosterone by ELISA in DEX-induced hypertension.
 |
Figure 3: Serum Renin and Angiotensin-II levels in rats with DEX-induced hypertension |
The data are shown as mean ± SD, n = 8. Statistical significance follows #p<0.05 for the DEX-group vs. control group; **p<0.01 for Captopril 40 mg/kg, M13T 90mg/kg, and M13T 180mg/kg vs. DEX-group.
The serum renin level in the DEX group indicated a 37.1% increase compared to the control group (p < 0.05). Additionally, angiotensin II levels in the DEX group significantly increased by 39.7% (p < 0.05). This indicates a pathological profile that is associated with an increase in blood pressure. In contrast, serum renin levels decreased by 36.4% in the captopril group and 46.6% in the M13T 90 mg/kg, 180 mg/kg groups, with a significant effect (p < 0.05). Likewise, serum angiotensin II levels were reduced by 46.4% in the captopril group, 47.2% in the M13T 90 mg/kg group, and 51.0% in the M13T 180 mg/kg group showing significant results (p < 0.05).
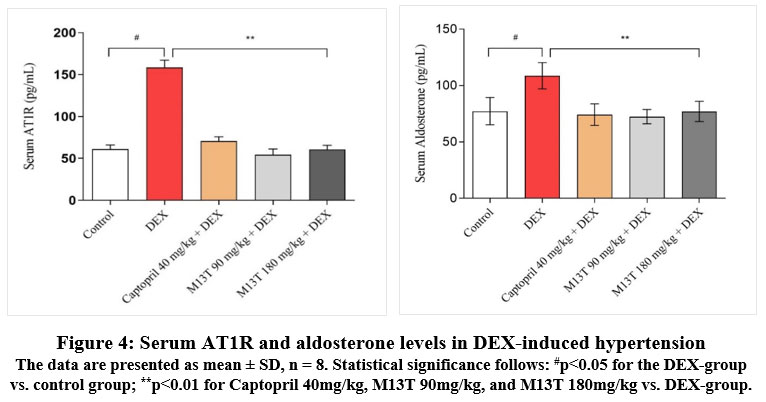 |
Figure 4: Serum AT1R and aldosterone levels in DEX-induced hypertension |
The data are presented as mean ± SD, n = 8. Statistical significance follows: #p<0.05 for the DEX-group vs. control group; **p<0.01 for Captopril 40mg/kg, M13T 90mg/kg, and M13T 180mg/kg vs. DEX-group.
As illustrated in the figure above, the serum AT1R level in the DEX group reflected a 61.5% increase relative to the control group’s (p < 0.01). Furthermore, the aldosterone level in the DEX group rose significantly by 28.9%, as opposed to the control group’s (p < 0.05). These findings suggest a pathological profile associated with increased blood pressure. In contrast, serum AT1R levels decreased significantly in the captopril group, showing a reduction of 55.6%, and in the M13T 90 mg/kg group, which experienced a 65.8% decrease. The M13T 180 mg/kg group also saw a 51.0% reduction, with all changes statistically significant (p < 0.01). Similarly, serum aldosterone levels were reduced by 31.7% in the captopril group, by 33.4% in the M13T 90 mg/kg group, and by 29.1% in the M13T 180 mg/kg group, all showing significant results (p < 0.01).
Discussion
This is the second study to examine the antihypertensive effects of M13T, and this time, we examined the antihypertensive effect in a dexamethasone-induced hypertension model. Following the methodology of Dignesh Patel and Sharon Leng, we evaluated M13T on blood pressure, serum renin, angiotensin-II, AT1R, and aldosterone levels.
Dexamethasone, a commonly used synthetic glucocorticoid, is known to elevate arterial hypertension in experimental animal models and humans20. Several studies have indicated that dexamethasone interacts with nitric oxide (NO) produced by the vascular endothelium critical vasodilator. This interaction disrupts the NO-redox balance and decreases NO bioavailability, which may affect the onset of hypertension28-30. Therefore, dexamethasone causes vasoconstriction by reducing NO levels31. Another possible explanation is that dexamethasone increases angiotensin-converting enzyme (ACE) levels32. Our previous study revealed that in L-NAME-induced hypertension, M13T (90 mg/kg) effectively restored suppressed NO production and lowered blood pressure by promoting vasodilation. Additionally, M13T (90 mg/kg) was identified to decrease angiotensin-converting enzyme activity5.
