Manuscript accepted on :29-07-2024
Published online on: 01-10-2024
Plagiarism Check: Yes
Reviewed by: Dr. Ramdas Bhat
Second Review by: Dr. Sunil Dutt Shukla
Final Approval by: Dr. Eman Refaat Youness
M. R. Suchitra* and Yogitha P. S
and Yogitha P. S
Department of Chemistry and Biosciences, SRC, SASTRA Deemed to Be University, Kumbakonam, India.
Corresponding Author E-mail: dietviji@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2951
Abstract
Introduction: Autism Spectrum Disorder (ASD) presents a multifaceted challenge with limited therapeutic options and a need for early biomarkers. Understanding disrupted signalling pathways offers promise for intervention and assessment. Methods: Literature review spanning genetic, pre-clinical, and patient studies elucidating pathways implicated in ASD pathogenesis, including melatonin, mTOR, Retinoic acid, Hedgehog, Notch and Wnt signalling. Results: Core components of key signalling pathways, such as melatonin, mTOR, Retinoic acid, Hedgehog, Notch and Wnt, are dysregulated in ASD. These pathways regulate crucial aspects of the nervous system, including immune function, neuronal growth, neurotransmission, and metabolism. Discussion: Manipulating these pathways could potentially modify ASD traits by influencing brain development and immune homeostasis. Targeting specific nodes within these pathways may offer novel therapeutic approaches for ASD management. Additionally, identifying biomarkers associated with pathway dysregulation could enable earlier diagnosis and monitoring of disease progression. Conclusion: Understanding the intricate interplay of signalling pathways in ASD pathogenesis provides insights into potential therapeutic targets and biomarkers. Further research into the manipulation of these pathways and their impact on ASD traits is warranted to advance personalized treatment strategies and improve outcomes for individuals with ASD.
Keywords
ASD; Autism; Hedgehog; Melatonin; mTOR; Notch; Retinoic acid; Signalling pathways; Wnt pathway
Download this article as:| Copy the following to cite this article: Suchitra M. R, Yogitha P. S. Unraveling Neurodevelopmental Disorders: Signalling Pathways as Potential Targets for Therapeutic Intervention in Autism Spectrum Disorder. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Suchitra M. R, Yogitha P. S. Unraveling Neurodevelopmental Disorders: Signalling Pathways as Potential Targets for Therapeutic Intervention in Autism Spectrum Disorder. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/47MGPkC |
Introduction
A neurodevelopmental illness called ASD is characterised by limitations in social communication, as well as by repetitive activities and narrow interests. The Diagnostic and Statistical Manual of Mental Disorders (DSM) by American Psychiatric Association, revised the diagnostic criteria for ASD (DSM – 5TH edition). There is a need to develop new biomarkers for early diagnosis, assess the disease stage for effective treatments that could potentially modify the course of the disorder. Signalling cascades governs the overall homeostasis of the nervous system. A systematic review in 2022 indicates a median prevalence of ASD in 1% children, with an underestimation of low and middle income nations and an upward trend in prevalence over time 1. Alteration and interaction of WNT signalling – canonical (CTNNB1), non-canonical (PRICKLE2), BMP/TGF – β, SHH (Hedgehog), FGF (fibroblast growth factor), RA (Retinoic acid) is most probably prone to autistic development2. In this systematic review, we can elaborate the signalling pathways which are responsible for the etiology of autistic development.
Methodology
A comprehensive literature review was conducted to identify studies focusing on cellular signalling pathways implicated in Autism Spectrum Disorder (ASD). Various experimental platforms, including genetic association analyses, pre-clinical animal models, and investigations utilizing patient samples, were examined to elucidate mutations affecting key signalling pathways such as melatonin, mTOR, and Wnt. Relevant studies addressing the role of these pathways in neuronal growth, neurotransmission, immune regulation, and other aspects of nervous system function were analysed.
Review of Literature
Signalling Pathways in ASD
Wnt Pathway
Wnts (Wintless-related integration site) are lipid-modified secreted glycoproteins that participate in the canonical Wnt/β-catenin, non-canonical planar cell polarity, and Wnt/Ca2+ signalling cascades. The growth and function of the mammalian central nervous system (CNS) are specifically known to be dependent on Wnt/β-catenin signalling, which is involved in several biological processes of CNS.3
The β-catenin destruction complex degrades β-catenin without Wnt ligands. Active Wnt signaling, facilitated by Frizzled and LRP5/6, increases nuclear β-catenin, enhancing target gene transcription. Non-canonical Wnt pathways raise Ca2+ levels via Ryk/Ror-Frizzled or activate RAC/JNK and RhoA/ROCK. Dishevelled (Dvl) links to ASD, crucial in Wnt signaling and synaptic vesicle clustering. Wnt7a/Dvl1 mutants show spine defects and neurotransmission issues, while Dvl1 knockout mice exhibit abnormal behavior despite normal neuroanatomy. GSK3 inhibitor treatment postnatally reduces autism-related behaviors and corrects abnormal development4.
