Manuscript accepted on :16-09-2024
Published online on: 27-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Pranjal Gujarathi
Second Review by: Dr. Rajan Singh
Final Approval by: Dr. Patorn Promchai
Ishmuratova Aziza Saydullayevna* , Abdugafurova Dilnoza Gulyamovna
, Abdugafurova Dilnoza Gulyamovna , Islomov Akmal Hushvaqovich
, Islomov Akmal Hushvaqovich , Mahmudov Lazizbek Umarjonovich, Baratov Ko’zijon Rabbim Ugli and Azimova Aziza Qodirali Kizi
, Mahmudov Lazizbek Umarjonovich, Baratov Ko’zijon Rabbim Ugli and Azimova Aziza Qodirali Kizi
A. S. Sadykov Institute of Bioorganic Chemistryof the Academy of Sciences of Republic.
Corresponding Author E-mail:Aziza_ishmuratova@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/3005
Abstract
Since ancient times, madder dye (Rubia tinctorum L.) has been used in Uzbekistan to help alleviate stone deposits in organs. In the experiment we conducted, we aimed to demonstrate that this plant can prevent the occurrence of kidney stone disease in rats using an induced model of the disease. The kidney stone disease model in rats was created by administering ethylene glycol and ammonium chloride to the animals over a period of 10 days. The substances contained in the madder dye plant mitigated the effects of the substances used to induce the disease. According to the study results, simultaneous oral administration of the madder dye extract prevented the development of kidney stone disease in rats. The extract of Rubia tinctorum L. was found to be highly effective, showing a significant nephroprotective effect due to its specific and rich composition of terpenes and terpenoids.
Keywords
Ethylene Glycol; Histopathology; Medicinal Plants; Rhizome of Plants; Rubia Tinctorum L.; Urolithiasis
Download this article as:| Copy the following to cite this article: Saydullayevna I. A, Gulyamovna A. D, Hushvaqovich I. A, Umarjonovich M. L, Ugli B. K. R, Kizi A. A. Q. The Study of the Biologically Active Effect of the Rubia Tinctorum L. Plant on Rats with Experimental Kidney Stone Disease and Issues of Introduction. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Saydullayevna I. A, Gulyamovna A. D, Hushvaqovich I. A, Umarjonovich M. L, Ugli B. K. R, Kizi A. A. Q. The Study of the Biologically Active Effect of the Rubia Tinctorum L. Plant on Rats with Experimental Kidney Stone Disease and Issues of Introduction. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4ei8LQ5 |
Introduction
Despite the rapid development of pharmacology and the emergence of effective modern medicines, we should not overlook the gifts of nature—medicinal plants that help in healing various ailments. One such “gift” is madder, whose therapeutic properties have been experimentally proven. Today, the extract from the roots of this plant is successfully used in scientific medicine for treating kidney stones and other diseases 1, 2. Traditionally, complex therapy regimens, including herbal medicines, are employed to treat urinary tract diseases. The use of medicinal plant-based drugs enhances the effectiveness of therapy, accelerates patient recovery, and reduces the likelihood of disease recurrence. Madder dye (Rubia tinctorum L.) is a medicinal plant whose rhizome and roots contain anthraquinones, sugars, ascorbic acid, iridoids, pectin substances, citric acid, malic acid, tartaric acid, proteins, and phenolic compounds 3, 4. These substances contribute to the dissolution of stones in the bladder and kidneys and help remove oxalic acid, phosphoric acid, and other salts. The dry extract of madder dye is used in official medicine as a diuretic and antispasmodic agent 1. Madder dye is endowed with valuable healing properties, and for medicinal purposes, it is recommended to use the fruits, rhizomes, leaves, and stems of the plant. The plant’s medicinal properties are attributed to the presence of cardenolides, triterpenoids, rubifolic acid, and rubiconmarium acid.
