Manuscript accepted on :29-11-2024
Published online on: 26-12-2024
Plagiarism Check: Yes
Reviewed by: Dr. Zena Sideeq Tawfeek
Second Review by: Dr. Akhtar Ali
Final Approval by: Dr. Prabhishek Singh
Prashanth Kumar Katta 1* , Mallikarjun Telsang 2
, Mallikarjun Telsang 2 and Pradeepkumar Narayanappa shiroorkar 2
and Pradeepkumar Narayanappa shiroorkar 2
1Conservative dentistry and Endodontics, College of Dentistry, King Faisal University, Al Ahsa, Kingdom of Saudi Araibia
2College of Medicine, King Faisal University, Kingdom of Saudi Araibia
Corresponding Author E-mail: drprashanthkumar@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/3041
Abstract
Objective: Globally, resistance bacteria have emerged as a result of the careless prescription of antimicrobial agents. This study's goal was to find out how frequently dentists in Saudi Arabia prescribed antibiotics. Methods: Dentists in Saudi Arabia were sent a one-page questionnaire. Participants in the questionnaire study totaled 607 dentists. Descriptive statistics and independence tests using chi-squares were used to analyze the data. Results: Most practitioner prescribed antibiotics for 3 days. In those who have never had any serious medical allergies (44.5%) chose amoxicillin associated with Clavulanic acid. A very widely used antibiotic with no history of allergies was amoxicillin 500mg. in case of patients with allergies, the most commonly prescribed antibiotic was Erythromycin 500mg (61.5%). Also, 50.3% dentists prescribe antibiotic in case of irreversible pulpitis. 97% dentists prescribes in patients diagnosed with acute apical abscess and systemic involvement, 65.3% dentists prescribed in case of apical periodontitis that is chronic with sinus tract. Conclusion: As the results suggest, most dentists chose the right antibiotic to use in orofacial infections. However, many dentists continue to indiscriminately administer antibiotics. Antibiotic resistance can be greatly exacerbated by prescribing antibiotics for non-infected individuals or, in certain situations, for small infections.
Keywords
Allergy; Acute Rheumatic Fever; Duration; Endodontic Surgeries; Resistance
Download this article as:| Copy the following to cite this article: Katta P. K, Telsang M, shiroorkar P. N. Antibiotics in endodontics: A survey using questionnaires to determine the frequency of antibiotics prescription by dentists in Saudi Arabia. Biomed Pharmacol J 2024;17(4). |
| Copy the following to cite this URL: Katta P. K, Telsang M, shiroorkar P. N. Antibiotics in endodontics: A survey using questionnaires to determine the frequency of antibiotics prescription by dentists in Saudi Arabia. Biomed Pharmacol J 2024;17(4). Available from: https://bit.ly/4iTsZ5d |
Introduction
Antibiotics are prescribed both prophylactically and to treat severe infections of the oral cavity in endodontics 1, 2. Importantly, a number of investigations3, 4, 5, have revealed a wide range of viewpoints among oral healthcare physicians about the appropriate use of antimicrobial agents. Antibiotic-resistant microbes have become much more prevalent as a result of this variance and the prescription of antibiotics for undiagnosed illnesses such as necrosis of the pulp or symptomatic permanent pulpitis. Therefore, it is imperative that dentists continue to prescribe antibiotics in a way that is suitable. It concerns the well-being of patients and health for everyone. The majority of infections of the dental pulp are contained within the tooth and are effectively treated with drainage, extraction, or well-established local surgical procedures avoiding the use of systemic or localized antibiotics. However, as a supplement to regional therapy, antibiotics may be recommended when there exists proof of widespread dissemination and the substantial, fast, and extensive dissemination of the infection6, 7.
Pulpitis and apical periodontitis are endodontic diseases that are caused by a polymicrobial mix of gram-negative, gram-positive, obligate anaerobic bacteria and facultative anaerobes 1. Nevertheless, despite the fact that numerous bacterial species were first discovered to be vulnerable to various antibiotic kinds, there has been a steady and progressive rise of resistant strains. An international problem is the indiscriminate use of antibiotics and the increase of strains of bacteria that are resistant to them. Regarding the microbiota of the oral cavity and the administration of antibiotics to treat infections of the mouth and teeth, this issue is equally significant 2, 3.