Dvl, central to Wnt signalling, links to ASD; Dvl1 KO mice exhibit abnormal behaviour’s with normal neuroanatomy. Wnt7a/Dvl1 mutants show impaired spine formation and neurotransmission. GSK3 inhibitor treatment rescues abnormal development and reduces autism behaviour’s.5 SFARI Gene lists CTNNB1 and TCF7L2 as high-confidence ASD genes. CTNNB1 has 54 uncommon de novo variants, and TCF7L2 has 23. Variants cluster around crucial interaction domains, requiring further refinement6.
TCF7L2 regulates oligodendroglial development, myelinogenesis, and cholesterol production, impacting ASD-related myelination anomalies7. ASD correlates with factors like TBL1X, TBL1XR, MED12, and T-Box TF1, which modulate Wnt/β-catenin-dependent transcription. Deficient activity dependent neuroprotective protein links to ASD traits8. In utero valproic acid (VPA) exposure in mice mirrors ASD molecular pathogenesis. DEPs overlap with ASD risk genes, notably in the Wnt/β-catenin pathway. Dysregulated Rnf146 in VPA-exposed mice associates with altered social behavior via heightened synaptic transmission8.
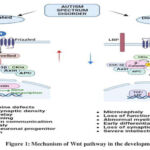 |
Figure 1: Mechanism of Wnt pathway in the development of ASD |
Haloperidol, clozapine, fluoxetine, and Ritalin, known to affect Wnt signaling, are utilized in therapeutic strategies for WNT-mediated autism. 10. Haloperidol boosts Wnt protein via D2 receptors, fluoxetine upregulates Wnt2 by reducing miR-16, and Ritalin modulates Akt/GSK3, implicating Wnt signaling11. Autism spectrum disorders (ASD) are linked to phenotypic alterations that are related with dysregulation of Wnt/β-catenin-dependent transcription.
mTOR (Mammalian Target of Rapamycin) Pathway
MTOR gene encodes for the mTOR pathway and its activation. This gene present in human chromosome 1. mTOR involves in signalling pathways to regulate apoptosis, autophagy, and replication of cells. Studies revealed the connection between the mTOR signalling pathway and several illnesses, such as rheumatic arthritis, osteoporosis, insulin resistance, and carcinoma. Microcephaly, macrocephaly, diminished Purkinje cells in the cerebellum, increased cell density, cortical dysgenesis, and aberrant migration patterns have all been observed in the brains of individuals with autism12.
Synaptic pruning has also been found to be disrupted in ASD. Elevated phosphorylation of mTOR and ribosomal protein S6, two of its downstream effectors, have been correlated with a higher spine density in ASD brains. By promoting LTP (Long term potentiation) of the synapses, the Akt/mTOR pathway is essential for the process of learning and memory formation. In valproic acid-induced ASD, it has been discovered that mTOR activity inhibition increases PI3K/Akt/mTOR-mediated autophagic pathway and enhances social interactions13. Using whole-genome linkage investigations, copy number variation screening, SNP analysis, and genome-wide studies, a number of ASD candidate genes have been discovered. Akt/mTOR signalling cascade and its downstream effectors, TSC1, TSC2, PTEN, and FMR1 are possible gene targets. The development of ASD symptoms may be related to the regulation of various cellular processes via the Akt/mTOR pathway, which has an impact on neurodevelopment14.
The PTEN GENE (Hippocampus, Macrocephaly, Synaptic Density):
Mutation (phosphatase and tensin homolog)or deletion leads to the abnormal or hyperactivation of mTOR pathway. Because the PTEN gene is the super regulator of mTOR pathway. Within that macrocephaly mediated autistic syndrome was developed by the mutation of PTEN genes. The PTEN gene mutation involved in mental retardation, seizures and Autism Spectrum Disorder. The PTEN gene act as antagonist for PIP2 to PIP3 conversion, so far this conversion initiates the activation and further processing for mTOR pathway. When there is a lack of PTEN gene, it leads to the hyperactivation or upregulation of mTOR pathway15. When the PTEN is inhibited P13K/Akt/mTOR pathway leads to the increased level of Akt and S6 phosphorylation in the brain, PTEN mutation are prone to developmental delays and mental health issues.
It has been discovered that variations in neuroligins, one kind of postsynaptic cell adhesion molecule, alter protein production. Neuroligins are essential for synaptogenesis and synaptic transmission. Upregulation of Akt/mTOR signalling led to increased protein synthesis and dendritic development in Nlgn3KO mice. Neurons were further treated with either rapamycin, an inhibitor of mTOR, or LY294002, an inhibitor of PI3K/Akt activity, to avoid the morphological defects seen in deletion animals. The research also revealed a novel association between NL3 and the Akt/mTOR signalling cascade.NL3 abnormalities and hyperactivation of Akt/mTOR signalling were primarily mediated by decreased PTEN expression and interactions between PTEN and NL3 and a scaffold protein called MAGI-2. It is evident from a number of the clinical trials discussed above that overactive mTOR resulting from PTEN mutations is the cause of ASD and other cognitive impairments16.