The rhizomes of this plant contain coumarins and several anthraquinones, including purpurin, lucidin, alizarin, rubiadin, ruberythric acid, pseudopurpurin, rubiadin primveroside, nordamcantol, fiscin, and mollugin. The aboveground part of the plant contains coumarins, flavonoids, and iridoids such as asperuloside and deacetylasperuloside 4. Infusions and decoctions made from the rhizomes of this plant are widely used in Korean, Indian, Tibetan, and Chinese medicine for conditions such as amenorrhea, various gynecological diseases, dysmenorrhea, leucorrhea, and endometritis. Rhizomes are included in drugs recommended as prototypes for regulating salt metabolism. Rubia tinctorum L. (the root of madder dye) belongs to the Rubiaceae family and is extensively used in folk medicine across Asia and Europe for treating various diseases, including kidney stones. The plant’s therapeutic properties against inflammation, oxidation, and bacterial infections have been confirmed through in vivo and in vitro studies. Madder dye inhibits stone formation, exhibits a diuretic effect, and has bactericidal properties 5, 6. The antimicrobial activity of madder was assessed using a low-diffusion method, and it was found that aqueous and alcoholic extracts of the plant are effective against some Gram-positive and Gram-negative bacteria, yeast, filamentous fungi, and actinomycetes 7. The use of madder dye is effective for treating calcium phosphate (vitlocite, apatite, hydroxyapatite, carbonatapatite) and struvite urolithiasis [6]. Rubia tinctorum L., a medicinal plant, is primarily used for treating kidney stone disease. A study was conducted to evaluate the effect of the active substances in this plant on kidney stones using a model induced in rats with ethylene glycol and ammonium chloride. The aim of the study was to investigate the protective effect of an ethanol extract of Rubia tinctorum L. and to identify the active substances in the extract associated with this property. To ensure the rational use of natural resources and to prevent further disruption of the existing balance in the natural environment, it is essential to create artificial plantations of medicinal plants. This approach not only helps in preserving a significant portion of plant diversity but also supports the cultivation of introduced plants. Expanding the base of regional plant resources through such cultivation meets a variety of human needs and helps sustain medicinal plant resources (Figure 1).
 |
Figure 1: Rhizome of Rubia tinctorum plant. |
Material and Methods
Chemicals
Methods for cultivating Rubia tinctorum seeds in vitro and from seeds are being explored. The primary goals of plant introduction programs are to enhance biodiversity with economically beneficial species, ensure the responsible and efficient use of natural resources, and preserve the genetic diversity of indigenous plant species. Currently, research is being conducted on the introduction of various plant groups, including ornamental, medicinal, edible, and forage species. Among these, medicinal plants are particularly important as they are sources of biologically active compounds. These compounds are crucial not only for medicine but also for the essential functions of plants. They participate in oxidation-reduction reactions, respiration, and photosynthesis, regulate growth and development, ensure tissue thermoregulation, and protect plants from diseases and pests.
The specific characteristics of medicinal plants, such as their metabolic processes and the selective accumulation of certain secondary metabolites associated with particular enzyme systems, are vital for plant adaptation to the environment. These characteristics also enable plants to adapt to new environmental conditions.
Medicinal plant breeding organizations are relatively few. Plants that are harvested for their roots do not germinate the following year, making the reproduction of such medicinal plants particularly urgent. The state has outlined several tasks for the sustainable use of natural medicinal plant reserves in Uzbekistan, including the establishment of plantations for specific medicinal plants and the increase in the processing and export of their raw materials. This emphasizes the importance of reproducing medicinal plants.
Currently, many scientists are conducting pioneering research on the cultivation and biology of medicinally valuable plants at the Institute of Bioorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan. Additionally, the laboratory of “Experimental Biology” at Gulistan State University is working on cultivating Rubia tinctorum (dyed madder) both in vitro and from seeds.
To initiate the process of cultivating Rubia tinctorum L. under in vitro conditions, the initial material is obtained from the tissues of actively growing green branches or mature cuttings. These samples undergo rigorous sterilization and are then washed in 70% ethanol to ensure that the green buds remain uncontaminated. The sterilized green branches or mature cuttings are placed in a nutrient medium within a biological test tube. Under simulated natural light conditions, the temperature is maintained at 25–28°C during the day and drops to 20–22°C in the evening. Rooting of the green shoots occurs within 8–10 days, while branching branches may require 40–60 days for successful establishment.