It is crucial that dentists act ethically because they recommended about 10% of the antibiotics used in elementary treatment 1. The most recent recommendations for prescribing practices linked to endodontic infections were released by the European Society of Endodontology (ESE) in 2018 in an effort to prevent antibiotic misuse. Antibiotics known as beta-lactams can trigger allergies 4, 5. Clindamycin (600 mg loading dose followed by 300 mg every 6 hours), clarithromycin (500 mg loading dose followed by 250 mg every 12 hours), or azithromycin (loading dose of 500 mg followed by 250 mg once a day) are substitutes if a true penicillin allergy is confirmed2-4. According to reports, amoxicillin is typically used to treat endodontic infections, but those who are allergic to penicillin should use clindamycin or erythromycin. Antibiotics are frequently prescribed by dentists worldwide for unwarranted illnesses such as pulpitis 6, 7.
In terms of microbiological considerations, doctors tailor their recommendations based on the potential bacteria that the infection may be caused by, keeping in mind that the majority originate from the mouth cavity, which is polymicrobic in nature 2, 3. In dental operations when there is a risk of endocarditis or other specific disorders, antibiotics are also recommended as a preventative strategy 8, 9.
The emergence of Clostridium difficile infection is a specific worry while using oral antibiotics. In 2011, C. difficile caused over half a million infections and was linked to about 29,000 fatalities 10.Of the antibiotics used to treat endodontic infections, cephalosporins, amoxicillin, and clindamycin are frequently linked to C. difficile infections, while metronidazole and macrolides have not been associated frequently 11.
Objective
This study’s goal was to find out how frequently dentists in Saudi Arabia prescribed antibiotics. Dentists in Saudi Arabia were sent a one-page questionnaire. Participants in the questionnaire study totaled 607 dentists. Descriptive statistics and independence tests using chi-squares were used to analyze the data.
Materials and Methods
A questionnaire was given to dental practitioners in Saudi Arabia. The study duration was for 4 months, including the collection of the data, inputting the data and statistical analysis. Based on previously released surveys, a questionnaire was created with additional criteria related to antibiotic prophylaxis. A total of 607 practitioners in Saudi Arabia answered the questionnaire adequately 12, 13. The survey consisted of eight questions with personal information, divided into two sections: the first section (2 questions) was a record of individual data such as gender and age, and the subsequent part (nine questions) included questions pertaining to understanding of prescribing antibiotics in infections of the teeth and prophylaxis [study design].
The Questionnaire and Data Collection
This study used a modified version of a questionnaire produced by the investigators as part of a survey of opinions and understanding about antibiotic use in endodontic infections 13, 14. The study’s questionnaire included inquiries on participants’ familiarity with the use of prophylactic and prescription antibiotics for endodontic infections. Nine questions in total, divided into two areas, asked about personal information, including gender and age. The second section (seven questions) asked about understanding of the use of antibiotics for prophylaxis and endodontic infections. Prior to the actual survey, a pilot study was conducted to test and assess the created tool. The pilot study included eighteen dentists. Some minor remarks provided by pilot participants were taken into account when creating the questionnaire’s final form.
Participants were made aware that this survey was confidential and anonymous and that no personal data, even the IP of the computer, was gathered. To eliminate any chance of identifying a study participant, data were gathered and evaluated collectively. To prevent having to respond more than once, in addition to cookies and captcha, the IP address of the device was monitored if the survey had previously been completed from that device.
Statistical analysis
An Excel file was used to compile the data (Microsoft Corp., Redmond, WA, USA). IBM SPSS Statistics 22 was used to perform the statistical analysis (IBM SPSS). The Fisher’s Freeman-Halton test and the chi-square test were employed for statistical analysis. The threshold for significant differences was 5% (p 0.05).
Results
Out of 607 respondents, male participants (n=288) contributed to 47.5%, and females (n=318) contributed to 52.5%. The participants’ average age was 46.4 years.