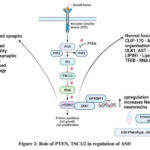 |
Figure 2: Role of PTEN, TSC1/2 in regulation of ASD |
The TSC Mutation in mTOR
TSC 1 (hamartin), TSC2 (tuberin) – tuberculosis complex. The gene mutation of TSC1 and TSC2 causes Tuberous Sclerosis complex genetic disorder. The mTORC1-dependent increased phosphorylation of S6, S6Ks, and 4E-BPs leads to altered protein synthesis. TSC affects all organ systems, with early involvement of the central nervous system. The TAND (TSC-associated neuropsychiatric diseases) spectrum is broad and includes mental, behavioural, and cognitive problems such anxiety, depression, and ADHD, as well as ASD. TSC is a widely recognised aetiology of syndromic ASD17.
The TSC1 and TSC2 gene mutation also leads to neuronal dysfunctions, impaired axonal regulation, abnormal dendritic morphogenesis, synapse formation and enhanced spine density are observed in the post-mortem investigations of ASD’s individuals. However, the mTOR inhibitor rapamycin reversed all of these effects18. Chrysophanol, piperine, resveratrol, curcumin, quercetin, luteolin, sulforaphane and epigallocatechin these are the compounds which have a capability of regulating mTOR pathway by decreases mTOR, Akt, interleukins (to reduce the inflammation and inflammatory reactions which prone to macrocephaly) and increases Bcl2 19.
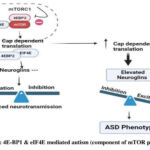 |
Figure 3: 4E-BP1 and eIF4E mediated autism (component of mTOR pathway) |
Role of Upregulated EIF4E in Development of Autism
The eukaryotic translation initiation factor 4E (eIF4E) is a possible target for ASD, as it is related with oxidative stress and is regulated by NGF-induced signalling. 4E-BP1 and eIF4E play key roles in the mTOR pathway. Dysfunctions in mTOR signalling, whether syndromic or idiopathic, lead to autism. Mutations in genes such as FMRP, NF1, PTEN, and TSC1/TSC2 cause syndromic autism, which includes fragile X syndrome, neurofibromatosis type 1, PTEN Hamartoma tumour syndrome, and tuberous sclerosis complex. Hyperactivation or hypoactivation of mTORC1 causes either translation initiation via P70S6K or translation blockage via 4EBP, resulting in ASD aetiology20.
p70S6K/eIF4B and 4E-BP1/eIF4E play crucial roles in dendritic spines because they are involved in the excitatory postsynapses identified in the fusiform gyrus of post-mortem brain samples from persons with ASD21. The eIF4F, eIF4A-mediated mTOR pathway plays a significant role in idiopathic autism development. Disruptions in mTOR signalling molecules contribute to ASD and idiopathic autism. Individuals with idiopathic autism exhibit decreased brain mTOR signalling, resulting in reduced synaptic activity, impaired cellular growth, disrupted protein production, and altered neuronal activity. Dysregulation of mRNA translation, mediated by 4E-BP1 binding and eIF4E modification of 5′ capped mRNA initiation, is crucial. eIF4E can modulate p70S6K and eIF4B expression, impacting mTOR-dependent translation22.
mTOR Pathway over Expression Leads to the Increased Synthesis of Inflammatory Cytokines
ASD-related disorders such macrocephaly, seizures, and learning disabilities may be brought on by dysregulation in the mTOR pathway. Autism development is also influenced by this route. A dysfunctional mTOR signalling pathway has been linked to 8–10% of autistic patients. Up to 58% of the genes that predispose someone to autism are either directly or indirectly connected to the mTOR signalling activity. These data collectively suggest that mTOR signalling is a convergent pathway associated with ASD. mTOR has also been connected to the regulation of the expression of genes of inflammatory cytokines secreted by various immune cells23. Numerous cells release CCL5, a proinflammatory chemoattractant cytokine that is involved in a number of physiological and pathological processes. There are four receptors that mediate it: CCR1, CCR3, CCR4, and CCR5. Additionally, human microglia that have been triggered by the proinflammatory neuropeptide neurotensin—which is present in children with ASD—release CCL524. So, the regulation of mTOR pathway is one of the therapeutic strategies was followed for the regulatory release of CCL5 which is downregulation of CCL5 is expected. So, here they used mTOR inhibitor to regulate the upregulated activity in ASD patients. The CCL5 a proinflammatory mediated increases the synaptic density and increased protein synthesis. Synapse overgrowth has been linked to learning disabilities, macrocephaly, and social and behavioural abnormalities. Normal head growth in children with ASD results in an overall larger brain size throughout infancy25. Rapamycin, an mTOR inhibitor, decreases CCL5 production, implicating mTOR in CCL5 expression. GSK-3β modulates CCL5 release induced by mTOR. GSK-3 phosphorylates tau protein, contributing to Alzheimer’s disease pathology. mTOR may inhibit GSK-3 phosphorylation at Ser9. NF-κB activation and CREB activity reduction are essential for CCL5 synthesis. Phosphorylated CREB may suppress CCL5 production by competing with NF-κB for CREB-binding protein binding, indicating their co-regulatory and antagonistic roles in CCL5 gene regulation26.