In cases where there is a shortage of initial raw material for introduction, plants cultivated in vitro can be propagated using one-bud cuttings, which are then replanted. These in vitro-developed plants are transferred to a controlled environment, such as a greenhouse, where they are grown on a substrate consisting of two layers: sand and wood sawdust. The substrate is composed of a lower layer of wooden sawdust and an upper layer of a simpler substrate. In hydroponic systems, the best results are achieved using large river sand or diorite as substrates 8-12.
For the first 12–13 days after planting, direct light should be excluded from reaching the plants. To increase humidity, a film should be placed over the substrate at a height of 30–60 centimeters. The film should be removed once a day for a few minutes to allow for ventilation while keeping the plant leaves moist. Depending on the temperature, watering should be done 1–2 times per day. If the greenhouse is equipped with artificial mist-generating devices, additional covering of the plants with film may not be necessary. During the initial 30–60 days, the root system will develop within the upper layer of the substrate. Depending on the specific biological characteristics of the variety and the planting time, mature branches in the greenhouse can reach a height of 0.4–0.5 meters. In the autumn, the seedlings are transplanted from the greenhouse into an open field. The process is illustrated in a cup-shaped room designed for the in vitro cultivation of dye-bearing rootstocks 8-12. The development and propagation of the technology for cultivating the dyed madder plant involve a series of meticulous steps. In fields designated for this purpose, 500 kilograms of organic fertilizers per hectare are distributed at a depth of 28–30 centimeters before plowing in early autumn, incorporating superphosphate. It is advisable to supplement this with 30 kilograms of nitrogen and potash fertilizers per hectare if the soil shows a deficiency in these nutrients. Additionally, the dye can be propagated from both seeds and rhizome cuttings. Before planting, the seeds are soaked for 12 hours in a solution of 0.002 percent potassium permanganate mixed with copper. In early spring, the prepared land is further cultivated. When soil temperatures reach 10–12°C, approximately 13–15 kilograms of fertilizer per hectare are applied in mid-March. The seeds should be planted at a depth of 4–5 centimeters. Under conditions of moderate soil temperature and adequate moisture, the germination rate should be between 75 and 80 percent, with seeds typically germinating within 10 to 12 days. When propagating using rhizome cuttings, it is essential that the tensile strength of the rhizomes is at least 80–90%. The rhizomes should be planted at a depth of 8–10 centimeters in early spring, using approximately 10–12 tons of rhizomes per hectare. The planting density should be 10–15 plants per meter. If rows of seeds or cuttings are spaced 60 centimeters apart, the plants will develop robust root systems in subsequent years, allowing for complete coverage of the soil surface [9]. The dyed madder plant requires irrigation seven to eight times during its first year, with this frequency decreasing in subsequent years. During the first year, it is also necessary to loosen the soil between plants and remove any weeds. The first application of fertilizer is carried out in May and June, with 30 kilograms of nitrogen and 20 kilograms of potassium per hectare. The second application occurs in August, providing 30 kilograms of nitrogen and 20 kilograms of superphosphate. Fertilizing should be done before irrigation. In the second and third years of cultivation, when the initial fruits begin to turn brown, they are harvested before fully ripening. The roots and rhizomes are extracted from the soil in late autumn or early spring by turning the soil with a tool approximately 30–35 centimeters deep. The extracted material is then rinsed with water and dried in a dryer at a temperature ranging from 45 to 50 degrees Celsius. Seeds of the dyed madder are harvested either manually or with specialized machinery. On average, between 