In the present study, serum renin, angiotensin II, AT1R, and aldosterone levels were significantly elevated in the context of DEX-induced hypertension. This finding is consistent with Hu Bi Si’s (2022) study33. In DEX-induced hypertension, renin activates ACE, resulting in the conversion of angiotensinogen into angiotensin-I and II, which subsequently increases aldosterone release. This results in elevated blood pressure through peripheral vasoconstriction and enhanced sodium reabsorption34. Aldosterone is a mineralocorticoid hormone important for controlling sodium and potassium levels in the kidneys, regulating blood pressure, and maintaining electrolyte balance35,36. In DEX-induced hypertension, the M13T (90 and 180 mg/kg) group significantly decreased serum renin, angiotensin II, AT1R, and aldosterone levels. By reducing the release of renin, AT1R, angiotensin-II, and aldosterone, M13T decreases sodium reabsorption in the renal pelvis, increases urine output, and lowers blood pressure.
The raw materials contained in M13T contain biologically active substances such as gallic acid, hydroxysafflower yellow A45, gingerol38, alantolactone, kaempferol, quercetin, hyperoside, isoquercetin, and rutin39,46. This antihypertensive effect may be ascribed to the biologically active compound quercetin in M13T. The primary components of M13T include Carthamus tinctorius L, Sambucus manshurica L, Quercus robur L, Zingiber officinale Roscoe, Pyrola incarnata, Terminalia bellirica Roxb, and Inula helenium L. These plants are rich in flavonol-O-glycosides, such as hyperoside and isoquercetin, and flavonoids, including kaempferol and quercetin37-46. Quercetin was identified in the M13T sample using the HPLC method, which recorded a retention time of 6.31 minutes. Our research determined that the quercetin content in M13T is 0.255 mg/g. Recent research has determined that quercetin has various effects, including anti-inflammatory, anti-hypertensive, hypoglycemic, neuroprotective, anti-cancer, anti-aging, and immune-enhancing properties47-49. Pereira’s study discovered that quercetin enhances vascular remodeling and reduces endothelial oxidative stress, decreasing SBP50. Similarly, Mackraj’s research found that quercetin improves urine output and lowers blood pressure by promoting sodium excretion in hypertension driven by the renin-angiotensin-aldosterone system (RAAS)51. Quercetin possesses considerable therapeutic potential in cardiovascular diseases, demonstrated through its antioxidant effects, myocardial fibrosis reduction, vascular endothelium protection, anti-arrhythmic and anti-heart failure properties, and ability to decrease blood pressure52.
A limitation of the study is that HPLC determined the biologically active substance quercetin and only one model of hypertension was used. Our future research should focus on isolating the biologically active compounds in Marchin-13 traditional prescription, conducting detailed chemical analyses using GC-MS, and investigating its cardioprotective effects through pharmacological studies.
Conclusion
More chemical and pharmacological research is needed on M13T, a formulation used in traditional Mongolian medicine for hypertension. This study found that M13T exerts antihypertensive effects by reducing serum levels of renin, angiotensin II, AT1 receptor (AT1R), and aldosterone. These effects are linked to the biologically active compounds present in M13T, specifically quercetin and gallic acid. To further understand the efficacy and safety of M13T, we will conduct a detailed chemical analysis to identify its components and concentrations. Additionally, an in-depth pharmacological investigation will assess its mechanisms of action, potential therapeutic benefits, and any side effects. This research aims to scientifically validate the traditional usage of M13T and contribute to the growing body of knowledge regarding herbal medicines in hypertension treatment.
Acknowledgment
We thank the Mongolian Institute of Traditional Medicine and Technology Research Center and the International School of Mongolian Medicine at MNUMS for supporting this study.
Funding Source
Foundation of Science and Technology, Mongolia, Project/Award number: Shut/Z-2017/05
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability Statement– This statement does not apply to this article.
Ethics Statement– This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement– This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration– This research does not involve any clinical trials
Author Contributions
( Anu Altangerel): Methodology, Data Collection, Analysis, Writing.
(Chimedragchaa Chimedtseren): Supervision, Writing, and Final approval.
(Zulgerel Dandii): Conceptualization, Supervised the study.
(Myadagbadam Urtnasan): Performed chemical research experiments.
(Dejidmaa B): Advised on pharmacological studies.
(Dagvatseren B): Supervision, Project Administration.
References
- Kario, K., Okura, A. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens Res. 2024;47:1099–1102.
CrossRef - David G. Harrison, Thomas M. Pathophysiology of Hypertension. Circulation Research, 2021;128(7):847-863
CrossRef - Li, Li, Chimedragchaa, Ch. Trends and Outcomes of Traditional Medicine Treatments for Arterial Hypertension and Rheumatic Diseases in Mongolia (2021-2023). Salud, Ciencia y Tecnología – Serie de Conferencias. 2024;3:985
CrossRef - Jigmeddanzanjamts, “Uzegsdiin baysgalan” Traditional Medical Source Book. China: “Inner Mongolian medical treasurers” printing house; 1999:162-163
- Altangerel A, Chimedtseren Ch. The Antihypertensive Effect of Marchin-13 Tang on L-NAME-induced Hypertension in Rats. Biomed Pharmacol J. 2024;17(2):1203-1212
CrossRef - Bunbupha S., Pakdeechote P. Carthamus Tinctorius L extract attenuates cardiac remodeling in L-NAME-induced hypertensive rats by inhibiting the NADPH oxidase-mediated TGF-β1 and MMP-9 pathway. Annals of Anatomy – Anatomischer Anzeiger.2019;222:120–128.