Prescription and Duration of Antibiotics
Most practitioners prescribed antibiotics for 3 days. There were no substantial variations between the participants in regard to their age and gender (p > 0.05). Most of the responders (44.5%) chose amoxicillin combined with Clavulanic acid in patients with no history of medical allergies or amoxicillin alone (36.4%). Clindamycin 300 mg and azithromycin 500 mg as the first-choice antibiotic by 8.9 and 6.3% respectively. Metronidazole 500mg by 3.8% dentists. The most frequently antibiotic prescribed with no history of allergies was amoxicillin 500mg. Metronidazole exhibits great action against the anaerobes nonetheless does not work against the aerobes, and hence must be used in concert with other medicines (antimicrobial combination) for treatment of infections in the mouth.
61.5% of dentists prescribe erythromycin 500mg in patients with a history of allergy to penicillin. 25.6% prescribed metronidazole 500mg, 7.5% prescribed Azithromycin 500mg and 5.3% Clindamycin 500mg.
Study design shows the The proportion of participants who prescribed antibiotics for different pulpal and periapical diagnosis (fig.1). For patients with Acute apical abscess having systemic involvement (body temperature >38°, uneasiness, lymphadenopathy 97%, localized fluctuant swellings, Acute apical abscess without systemic involvement 53%, Chronic apical periodontitis with sinus tract 65%, Irreversible pulpitis 50.3%, Chronic apical periodontitis and no sinus tract 15%, Apical acute periodontitis 13%. 91.7% prescribed for 3 number of days and 8.1% prescribed for 5 days.
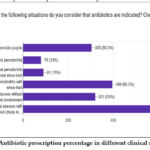 |
Figure 1: Antibiotic prescription percentage in different clinical situations |
Systemic situations for which antibiotics are indicated for prophylaxis included those prone to bacterial endocarditis or endocarditis in the past and acute rheumatic fever, 82.5% and 79.7%, respectively. Myocardial infarction 76.1%, congenital heart disease 53.9%, heart transplant 48.9%, Prosthetic heart valve 38.1%, unstable angina pectoris and leukemia 20.9% and 20.4%, respectively. Other organ transplants 15.7% (fig.2).
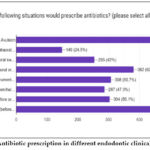 |
Figure 2: Antibiotic prescription in different endodontic clinical scenarios |
Avulsion and endodontic surgeries (before or after) 78.9%, Incision & Drainage of a edematous oral swelling + external edema present 62.9%, Post-op pain following root canal preparation or obturation 50.7%, Perforation repair (before or after) 50.1%, Retreatment of gutta percha/silver point 47.3%, I & D of a diffuse intraoral edema, no external edema 42%, Incision & Drainage of a localized intraoral edema, no external edema 24.5% (fig. 3).
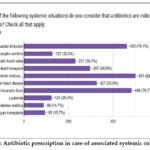 |
Figure 3: Antibiotic prescription in case of associated systemic conditions |
Recommendation for usage of antibiotics at your clinic/hospital/college: 85.2% have guidelines for antibiotic usage; remaining 14.8% did not have guideline for antibiotic usage.
Prescribing Antibiotics is of no use after 2-3 days. 86.5% change antibiotics, 6.3% add a second antibiotic, and 7.7% extend the duration of the current antibiotic.
Study design: Survey to gauge understanding and comprehension of prescribing patterns
1. Gender
Male
Female
2. Age bracket
25-35
36-45
46 and above
Which is the antibiotic that is most commonly prescribed with no history of allergies?
Amoxicillin 500mg
Amoxilillin + clavulanic acid 625 mg
Clindamycin 300mg
Azithromycin 500 mg
Metronidazole 500mg
For how many number of days antibiotics would be prescribed?
3 days
5 days
7 days
Which antibiotic is prescribed in patients with a history of allergy to penicillin?
Erythromycin 500mg
Clindamycin 500mg
Azithromycin 500mg
Metronidazole 500mg
In which cases do you believe antibiotics are appropriate? Check everything that applies:
Irreversible pulpitis
Apical acute periodontitis
Chronic apical periodontitis without sinus tract
Chronic apical periodontitis with sinus tract
Acute apical abscess without systemic involvement
Acute apical abscess with systemic involvement (body temperature >38°, malaise, localized fluctuant swellings, lymphadenopathy)
How many days?
3 days
5 days
7 days
Which one of the aforementioned systemic conditions do you think warrants the use of antibiotics as a preventative measure? Verify everything that pertains.