Diagram illustrating a possible method for CCL5 release that involves mTOR pathway activation. In our study, people with ASD had active mTOR signalling. The down-regulation of GSK-3β at Ser9 and the reduction of CREB at Ser133, which results in a decrease in CREB binding to CREBBP, are both influenced by phosphorylated mTOR at serine residue 2448. Thus, additional CREBBP that is accessible may bind to NF-κB, encouraging the synthesis of CCL5. The components of the signalling pathway that are up-regulated (red) and down-regulated (blue) are represented by corresponding arrows.
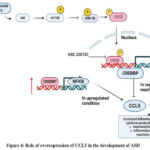 |
Figure 4: Role of overexpression of CCL5 in the development of ASD |
Melatonin Pathway
The chemical composition of the circadian rhythm-responsive hormone melatonin (N-acetyl-5-methoxytryptamine) was identified in 1958. Although it is also produced by the bone marrow, retina, lymphocytes, skin, pancreas, glial cells, Harderian gland and kidney, melatonin is principally produced by the pineal gland.
Using tryptophan hydroxylase as a mediator, tryptophan, the precursor to melatonin, is transformed into 5-hydroxytryptophan, which forms melatonin. AAD (amico acid decarboxylase) converts 5-hydroxytryptophan to serotonin, which is then converted by arylalkylamine N-acetyltransferase (AANAT) into N-acetylserotonin (NAS). N-acetylserotonin, the acetylated form of serotonin, is converted into melatonin (HIOMT) by the enzyme hydroxyindole O-methyltransferase. Melatonin is also critical to the foetus’s growth. Since the pineal gland develops after birth, the foetus is dependent on the mother’s melatonin27.
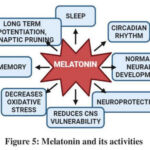 |
Figure 5: Melatonin and its activities |
Melatonin has an impact on placental function because it may pass across bodily barriers, such as the blood-placenta barrier, without being denaturized. Melatonin enters the foetal blood throughout pregnancy and crosses the placenta, giving the foetus information about the photoperiod 28. The DDC (AADC), AANAT and ASMT genes which encodes for the enzymes, involved in the melatonin synthesis. The polymorphic variants or any mutation in the genes leads to the insufficient synthesis melatonin and involved in many neuropsychiatric disorders29.
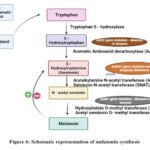 |
Figure 6: Schematic representation of melatonin synthesis |
Normal neuronal firing is the result of electrical impulses and neurotransmitters, which allow neurons to communicate with one another. Understanding a neuron’s components, such as its soma, dendrites, and axons, is crucial for a better understanding of this process. The MT1 receptor in the SCN and other limbic system regions is activated by melatonin receptor activation, which reduces neuronal activity and may be how melatonin promotes sleep sufficient level leads to the hyperactivity of neurotransmission was seen in ASD, ID etc., These two transcription factors are activated by the PKC, the PKC was activates which is phosphorylated by the melatonin. The clock genes are transcribed, to maintain the circadian rhythm30. Part of GDNF’s function is to sustain sensory, parasympathetic, and enteric neurons as well as the retention and neural defence of catecholaminergic and dopaminergic populations. It also contributes to the morphogenesis of the kidney and digestive system, as well as the division, migration, and maturation of brain cells. If the melatonin was absent or insufficiency leads to the dysregulated mechanisms in circadian rhythm, synaptic plasticity regulation and diminishes brain growth which associates the development of ASD. The melatonin mainly involved in the regulation of neuron firing. Because when the neuron firing goes upregulated, the continuous electrical impulses are transferred concordantly, moves positively charged molecules (K+, Na+) through cell membrane. When the melatonin comes it regulates, the neuron firing tends to the hyperexcitability to the normal condition and then tends to sleep state31.
ASD children’s sleep problems are often treated with melatonin, yet trustworthy clinical evidence is still not provided for this treatment. In a phase 3 randomised, placebo-controlled clinical research, 196 children with ASD received 1-, 4-, or placebo doses of melatonin once day before bedtime as part of proper sleep hygiene therapy. Sleep onset latency (SOL), measured with the electronic sleep diary, was the main result. As comparison to the placebo group, SOL dramatically decreased in the 1- and 4-mg melatonin groups. Children with ASD can use this melatonin treatment plan as a realistic clinical strategy to deal with newly developed challenges32. Wild type mice and Cntnap2 gene mutation was caused in mice model (2-3 months old). To bring ASD phenotypes, for the melatonin treatment (3.0mg/kg) mixed with small amount of condensed milk (10mg condensed milk/g of b.w.) at a daily dose manner for 24 days. They investigated the effects of nighttime exposure to low light. Based on the behavioural statistics the melatonin treated mice model showed the relaxed and prolonged napping time, reduced rapid eye movement, lower hyper locomotor activity and initiates the normal circadian rhythm33.