CrossRef - Emmanuel N W., Jing L., Elijah M M. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae). Journal of Ethnopharmacology. 2022; 292:115102.
CrossRef - Rohan S P. Phytochemistry, Pharmacological Activities and Intellectual Property Landscape of Gardenia jasminoidesEllis: a Review. Pharmacognosy Journal. 2015;7(5):254-265.
CrossRef - Mohammad Abu B N., Md. Abdul M. Rubia cordifolia-phytochemical and Pharmacological evaluation of indigenous medicinal plant: A review. International Journal of Physiology, Nutrition and Physical Education. 2018;3(1):766-771.
- Witkowska-Banaszczak E. The genus Trollius-review of pharmacological and chemical research. Phytother Res. 2015;29(4):475-500.
CrossRef - Ștefănescu R, Ciurea CN. Quercus robur Older Bark-A Source of Polyphenolic Extracts with Biological Activities. Applied Sciences. 2022; 12(22):11738.
CrossRef - Buza V., Matei M.C., Ștefănuț L.C. Inula helenium: A literature review on ethnomedical uses, bioactive compounds, and pharmacological activities. Lucrări Ştiinţifice Seria Medicină Veterinară. 2020; 63(1):53-59.
- Kanika D., Gurpreet K. Species of Arnebia Genus Found in the Western Himalayas: Arnebia euchroma (Royle ex Benth.), Arnebia benthamii (Wall. Ex G Don) Johnston, Arnebia guttata Bunge. Immunity Boosting Medicinal Plants of the Western Himalayas.2023; 77–105
CrossRef - Li, C., Li, J. Vasculoprotective effects of ginger (Zingiber officinale Roscoe) and underlying molecular mechanisms. Food & Function. 2021;12(5):1897–1913.
CrossRef - Khan A.-U., Gilani A H. Pharmacodynamic evaluation of Terminalia bellerica for its antihypertensive effect. Journal of Food and Drug Analysis. 2008;16(3):6-14
CrossRef - Sornwatana T., Bangphoomi K. Chebulin: Terminalia chebul Retz. A fruit-derived peptide with angiotensin-I-converting enzyme inhibitory activity. Biotechnol Appl Biochem.2015; 62(6):746-53.
CrossRef - Xiong X, Yang X. Chinese herbal formulas for treating hypertension in traditional Chinese medicine: perspective of modern science. Hypertension Research. 2013;36(7):570-9.
CrossRef - World Health Organization. Traditional medicine strategy: 2014-2023. Hong Kong SAR, China. 2013;21-24
- Rafieian K. M. Medicinal plants and the human needs. Journal of Herb Med Pharmacology. 2012;1(1):1-2.
- Goodwin, J.E., Geller, D.S. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012; 27: 1059–1066.
CrossRef - Ong SL, Zhang Y, Whitworth JA. Mechanisms of dexamethasone-induced hypertension. Current Hypertension Reviews. 2009;15(1):61-74.
CrossRef - Yao Y, Mi W, Cao G. The absorption characteristics of nonvolatile components in a water extraction from Amomi fructus as determined by in situ single-pass intestinal perfusion and high-performance liquid chromatography. Frontiers in Pharmacology. 2020; 5(11):711.
CrossRef - Patel, D., Patil, R. Antihypertensive activity of Beta vulgaris on dexamethasone induced hypertension in rats. Pharmaceutical and Biological Evaluations, 2017; 4(1):37-46.
CrossRef - Ong, S. L. H., Zhang, Y. Hemodynamics of dexamethasone-induced hypertension in the rat. Hypertension Research. 2009; 32(10):889-894.
CrossRef - Ugusman, A., Md Fadze, N. Piper sarmentosum attenuates dexamethasone-induced hypertension by stimulating endothelial nitric oxide synthase. Res. Pharm. 2020; 24:1-9.
CrossRef - Belemnaba L., Nitiéma M. Preclinical Evaluation of the Antihypertensive Effect of an Aqueous Extract of Anogeissus leiocarpa (DC) Guill et Perr. Bark of Trunk in L-NAME-Induced Hypertensive Rat. J Exp Pharmacol. 2021;13:739-754
CrossRef - DeMers D, Wachs D. Physiology, mean arterial pressure. 2019.