80 and 100 kilograms of seeds and between 13 and 14 centners of dry root material can be obtained from each hectare of cultivated land. We have conducted initial introduction trials with the studied varieties of medicinal plants, considering their ontogenetic and phenological characteristics. Our introduction studies on dyed madder revealed that the species, when grown in the Sirdaryo region of Uzbekistan, undergo all stages of ontogenesis, enter the reproductive phase, and produce viable seeds. In other words, the samples we studied demonstrate successful adaptation. Considering the resilience of the introduced specimens from various life forms and diverse geographical origins, these medicinal crops can be regarded as potential sources of plant resources, contributing to the expansion of the region’s resource base. Moreover, the conservation of medicinal plants, whether in their natural habitats or cultivated settings, is essential for preserving the valuable components of the region’s phytodiversity. The mineral element content in the seeds of Rubia tinctorum L. was determined using the X-ray fluorescence spectrometry method. In developing effective medicines from locally available raw materials, it is essential to implement comprehensive measures to conduct scientific research at the highest level and to provide the domestic pharmaceutical market with high-quality products. Significant practical results have been achieved in developing competitive preparations derived from natural plant-based materials based on these measures. Exploring the biology of dyed madder (Rubia tinctorum L.) is particularly important, as it lays the foundation for creating local materials necessary for producing affordable and high-quality substitutes for imported natural medicines from domestic resources. These preparations can be differentiated from the medicinal plant Rubia tinctorum L. and find applications in both medical practice and traditional medicine. Plants have the remarkable ability to assimilate a wide range of elements from their environment, both in minute quantities and in substantial amounts. However, only 19 elements are identified as essential for plant growth and development, and cannot be substituted by other elements. These critical elements include carbon, hydrogen, oxygen, nitrogen, phosphorus, sulfur, potassium, calcium, magnesium, iron, manganese, copper, zinc, molybdenum, boron, chlorine, sodium, silicon, and cobalt. Of these, 16 are classified as mineral elements. Carbon, hydrogen, and oxygen are absorbed by plants in the forms of CO2, O2, and H2O, respectively. Plants acquire water and all essential mineral elements through their roots from the soil. Minerals exist in soil solutions in both organic and inorganic forms, as well as adsorbed onto soil colloids. The uptake of ions by plants is influenced not only by their availability in the soil but also by factors such as the concentration of specific ions in the soil solution, their mobility within the soil matrix, and soil chemical reactions. The majority of the constituents of plant matter consist of four fundamental elements: carbon, hydrogen, oxygen, and nitrogen (Table 1).
Table 1: The amount of macro-and microelements in the root of the plant Rubia tinctorum L.
|
№ |
Elements |
The amount, mg/g |
№ |
Elements |
The amount mg/g |
№ |
Elements |
The amount mg/g |
|
1 |
Al |
451,110 |
15 |
Zn |
7,699 |
29 |
As |
0,258 |
|
2 |
Ba |
41,515 |
16 |
P |
2139,666 |
30 |
Zr |
0,422 |
|
3 |
Bi |
0,020 |
17 |
Pb |
1,609 |
31 |
Nb |
0,037 |
|
4 |
Ca |
94970,086 |
18 |
Ni |
5,889 |
32 |
Mo |
0,242 |
|
5 |
Fe |
874,553 |
19 |
Be |
0,019 |
33 |
Ag |
0,038 |
|
6 |
K |
S |
20 |
B |
26,873 |
34 |
Cd |
2,522 |
|
7 |
Li |
1,128 |
21 |
Si |
541,173 |
35 |
In |
0,001 |
|
8 |
Mg |
2402,037 |
22 |
S |
161,204 |
36 |
Cs |
0,079 |
|
9 |
Na |
734,728 |
23 |
Ti |
27,613 |
37 |
Ta |
0,005 |
|
10 |
Mn |
61,990 |
24 |
Gr |
3,924 |
38 |
W |
0,154 |
|
11 |
Rb |
11,740 |
25 |
Co |
0,437 |
39 |
Re |
0,001 |
|
12 |
Se |
0,121 |
26 |
Cu |
6,610 |
40 |
Hg |
-1,417 |
|
13 |
Sr |
602,121 |
27 |
Ga |
1,917 |
41 |
TI |
0,041 |
|
14 |
V |
1,023 |
28 |
Ge |
0,008 |
42 |
U |
0,049 |