The melatonin also involved in the phosphorylation of other compounds to initiate another signalling pathway which was mediated by the phosphorylation of PKC. The melatonin treatment in valproic acid induced ASD BTBR T+tf/J mice model. In that condition threonine/serine kinases are reduced in hippocampus, so the melatonin reverses the synthesis of kinases to maintain the long -term potentiation. Which mediated by the phosphorylation of PKC, this PKC increases the CAMKII, NMDAR1 and PKA’s phosphorylation for the further gene transcription (CREB, BDNF) for the memory formation, cell regulation and synaptic plasticity34.
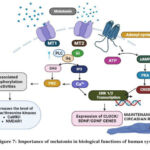 |
Figure 7: Importance of melatonin in biological function of human system |
Potential biomarkers for the autism in melatonin pathway is N-acetyl serotonin (NAS), 6-sulphatoxymelatonin (aMT6s) in urine, serotonin, higher level of 5-HT in total blood volume, 14-3-3 protein and miRNA-451. Melatonin insufficiency in ASD patients may be caused by alterations in 14-3-3 dependent translation in vivo, according to reports indicating miR-451 might suppress the 14-3-3 protein, which controls the activity of AANAT and ASMT. Melatonin is not currently thought of as a clinical biomarker for autism, but the drop in melatonin seen in autism suggests that it may one day be used as one35.
Retinoic Acid Pathway
ASD and neurological system development are impacted by retinoic acid (RA). By attaching to nuclear receptors, RA signaling controls the transcription of genes. This pathway’s dysregulation may be a contributing factor to ASD, indicating therapeutic promise through RA signaling modulation for the treatment of ASD and comprehension of its origin36. RA restores RARα signaling, reduces microglial activation, and normalizes polarization, alleviating VPA-induced autism-like behaviors37. RA supplementation in Fmr1 KO mice rescues social novelty behaviour but not sociability or hyperactivity; restores neuronal gene expression38. Beta-carotene supplementation in BALB/c and BTBR mice at birth improves autistic-like behaviors, increasing social interactions and CD38 and oxytocin levels. Dosage: 0.1–5.0 mg/kg orally for 7 days. Enhanced brain neuroplasticity via increased expression of CD38, oxytocin, oxytocin receptor, BDNF, and retinoic acid receptor genes. Promising for clinical studies 39.
Hedgehog Pathway
SMO-SHH dysregulation in ASD leads to GLI3 repression, causing oxidative stress, neuroinflammation, and apoptosis. Modulating SHH pathway may treat ASD40. PUR, a Smo-Shh agonist, protects against ICV-PPA-induced brain intoxication, restoring balance, potentially preventing demyelination, and improving ASD behaviors41. SAG alleviates anxiety in HFD-fed mice by rebalancing mitochondrial dynamics42. Interactions between mutations, teratogens, and Hedgehog (Hh) signaling via the MMM complex (MOSMO, MEGF8, MGRN1) influence birth defect outcomes. Deficiency of MOSMO elevates SMO and Hh signaling, causing defects. Teratogen inhibition of SMO reduces defect severity in Mosmo-/- embryos, suggesting potential therapeutic targets for birth defects and ASD43.
Notch Signalling Pathway
Shh/Wnt pathways’ interplay affects BBB integrity and neuroinflammation in ASD. Understanding their role offers potential therapeutic targets for symptom management while preserving normal development44. Prenatal valproic acid exposure induces autistic-like behaviors in rats, accompanied by increased expression of Notch pathway-related molecules (Notch1, Jagged1, NICD, and Hes1) in the prefrontal cortex, hippocampus, and cerebellum45. BTBR mice, modeling autistic-like behaviors, exhibit constitutively activated Notch signaling in immune cells and liver. Increased expression of Dll4, Notch1-3, and RBPJ, along with altered downstream transcription factors, suggests immune dysregulation46. In VPA-induced autism rats, DAPT reduces Notch1/Hes1, NRG1/phosphorylated ErbB4 levels, and autism-like behaviors; AG1478 improves behaviors and reduces microglial activation47. NAC reverses VPA-induced autism symptoms by restoring autophagy and reducing Notch-1/Hes-1 pathway activity, offering therapeutic potential for autism48.
Results and Discussion
Dysregulation of key signalling pathways is frequently observed in ASD. These pathways govern various aspects of nervous system function, suggesting their involvement in ASD pathogenesis. Timely manipulation of these pathways could potentially influence brain development and immune homeostasis, impacting ASD traits.
Clinical Relevance
Understanding the dysregulated signalling pathways implicated in ASD offers potential targets for therapeutic intervention, timely manipulation and the development of novel biomarkers for earlier diagnosis and monitoring disease progression mainly at neurodevelopmental phase.