- Ong S.L.H., Vickers J.J. Role of xanthine oxidase in dexamethasone-induced hypertension in rats. Clin Exp Pharmacol Physiol. 2007;34:517–519.
CrossRef - Zhang Y., Croft K.D.The antioxidant tempol prevents and partially reverses dexamethasone-induced hypertension in the rat. Am J Hypertens. 2004; 17:260–265.
CrossRef - Rojas A., Figueroa H. Oxidative stress at the vascular wall: Mechanistic and pharmacological aspects. Arch Med Res. 2006; 37:436–448.
CrossRef - Wallwork C.J., Parks D.A. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res. 2003; 66:30–37.
CrossRef - Barreto-Chaves M.L.M., Heimann A. Stimulatory effect of dexamethasone on angiotensin-converting enzyme in neonatal rat cardiac myocytes. Braz J Med Biol Res. 2000; 33:661–664.
CrossRef - Hu Bi Si Ha La Tu., Khaliunaa T. Results of the Study for the Antihypertensive Effect in the Panzeria Alaschanica Kupr Plant. Cent Asian J Med Sci. 2022; 8(2):102-11
CrossRef - Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. Journal of managed care pharmacy. 2007;13(8):9-20.
CrossRef - Rozansky DJ. The role of aldosterone in renal sodium transport. Paper presented at Seminars in nephrology. 2006.
CrossRef - Scott JH., Menouar MA, Dunn RJ. Physiology, aldosterone. 2017.
- Ghasemzadeh, A., Jaafar, H.Z.E. Changes in antioxidant and antibacterial activities as well as phytochemical constituents associated with ginger storage and polyphenol oxidase activity. BMC Complementary Alternative Medicines. 2016;16(1):25–32.
CrossRef - Styawan, A.A., Susidarti, R.A. Review on ginger (Zingiber officinale Roscoe): phytochemical composition, biological activities, and authentication analysis. Food Research; 2022; 6(4):443 – 454.
CrossRef - Monica N., Sigrid E. The Analysis of Flavonoids from Inula helenium L. Flowers and Leaves. Acta Medica Marisiensis, 2011; 57(4).
- Gavasheli NM, Moniava II. Flavonoids from P. incarnata. Khimiya Prirodnykh Soedinenii. 1974; 10:95-96.
CrossRef - Marcin O., Anna P, Anna P J. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Brazilian Journal of Pharmacognosy. 2018; 28(2):179–191.
CrossRef - Sobeh M., Mahmoud MF. Chemical composition, antioxidant and hepatoprotective activities of methanol extracts from leaves of Terminalia bellirica and Terminalia sericea (Combretaceae). Peer J. 2019; 7:6322
CrossRef - Myadagbadam U., Purevsuren S. Standardization study of khurtsiin deed-6 traditional medicine. Pharmacognosy Journal. 2022; 14(3):610-621.
CrossRef - Burlacu, E., Nisca, A. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests, 2020; 11(9):904.
CrossRef - Fadhil MA., Kadhim EJ. Phytochemical investigation of some phenolic compounds in Carthamus tinctorius that grown naturally in Iraq. Systematic Reviews in Pharmacy. 2020;11(11):1869-1874.
- Ligaa U., Davaasuren B, Ninjil N. Medicinal plants of Mongolia are not used in Western and Eastern medicine. JKC printing; Ulaanbaatar. 2006; 91-92.
- Reyes F M, Carrasco P C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019; 20(13):3177.
CrossRef - Li Y., Cao R. Research progress on structural modification and biological activity of quercetin. Chinese Tradit Herb Drugs. 2023;54(5):1636–53.
- Shen P, Lin W. Potential implications of quercetin in autoimmune diseases. Front Immunol. 2021; 12:689044.
CrossRef - Pereira SC., Parente JM. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis. 2018; 270:146–53.
CrossRef - Mackraj I, Govender T, Ramesar S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J Cardiovasc Pharmacol. 2008;51(3):239–45.
CrossRef - Zhang W, Zheng Y. Research progress of quercetin in cardiovascular disease. Frontiers in Cardiovascular Medicine. 2023; 16(10):1203713.
CrossRef
Abbreviations
ANGII – Angiotensin II; M13T – Marchin-13 traditional prescription; RAAS – Renin-angiotensin-aldosterone system; NO – Nitric oxide; ACE – Angiotensin converter enzyme; AT1R – Angiotensin 1 receptor; ITMT – Institute of Traditional Medicine and Technology; DEX – Dexamethasone; SBP – Systolic blood pressure; DBP – Daistolic blood pressure; MAP – Mean arterial pressure