Myocardial infarction
Unstable angina pectoris
Prosthetic heart valve
Heart transplant
History/risk of bacterial endocarditis
Congenital heart disease
Acute rheumatic fever
Leukemia
Diabetus mellitus
Other organ transplants
Which of these scenarios would an antibiotic prescription be given for? (please select all that are applicable)
Avulsion
I & D of a localized intraoral edema, without external edema
I & D of a diffuse intraoral edema, no external edema
I & D of a diffuse intraoral oral edema + external edema present
Pain following cleaning and shaping or obturating
Retreatment of gutta percha/silver point
Repair of Perforation (post-op or prior to)
Surgeries for Endodontic reasons (post-op or prior to)
If your prescribed antibiotic is not effective after 2 or 3 days, what would you do?
Change antibiotics
Add a second antibiotic
Extend the duration of the current antibiotic
Do you have policies on the use of antibiotics at your office, hospital, or college?
Yes ▢ No ▢
| Age | |
| Male | 288 |
| Female | 318 |
Discussion
In the past, The instrument used for the survey was effective in gathering relevant data on the endodontics practice. The study’s large, 607-person sampling population participated. The majority of this target audience were endodontists in practice. A survey’s overall response rate can be regarded as an acceptable rate of return. The survey’s questions were created to gather a range of data regarding the types of antibiotics prescribed and the endodontists’ prescribing practices, as established by age, sex, number of days the drug was prescribed, and conditions for which the drug was prescribed. In terms of antibiotic therapy, the infection needs to be systemic or persistent (i.e., high body temperature, edema, enlarged lymph nodes, lockjaw, or restlessness in a patient who is apparently healthy) to warrant the use of antibiotics. Additionally, a patient with a damaged immune system or a sick patient is more likely to require antibiotics 14, 15, 16. The survey’s list of antibiotics identifies those that Saudi Arabian dentists most frequently recommend for the treatment of orofacial infections.
To summarize the percentages of responses on the usage of antibiotics and the circumstances in which respondents believed the antibiotic(s) usage happens to be suitable, frequency distributions were created. Considering the data’s non-normal distribution, the test used to investigate was chi-square tests for testing the statistical significance of these frequency distributions, while other tests like U-Mann-Whitney tests were used to analyze numerical data. The likelihood-ratio of chi-square was employed to analyze the power sample, and the threshold for statistical significance was established at 5% (P 0.05). Endodontic infections often start quickly and last for a brief period of time, 3 to 7 days or less, especially if the treatment of the source is done or removed. In this survey, antibiotic prescriptions were written for a mean range of 5.8 days, with a range of 4-9 consecutive days. When there is adequate proof that host defenses of the patient have successfully fought off the bacteria, appropriate dosage and the duration of an antibiotic are sufficient. The medicine should be stopped when the infection is healing or has healed 17, 18.
Unnecessarily prescribing antibiotics to patients with pulpitis that is irreversible (50.3%), pulps that are necrotic without systemic complications (97%), and also acute apical abscess with sinus tracts (65.3%) was another alarming finding in the survey. The typical course of treatment in these situations is endodontic therapy without the need for surgery and without antibiotics 19. With 53% of the responders prescribing antibiotics, the incidence for the prescription of antibiotics for acute apical abscess without any systemic complications cases was significantly more. In particular clinical circumstances, the choice of whether or not to administer an antibiotic should be supported by evidence 20.
Although it is widely acknowledged that clindamycin is the best option for treating individuals who are allergic to penicillin, just a tiny fraction of our respondents (25.6%) chose to follow this advice because a sizable portion preferred to recommend erythromycin (61.5%). 21, 22 The latter is a macrolide with action that is similar to penicillin’s spectrum, whereas clindamycin has a broader spectrum but a narrower specificity and is more effective against oral infections. In cases of penicillin allergy, 7.6% of responders used azithromycin. It is an erythromycin derivative that is semi-synthetic with more antibacterial activity and higher tissue penetration than the latter.
44.5% of those who responded to our survey recommended a combinationn of Amoxicillin and Clavulanic Acid as the main drug for infections of the tooth23. Active components of this combination drug are amoxycillin and clavulanic acid. With the addition of beta-lactamase coverage, its spectrum is the same as that of amoxicillin. On the other hand, 36.4% of the practitioners opted to administer only amoxicillin as the drug of choice.