The root of Rubia tinctorum L. contains 42 chemical elements, with their quantities determined through analysis for heavy metals, macroelements, and microelements. Notably, the root’s content of calcium (Ca) is 94,970.086 mg/g, magnesium (Mg) is 2,402.037 mg/g, and phosphorus (P) is 2,139.666 mg/g, which are significantly higher compared to other elements. Rubia tinctorum L. contains anthracene derivatives of the alizarin series and ruberitrinic acid, which are used for their pain-relieving, urinating, antimicrobial, and kidney-stone-dissolving effects. Ruberitrinic acid helps acidify urine and is used to dissolve phosphate stones. It also alleviates urinary tract and kidney pain, facilitating the passage of small stones, and has a diuretic effect that helps lower kidney stone levels. Additionally, Rubia tinctorum L. lowers tension and strengthens the movement of smooth muscles, aiding in the painless release of small stones and sand in kidney-stone disease. The use of this remedy results in reduced pain and improved patient condition. Currently, phytopreparations with such properties are used in urology, but there is a shortage of kidney-stone disease treatments registered in the State Register of Uzbekistan. In this research, we initially explored whether it was possible to prevent the development of nephrolithiasis in an experimental rat model exposed to a 0.75% ethanol extract of Rubia tinctorum L. combined with a 2% solution of ammonium chloride. We also assessed the potential antioxidative properties of these extracts and their polyphenol content. Administration of ethylene glycol and ammonium chloride over a period of 10 days resulted in the formation of calcium oxalate crystals in the urine. In the rat model exposed to ethylene glycol and ammonium chloride, the application of an extract rich in antioxidants and polyphenols significantly reduced the levels of calcium oxalates and effectively prevented kidney tissue damage associated with the disease. The experimental model of kidney-stone disease was established in accordance with the method described for rats 13. The collection of plant material for our research was conducted in June 2023 in the Navoi region of Uzbekistan. The plant, Rubia tinctorum L., was carefully selected and prepared for further analysis. The botanical classification of this plant was confirmed by Professor Kushiev X.X., a distinguished expert in the field of botany from the Department of Biology at the Faculty of Natural and Life Sciences at Gulistan University in Uzbekistan. The preparation of the extract from Rubia tinctorum involved converting the dried roots into a powder, which was then extracted with 70% ethanol at temperatures ranging from 75°C to 79°C for 15 hours using a Soxhlet extractor. The resulting extract was evaporated at 45°C to yield a concentrated extract with a 12.75% concentration. To isolate successive fractions of the extract, ethyl acetate, hexane, butanol, and distilled water were used. These polar solvents facilitated the separation of different components, achieving the final concentration of 12.75% by weight. After a three-day adaptation period, the male rats were divided into four groups of six. The first group consisted of healthy animals and was provided only with distilled water for ten days. The second group, the control group, received distilled water supplemented with 0.75% ethylene glycol and a 2% ammonium chloride solution for ten days. The third group was given a histone preparation along with distilled water containing 0.75% ethylene glycol and a 2% ammonium chloride solution for ten days. Finally, the fourth group was administered Rubia tinctorum L. extract along with distilled water enriched with 0.75% ethylene glycol and a 2% concentration of ammonium chloride for ten days. The substances studied were introduced into the animals’ stomachs via a special probe for 10 days. The animals were divided into six groups, with the healthy group receiving only water. The experimental groups were induced with kidney-stone disease. On the 11th day after the introduction of the substances, the kidneys of the animals were collected and prepared for histological examination.