Limitations and Future Directions
Despite advancements, challenges persist in comprehensively grasping the intricacies of ASD pathogenesis and translating discoveries into viable therapies. Moving forward, research should prioritize unraveling the intricate interactions among signalling pathways, pinpointing dependable biomarkers, and crafting precise interventions tailored to the varied requirements of ASD individuals.
Environmental Factors and Autism
Environmental factors, such as prenatal infections, medication, perinatal complications, air pollution, heavy metal exposure, vaccines and socioeconomic status, interact with genetic predispositions to influence ASD risk. Understanding these factors’ roles is crucial for developing preventive strategies and interventions to mitigate ASD’s impact.
Conclusion
ASD involves disruptions in crucial genetic mutations and signalling pathways essential for brain maturation, including Wnt, Hedgehog, PI3K/AKT/mTORC1, and ERK1/2 pathways, alongside factors like melatonin and retinoic acid. Overactivity in these pathways, often caused by factors like drug exposure during pregnancy, environmental influences, or hereditary factors, disturbs brain development, contributing to ASD. Recent research explores nutraceutical, diet-based therapies targeting these pathways. Advanced techniques like chromosomal microarray ,NGS aid diagnosis, and iPSC-derived neuronal cells offers insights into impaired pathways. Top of Form
Acknowledgement
The author would like to thank Dr. S. Parthasarathy, Associate Professor, Mahatma Gandhi medical college, Puducherry for helping in editing the article. The author is also grateful to SASTRA UNIVERSITY, Kumbakonam for providing a platform to do further research on ASD which is the main provoking factor to go into the insights of the neurodevelopmental disorder and hence to collect a review on the condition.
Conflict of Interest
The author(s) declares no conflict of interest
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Authors Contribution
Both the authors have significantly contributed and qualify for authorship.
References
- Zeidan J, Fombonne E, Scorah J, et al. Global prevalence of autism: A systematic review update. Autism Res. 2022;15(5):778-790. doi:10.1002/aur.2696
CrossRef - Kumar S, Reynolds K, Ji Y, Gu R, Rai S, Zhou CJ. Impaired neurodevelopmental pathways in autism spectrum disorder: a review of signalling mechanisms and crosstalk. J Neurodev Disord. 2019;11(1):10. doi:10.1186/s11689-019-9268-y
CrossRef - Alkailani MI, Aittaleb M, Tissir F. WNT signalling at the intersection between neurogenesis and brain tumorigenesis. Front Mol Neurosci. 2022;15:1017568. doi:10.3389/fnmol.2022.1017568
CrossRef - Belinson H, Nakatani J, Babineau BA, et al. Prenatal β-catenin/Brn2/Tbr2 transcriptional cascade regulates adult social and stereotypic behaviors. Mol Psychiatry. 2016;21(10):1417-1433. doi:10.1038/mp.2015.207
CrossRef - Wang T, Hoekzema K, Vecchio D, et al. Author Correction: Large-scale targeted sequencing identifies risk genes for neurodevelopmental disorders. Nat Commun. 2020;11(1):5398. doi:10.1038/s41467-020-19289-5
CrossRef - Dias C, Pfundt R, Kleefstra T, et al. De novo variants in TCF7L2 are associated with a syndromic neurodevelopmental disorder. Am J Med Genet A.2021;185(8):2384-2390. doi:10.1002/ajmg.a.62254.
CrossRef - Li Q, Becker B, Jiang X, et al. Decreased interhemispheric functional connectivity rather than corpus callosum volume as a potential biomarker for autism spectrum disorder. Cortex. 2019;119:258-266. doi:10.1016/j.cortex.2019.05.003
CrossRef - Arnett AB, Rhoads CL, Hoekzema K, et al. The autism spectrum phenotype in ADNP syndrome. Autism Res. 2018;11(9):1300-1310. doi:10.1002/aur.1980
CrossRef - Park G, Jang WE, Kim S, et al. Dysregulation of the Wnt/β-catenin signalling pathway via Rnf146 upregulation in a VPA-induced mouse model of autism spectrum disorder. Exp Mol Med.2023;55(8):1783-1794. doi:10.1038/s12276-023-01065-2.
CrossRef - Salcedo-Arellano MJ, Cabal-Herrera AM, Punatar RH, Clark CJ, Romney CA, Hagerman RJ. Overlapping Molecular Pathways Leading to Autism Spectrum Disorders, Fragile X Syndrome, and Targeted Treatments. Neurotherapeutics. 2021;18(1):265-283. doi:10.1007/s13311-020-00968-6
CrossRef - Bae SM, Hong JY. The Wnt Signalling Pathway and Related Therapeutic Drugs in Autism Spectrum Disorder. Clin Psychopharmacol Neurosci. 2018;16(2):129-135. doi:10.9758/cpn.2018.16.2.129
CrossRef - Panwar V, Singh A, Bhatt MLB, et al. Multifaceted role of mTOR (mammalian target of rapamycin) signalling pathway in human health and disease. Signal Transduction and Targeted Therapy. 2023;8(1). doi:10.1038/s41392-023-01608-z
CrossRef - Lieberman OJ, Cartocci V, Pigulevskiy I, et al. mTOR Suppresses Macroautophagy During Striatal Postnatal Development and Is Hyperactive in Mouse Models of Autism Spectrum Disorders. Front Cell Neurosci. 2020;14:70. Published 2020 Mar 31. doi:10.3389/fncel.2020.00070
CrossRef - Rosina E, Battan B, Siracusano M, et al. Disruption of MTOR and MAPK pathways correlates with severity in idiopathic autism. Transl Psychiatry. 2019;9(1):50. doi:10.1038/s41398-018-0335-z.