The majority of the dentists recommended a three-day treatment for most of the infections, which is the commonly acknowledged recommendation for oro-facial infections 24, 25. The results are consistent with other research in which at least a three-day regimen was recommended 6, 26. Infections of endodontic origin often start quickly and last for no more than two to seven days 28, 29, especially if the infectious aetiology is treated. It’s also noteworthy that 86.5% of the dentists chose to change antibiotics if it wasn’t working. It has been observed that prolonged or inadequate use of medications can contribute to the development of resistant bacteria 30, 31.
Patients’ demands, referring doctors’ expectations, or the fact that a patient cannot visit a dentist because of a vacation or non-working days should not have any bearing on the choice to prescribe antibiotics 32. In order to meet the demands of both the patients and the doctor who is referring the case, it was previously demonstrated that most of the endodontists sensed they were forced to recommend antibiotics in case of all endodontic problems 33, 34. These are the justifications for improper antibiotic use.
Consequently, use antibiotics only as supportive therapy where there are signs of entire bodily manifestations (fever, discomfort, staphylococcus infection or streptococcus infection, and/or swollen lymph nodes) after appropriate endodontic preparation of the root canal and pus lancing if there is edema. 35, 36. Additionally, preventative medication should be given to patients who are immunodeficient or prone to predisposed circumstances similar to a history of endocarditis. It is significant to highlight that there is no evidence of a therapeutic advantage from administering antibiotics in the absence of the aforementioned factors 37, 38.
The selection of the antibiotic drug and dosage regimen is normally determined empirically in situations when there is a therapeutic indication.
Despite the fact that penicillin and amoxicillin are the two antibiotics that are most frequently administered, their pattern of side effects is such that it includes everything from gastrointestinal problems to severe anaphylactic allergic reactions. 39
Endodontic infections should be treated with a dose of 600 milligrams as an initial higher dose, followed by 300 milligrams every 6 hours. In children, however, the dosage ought to be modified to 10-30 mg for every kg (dose per body weight), split into 4 equal doses of measurement. No dentist has prescribed a loading dose, and the dosage prescribed is also more than the recommended dosage. 40, 41
Most of the respondents have the guidelines for prescribing antibiotics (85.2%) (fig. 4). Lack of expertise, patient satisfaction, and associated societal variables are some of the causes of dentists’ incorrect antibiotic prescribing practices 42, 43.
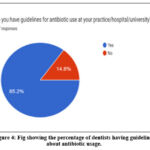 |
Figure 4: Fig showing the percentage of dentists having guidelines about antibiotic usage. |
The process of inflammation that causes endodontic discomfort is typically linked to microbial discomfort, but it can also be caused by physical or chemically induced irritation of the canal. By enhancing the permeability of vessels in these areas, these mediators of inflammation cause nociceptors to sprout, trigger nociceptors that terminate in the pulp, periodontal ligament, or periradicular bone, and cause edema and elevated tissue pressure. However, it’s unclear if antibacterial agents can successfully lessen the pain that these inflammatory illnesses cause. Antibiotics are typically provided in conjunction with regional therapy to enhance host defense systems in cases when immune systems do not seem to be able to restrict the dissemination of microbial components 44.
Conclusion
As previously said, dentists and other professionals need to weigh the risks and benefits of antibiotics and speak with their patients to decide whether they are appropriate in a certain situation. The results of this study confirm the importance of clinical expertise and training in line with current international standards on the proper indications for administering antibiotics. In order to ensure the efficiency of antibiotics in treating and preventing infections as well as combating antimicrobial resistance, dental education should continue to place greater focus on prescribing antibiotics in certain situations, even though the results showed optimal competency.
The study suggests that increasing dentists’ knowledge of antibiotics, especially with regard to their practical use, and prioritizing universal healthcare in the fight against antibiotic resistance are crucial.
Acknowledgment
The author thank the Deanship of Scientific Research, King Faisal University, college of dentistry, kingdom of Saudi Arabia, for supporting this project electronic supplementary material.
Funding Sources
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT1,898].
Conflicts of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Statement
This study protocol was reviewed and approved by Deanship of scientific research , King Faisal University, Kingdom f Saudi Arabia, approval number FU-REC-2023-MAR-ETHICS722
Informed Consent Statement
The author(s) received no financial support for the research, authorship, and/or publication of this article
Clinical Trial Registration
This research does not involve any clinical trials
Author’s contribution
Study conception and design data collection: Dr. Prashanth Kumar Katta
Analysis and interpretation of results: Dr. Mallikarjun Telsang , Dr Pradeep Kumar Narayanappa Shiroorkar, Dr. Prashanth Kumar Katta
Draft manuscript: Dr. Mallikarjun Telsang , Dr Pradeep Kumar Narayanappa Shiroorkar, Dr. Prashanth Kumar Katta
References
- Solomonov M, Batashvili G, Shuster A, Slutzky, H., Moshonov, J., Buchkovskii, O., Lvovsky, A., Azizi, H., Levin, A., Ben Itzhak, J., & Shemesh, A.. Systemic antibiotics in endodontics-International questionnaire study: Choice of types, dosage, loading and duration. Aust Endod J. 2023;49 Suppl 1:58-63.
CrossRef - Shemesh A, Batashvili G, Shuster A, Slutzky, H., Moshonov, J., Buchkovskii, O., Lvovsky, A., Azizi, H., Levin, A., Itzhak, J. B., & Solomonov, M.. International questionnaire study on systemic antibiotics in endodontics. Part 1. Prescribing practices for endodontic diagnoses and clinical scenarios. Clin Oral Investig. 2022;26(3):2921-2926.
CrossRef - Sović J, Šegović S, Pavelić B, Bago I, Šutej I, Tomašić I. Patterns of Antibiotic Prescription in Endodontic Therapy in the Republic of Croatia. Antibiotics (Basel). 2024;13(7):645.
CrossRef - Rutherford SJ, Glenny AM, Roberts G, Hooper L, Worthington HV. Antibiotic prophylaxis for preventing bacterial endocarditis following dental procedures. Cochrane Database Syst Rev. 2022;5(5):CD003813.
CrossRef - Manciocchi E, Xhajanka E, D’Addazio G, Tafuri, G., Santilli, M., Rexhepi, I., Caputi, S., & Sinjari, B. Antibiotic prescribing patterns among dentists in Italy and Albania: A comparative questionnaire analysis. Heliyon. 2024;10(13):e33575.
CrossRef - Jenny A., Nagar P., Raghunath V.N., Lakhotia R.K.F.N., Ninawe N. Prescription pattern of antibiotics among general dental practitioners in Karnataka—a cross-sectional survey. Dental Journal of Advance Studies. 2022;10:9–14.
CrossRef - Contaldo M., D’Ambrosio F., Ferraro G.A., Di Stasio D., Di Palo M.P., Serpico R., Simeone M. Antibiotics in dentistry: A narrative review of the evidence beyond the myth. Int. J. Environ. Res. Public Health. 2023;20:6025.
CrossRef - D’Ambrosio F., Di Spirito F., Amato A., Caggiano M., Lo Giudice R., Martina S. Attitudes towards antibiotic prescription and antimicrobial resistance awareness among Italian dentists: What are the milestones? Healthcare. 2022;10:1585.
CrossRef - Mustafa L., Islami H., Sutej I. Administration of systemic antibiotics for dental treatment in Kosovo major dental clinics: A national survey. Eur. J. Dent. 2022;16:430–436.
CrossRef - Šutej I., Lepur D., Bašić K., Šimunović L., Peroš K. Changes in medication prescribing due to COVID-19 in dental practice in Croatia—National study. Antibiotics. 2023;12:111.
CrossRef - Contaldo M., D’Ambrosio F., Ferraro G.A., Di Stasio D., Di Palo M.P., Serpico R., Simeone M. Antibiotics in dentistry: A narrative review of the evidence beyond the myth. Int. J. Environ. Res. Public Health. 2023;20:6025.
CrossRef - D’Ambrosio F., Di Spirito F., Amato A., Caggiano M., Lo Giudice R., Martina S. Attitudes towards antibiotic prescription and antimicrobial resistance awareness among Italian dentists: What are the milestones? Healthcare. 2022;10:1585.