CrossRef - Popova NV, Jücker M. The Role of mTOR Signalling as a Therapeutic Target in Cancer. Int J Mol Sci. 2021;22(4):1743. Published 2021 Feb 9. doi:10.3390/ijms22041743
CrossRef - Xu J, Du YL, Xu JW, et al. Neuroligin 3 regulates dendritic outgrowth by modulating Akt/mTOR signalling. Front Cell Neurosci.2019;13:518. doi:10.3389/fncel.2019.00518.
CrossRef - Bassetti D, Luhmann HJ, Kirischuk S. Effects of Mutations in TSC Genes on Neurodevelopment and Synaptic Transmission. Int J Mol Sci. 2021;22(14):7273. Published 2021 Jul 6. doi:10.3390/ijms22147273
CrossRef - Pagani M, Barsotti N, Bertero A, et al. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat Commun.2021;12(1):6084. doi:10.1038/s41467-021-26131-z.
CrossRef - Thomas SD, Jha NK, Ojha S, Sadek B. mTOR Signalling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules. 2023;28(4):1889. Published 2023 Feb 16. doi:10.3390/molecules28041889
CrossRef - Ganesan H, Balasubramanian V, Iyer M, et al. mTOR signalling pathway – A root cause for idiopathic autism?. BMB Rep. 2019;52(7):424-433. doi:10.5483/BMBRep.2019.52.7.137
CrossRef - Nicolini C, Fahnestock M. The valproic acid-induced rodent model of autism. Exp Neurol.2018;299(A):217-227. doi:10.1016/j.expneurol.2017.04.017.
CrossRef - Okondo MC, Rao SD, Taabazuing CY, et al. Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem Biol. 2018;25(3):262-267.e5. doi:10.1016/j.chembiol.2017.12.013
CrossRef - Bhandari R, Paliwal JK, Kuhad A. Neuropsychopathology of autism spectrum disorder: complex interplay of genetic, epigenetic, and environmental factors. Adv Neurobiol.2020;24:97-141. doi:10.1007/978-3-030-30402-7_4.
CrossRef - Li M, Sun X, Zhao J, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17(7):753-764. doi:10.1038/s41423-019-0279-0
CrossRef - Wong CW, Or PMY, Wang Y, et al. Identification of a PTEN mutation with reduced protein stability, phosphatase activity, and nuclear localization in Hong Kong patients with autistic features, neurodevelopmental delays, and macrocephaly. Autism Res. 2018;11(8):1098-1109. doi:10.1002/aur.1950
CrossRef - Wang B, Qin Y, Wu Q, et al. mTOR Signalling Pathway Regulates the Release of Proinflammatory Molecule CCL5 Implicated in the Pathogenesis of Autism Spectrum Disorder. Front Immunol. 2022;13:818518. Published 2022 Mar 29. doi:10.3389/fimmu.2022.818518
CrossRef - Wang L, Deng Y, Gao J, et al. Biosynthesis of melatonin from l-tryptophan by an engineered microbial cell factory. Biotechnology for Biofuels and Bioproducts. 2024;17(1). doi:10.1186/s13068-024-02476-7
CrossRef - Jin Y, Choi J, Won J, Hong Y. The Relationship between Autism Spectrum Disorder and Melatonin during Fetal Development. Molecules. 2018;23(1):198. Published 2018 Jan 18. doi:10.3390/molecules23010198
CrossRef - Moskaleva PV, Shnayder NA, Nasyrova RF. Assotsiatsiya polimorfizmov genov DDC (AADC), AANAT I ASMT, kodiruyushchikh fermenty sinteza melatonina, sriskom razvitiya psikhonevrologiche skikh rasstroistv [Association of polymorphic variants of DDC (AADC), AANAT and ASMT genes encoding enzymes for melatonin synthesis with the higher risk of neuropsychiatric disorders]. Z Nevrol Psikhiatr Imeni S.S. Korsakova. 2021;121(5):151-157. doi:10.17116/jnevro 2021121041151.