CrossRef - Mustafa L., Islami H., Sutej I. Administration of systemic antibiotics for dental treatment in Kosovo major dental clinics: A national survey. Eur. J. Dent. 2022;16:430–436.
CrossRef - Galić M, Miletić I, Poklepović Peričić T, Rajić, V., Većek Jurčević, N. N., Pribisalić, A., & Medvedec Mikić, I. Antibiotic Prescribing Habits in Endodontics among Dentists in the Federation of Bosnia and Herzegovina-A Questionnaire-Based Study. Antibiotics (Basel). 2024;13(9):876.
CrossRef - Méndez-Millán JA, León-López M, Martín-González J, Saúco-Márquez JJ, Cabanillas-Balsera D, Segura-Egea JJ. Antibiotic Over-Prescription by Dentists in the Treatment of Apical Periodontitis: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2024;13(4):289.
CrossRef - Kissa J, Chemlali S, Gharibi A. Systemic antibiotic prescribing patterns of dentists in Morocco: A questionnaire study. Ann Afr Med. 2023;22(3):293-299.
CrossRef - Duncan HF, Kirkevang LL, Peters OA, El-Karim, I., Krastl, G., Del Fabbro, M., Chong, B. S., Galler, K. M., Segura-Egea, J. J., Kebschull, M., & ESE Workshop Participants and Methodological Consultant. Treatment of pulpal and apical disease: The European Society of Endodontology (ESE) S3-level clinical practice guideline. Int Endod J. 2023;56 Suppl 3:238-295..
CrossRef - Cope AL, Francis N, Wood F, Thompson W, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev. 2024;5(5):CD010136.
CrossRef - Dias NM, Moreno JO, Alves FR, Gonçalves LS, Provenzano JC. Antibiotic indication in endodontics by Colombian dentists with different levels of training: a survey. Indicação dos antibióticos em Endodontia por dentistas colombianos com diferentes níveis de formação: uma pesquisa por questionário. Acta Odontol Latinoam. 2022;35(3):198-205.
CrossRef - Khijmatgar S, Bellucci G, Creminelli L, Tartaglia GM Jr, Tumedei M. Systemic Antibiotic Use in Acute Irreversible Pulpitis: Evaluating Clinical Practices and Molecular Insights. Int J Mol Sci. 2024;25(2):1357.
CrossRef - [21] Khaloufi O., Khalaf L.H., Akerzoul N., Hassani F.Z.I.M., Toure B. Attitudes of dental practitioners from Northern Morocco on the prescription of antibiotics during endodontic treatment: A survey. Saudi Endod. J. 2022;12:316–321.
CrossRef - Parirokh M, Saffarzadeh A, Nakhaei N, Abbott P. The Outcome of Prescribing Antibiotics for the Management of Patients with Endodontic Infections. Eur Endod J. 2023;8(3):194-200.
CrossRef - Vengidesh R, Kadandale S, Ramachandran A, Srinivasan, S., Parthasarathy, R., Thanikachalam, Y., & Kumar, P. Antibiotic Prescription Patterns for Endodontic Procedures in India: A Knowledge, Attitude, and Practices (KAP) Survey. Cureus. 2023;15(4):e37804.
CrossRef - Khalil, D.; Baranto, G.; Lund, B.; Hultin, M. Antibiotic utilization in emergency dental care in Stockholm 2016: A cross sectional study. Acta Odontol. Scand. 2022, 80, 547–553.
CrossRef - López-Marrufo-Medina, A.; Domínguez-Domínguez, L.; Cabanillas-Balsera, D.; Areal-Quecuty, V.; Crespo-Gallardo, I.; Jiménez-Sánchez, M.C.; López-López, J.; Segura-Egea, J.J.; Martin-Gonzalez, J. Antibiotics prescription habits of Spanish endodontists: Impact of the ESE awareness campaign and position statement. J. Clin. Exp. Dent. 2022, 14, e48–e54.
CrossRef - Kaushik A, Rana N, Ashawat MS, Ankalgi A, Sharma A. Alternatives to β-Lactams as Agents for the Management of Dentoalveolar Abscess. Curr Top Med Chem. 2024;24(21):1870-1882.