CrossRef - Dong S, Kifune T, Kato H, et al. Effects of melatonin on dopaminergic neuron development via IP3-mediated mitochondrial Ca2+ regulation in autism spectrum disorder. Biochem Biophys Res Commun. 2023;681:7-12. doi:10.1016/j.bbrc.2023.09.050
CrossRef - Feybesse C, Chokron S, Tordjman S. Melatonin in Neurodevelopmental Disorders: A Critical Literature Review. Antioxidants (Basel). 2023;12(11):2017. Published 2023 Nov 20. doi:10.3390/antiox12112017
CrossRef - Hayashi M, Mishima K, Fukumizu M, et al. Melatonin Treatment and Adequate Sleep Hygiene Interventions in Children with Autism Spectrum Disorder: A Randomized Controlled Trial. J Autism Dev Disord. 2022;52(6):2784-2793. doi:10.1007/s10803-021-05139-w
CrossRef - Wang HB, Tahara Y, Luk SHC, et al. Melatonin treatment of repetitive behavioral deficits in the Cntnap2 mouse model of autism spectrum disorder. In: Neurobiol DisKimY, BlockGD, GhianiCA, LohDH, ColwellCS, eds.2020;145:105064. doi:10.1016/j.nbd.2020.105064.
CrossRef - Mohanan AG, Gunasekaran S, Jacob RS, Omkumar RV. Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Mediating Function and Dysfunction at Glutamatergic Synapses. Front Mol Neurosci. 2022;15:855752. Published 2022 Jun 20. doi:10.3389/fnmol.2022.855752
CrossRef - Wu ZY, Huang SD, Zou JJ, et al. Autism spectrum disorder (ASD): Disturbance of the melatonin system and its implications. Biomed Pharmacother. 2020;130:110496. doi:10.1016/j.biopha.2020.110496
CrossRef - Liu Z, Wang J, Xu Q, Hong Q, Zhu J, Chi X. Research Progress in Vitamin A and Autism Spectrum Disorder. Behav Neurol. 2021;2021:5417497. Published 2021 Dec 7. doi:10.1155/2021/5417497
CrossRef - Luo L, Li T, Wu Q, et al. Retinoic acid administration normalizes aberrant microglial activation via regulating TREM2 transcription in the PFC of valproic acid induced autism rat. Neurosci Lett. 2023;803:137193. doi:10.1016/j.neulet.2023.137193
CrossRef - Yang L, Xia Z, Feng J, et al. Retinoic Acid Supplementation Rescues the Social Deficits in Fmr1 Knockout Mice. Front Genet. 2022;13:928393. Published 2022 Jun 17. doi:10.3389/fgene.2022.928393
CrossRef - Avraham Y, Berry EM, Donskoy M, et al. Beta-carotene as a novel therapy for the treatment of “Autistic like behavior” in animal models of Autism. Behav Brain Res. 2019;364:469-479. doi:10.1016/j.bbr.2017.09.041
CrossRef - Rahi S, Mehan S. Understanding Abnormal SMO-SHH Signaling in Autism Spectrum Disorder: Potential Drug Target and Therapeutic Goals. Cell Mol Neurobiol. 2022;42(4):931-953. doi:10.1007/s10571-020-01010-1
CrossRef - Rahi S, Gupta R, Sharma A, Mehan S. Smo-Shh signaling activator purmorphamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum Exp Toxicol. 2021;40(11):1880-1898. doi:10.1177/09603271211013456
CrossRef - Sun D, Deng J, Wang Y, et al. SAG, a sonic hedgehog signaling agonist, alleviates anxiety behavior in high-fat diet-fed mice. Brain Res Bull. 2023;195:25-36. doi:10.1016/j.brainresbull.2023.01.014
CrossRef - Kong JH, Young CB, Pusapati GV, et al. Gene-teratogen interactions influence the penetrance of birth defects by altering Hedgehog signaling strength. Development. 2021;148(19):dev199867. doi:10.1242/dev.199867
CrossRef - Gozal E, Jagadapillai R, Cai J, Barnes GN. Potential crosstalk between sonic hedgehog-WNT signaling and neurovascular molecules: Implications for blood-brain barrier integrity in autism spectrum disorder. J Neurochem. 2021;159(1):15-28. doi:10.1111/jnc.15460
CrossRef - Zhang Y, Xiang Z, Jia Y, He X, Wang L, Cui W. The Notch signaling pathway inhibitor Dapt alleviates autism-like behavior, autophagy and dendritic spine density abnormalities in a valproic acid-induced animal model of autism. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109644. doi:10.1016/j.pnpbp.2019.109644
CrossRef - Yao Y, Uddin MN, Manley K, Lawrence DA. Constitutive activation of Notch signalling and T cell activation characterize a mouse model of autism. Cell Biochem Funct. 2022;40(2):150-162. doi:10.1002/cbf.3684
CrossRef - Deng Y, Ma L, Du Z, et al. The Notch1/Hes1 pathway regulates Neuregulin 1/ErbB4 and participates in microglial activation in rats with VPA-induced autism. Prog Neuropsychopharmacol Biol Psychiatry. 2024;131:110947. doi:10.1016/j.pnpbp.2024.110947
CrossRef - Zhang YH, Wang T, Li YF, Deng YN, He XL, Wang LJ. N-acetylcysteine improves autism-like behavior by recovering autophagic deficiency and decreasing Notch-1/Hes-1 pathway activity. Exp Biol Med (Maywood). 2023;248(11):966-978. doi:10.1177/15353702231179924
CrossRef