CrossRef - León-López, M.; Cabanillas-Balsera, D.; Martín-González, J.; Montero-Miralles, P.; Saúco-Márquez, J.J.; Segura-Egea, J.J. Prevalence of root canal treatment worldwide: A systematic review and meta-analysis. Int. Endod. J. 2022, 55, 1105–1127
CrossRef - Immich F, Cotti E, Pirani C, Rossi-Fedele G. What is new in the 2023 European Society of Endodontology S3-level clinical practice guidelines?. Int Endod J. 2024;57(8):1059-1064.
CrossRef - Rodríguez-Fernández A, Vázquez-Cancela O, Piñeiro-Lamas M, Herdeiro MT, Figueiras A, Zapata-Cachafeiro M. Magnitude and determinants of inappropriate prescribing of antibiotics in dentistry: a nation-wide study. Antimicrob Resist Infect Control. 2023;12(1):20.
CrossRef - Vázquez-Cancela O, Zapata-Cachafeiro M, Herdeiro MT, Figueiras A, Rodríguez-Fernández A. Dentists’ knowledge, attitudes and perceptions of antibiotic prescribing: A systematic review. Prev Med. 2024;185:108043.
CrossRef - Kjome RLS, Bjønnes JAJ, Lygre H. Changes in Dentists’ Prescribing Patterns in Norway 2005-2015. Int Dent J. 2022;72(4):552-558.
CrossRef - Haidar ZS. Antibiotic Stewardship: Integrating a Crucial Element for Dental Practices, Education, and Patient Care. Int Dent J. 2023;73(5):595-597.
CrossRef - Sirinoglu Capan B, Duman C, Kalaoglu EE. Antibiotic prescribing practices for prophylaxis and therapy of oral/dental infections in pediatric patients – results of a cross-sectional study in Turkey. GMS Hyg Infect Control. 2023;18:Doc11.
- Kyles BJ, Spivakovsky S. Toward the development of an antibiotic stewardship competency in dental education. J Dent Educ. 2022;86(7):883-886.
CrossRef - Thompson W, Teoh L, Pulcini C, Sanderson, S., Williams, D., Carter, V., Pitkeathley, C., & Walsh, T. International Consensus on a Dental Antibiotic Stewardship Core Outcome Set. Int Dent J. 2023;73(3):456-462.
CrossRef - Thornhill M.H., Dayer M., Prendergast B.D., Lockhart P., Baddour L. Antibiotic Prophylaxis in Dentistry. Clin. Infect. Dis. 2023;76:960–961.
CrossRef - Monsef E, Goodman X, Patil R, White SN. Dentists’ knowledge of non-surgical root canal treatment, a systematic review. J Dent. 2024;145:104975.
CrossRef - Özdemir Kabalak M, Aytac EN, Tarhan N, Karabulut E, Keceli HG. Potential barriers to the rational antibiotic use in dental and periodontal practice: A questionnaire-based online survey. Dent Med Probl. 2024;61(3):373-383.
CrossRef - Teklay G, Tefera H. Appropriateness of antibiotic prescribing among patients treated for dental diseases in Mekelle city, Northern Ethiopia: a cross sectional study. J Health Popul Nutr. 2024;43(1):153.
CrossRef - Teoh L, Taylor M, Ierano C, McCullough M, Thursky K, James R. Appropriateness of antimicrobial prescribing for oral and dental conditions in Australian hospitals: 2013 to 2022. J Dent. 2024;148:105241.
CrossRef - Alqadi SF, Almuzaini SA, Algarni AA, Ayed Y, Alghamdi NS, Ain TS. The impact of clinical audit on antibiotic prescribing in dental practice at Taibah University Dental Hospital. J Clin Pediatr Dent. 2024;48(5):138-142.
CrossRef - Domínguez-Domínguez L, Castelo Baz P, Cabrera-Fernandez A, Cabanillas-Balsera, D., Pabon-Carrasco, M., Segura-Egea, J. J., & Martin-Gonzalez, J. Patients’ Opinions on Antibiotics in the Treatment of Dental Infections: A Cross-Sectional Survey. J Clin Med. 2024;13(7):2099.
CrossRef - Koosha F, Cymerman J, Manders T, Simon M, Walker S, Rafailovich M. Non-cytotoxic Root Canal Dressing with Improved Antimicrobial Efficacy. J Endod. 2023;49(2):205-211.
CrossRef