Results and Discussion
Histopathological Analysis
After decapitation, the rats’ kidneys were placed in a 10% formaldehyde solution, dehydrated with ethanol solutions, impregnated with ethanol-benzene, and sliced into 4-micron-thick sections using a Microtome CUT 5062. The sections were stained with hematoxylin and eosin and then analyzed under a light microscope. In the group of animals injected with Madder oil extract, the following pathomorphological changes were observed in the rat kidney tissue: expansion of the glomerular capsule cavity in the cortical region and an increase in eosinophilic, oxyl-rich tumor fluid in the kidneys (black arrows) 14. The capillaries exhibited overflow and adhesion of red blood cells (white arrows) (A); degenerative and necrotic changes were seen in the diapedesis of erythrocytes (white arrows) and surrounding tissues, resulting from the unusual appearance of the glomeruli and impaired capillary wall permeability (B). Damage to the glomerular capsule and vascular wall led to the release of extravasate (C). These changes resulted in the detection of glomerulosclerosis and hemosiderin pigments (white arrows) (D). Vacuole degeneration and necrosis of the nephrothelium were observed in the bone marrow region. Cellular detritus, desquamation, and an oxyl plug were visible in the canal cavity 15. Vasodilation, fullness, and adhesion of erythrocytes (A) were noted, along with mononuclear infiltration and fibroblast proliferation in the interchannel space (C). Additionally, vacuole degeneration and necrosis of the epithelium of the collecting tubes (C) were observed, with a dense, homogeneous, hyaline-like plug found in the tube cavity of the papillary sphere (D). In a group of animals injected with Madder dye extract into the kidney tissue, nonspecific dystrophic and necrobiotic changes, tumors resulting from glomerular filtration disorders, and extravasates and cylinders such as hyaline ones were diagnosed 16. However, symptoms of nephrolithiasis, specifically salt crystals, were not detected (figure 2).
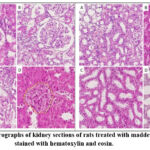 |
Figure 2: Micrographs of kidney sections of rats treated with madder dye extract, stained with hematoxylin and eosin. |
Conclusion
The results of our studies indicate that the oral administration of Rubia tinctorum L. extract effectively prevented the development of kidney-stone disease in rats. The extract of Rubia tinctorum L. was found to be particularly effective, demonstrating a significant nephroprotective effect due to its specific and rich composition of terpenes and terpenoids. Our findings suggest that Rubia tinctorum L. can serve as an effective alternative to existing medications for kidney-stone diseases and is recommended as a viable remedy.
Acknowledgement
The author like to thank Applied Research Program of the Ministry of Higher Education, Science and Innovation Republic of Uzbekistan.
Conflict of Interest
The authors do not have any conflict of interest.
Funding Source
The research is funded by the Innovative Development Agency under the Ministry of Higher Education, Science and Innovation, grant number project А-М3-2019-41
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
References
- Cuoco G., Mathe C., Archier P., Vieillescazes C. Characterization of madder and garancine in historic French red materials by liquid chromatography-photodiode array detection. Journal of cultural heritage. 2011; 12(1): 98-104.
CrossRef - Westendorf J., Poginsky B., Marquard H., Groth G., Marquard H. The genotoxicity of lucidin, a natural component of Rubia tinctorum L., and lucidinethylether, a component of ethanolic Rubia extracts. Cell Biology and Toxicology. 1988; 4:225-239.
CrossRef - Clementi C., Nowik W., Romani A., Cibin F., Favaro G. A spectrometric and chromatographic approach to the study of ageing of madder (Rubia tinctorum L.) dyestuff on wool. Analytica chimica acta. 2007; 596(1): 46-54.
CrossRef - Derksen G. C., Niederländer H. A., Van Beek T. A. Analysis of anthraquinones in Rubia tinctorum L. by liquid chromatography coupled with diode-array UV and mass spectrometric detection. Journal of chromatography A. 2002; 978(1-2): 119-127.
CrossRef - Ozen E., Yeniocak M., Goktas O., Alma M. H., Yilmaz, F. Antimicrobial and antifungal properties of madder root (Rubia tinctorum) colorant used as an environmentally-friendly wood preservative. Bioresources. 2014; 9(2): 1998-2009.
CrossRef - Agnhage T., Zhou Y., Guan J., Chen G., Perwuelz A., Behary N., Nierstrasz V. Bioactive and multifunctional textile using plant-based madder dye: Characterization of UV protection ability and antibacterial activity. Fibers and polymers. 2017; 18: 2170-2175.
CrossRef - Kalyoncu F., Cetin B., Saglam, H. Antimicrobial activity of common madder (Rubia tinctorum L.). Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2006; 20(6): 490-492.
CrossRef - Kurkin V. A., Shmygareva A. A., Rybalko M. V., Daeva E. D., Kadentsev, V. I. Xanthopurposide, A New Anthraglycoside from Rubia tinctorum Rhizomes. Chemistry of natural compounds. 2021; 57: 14-15.
CrossRef - Marhoume F. Z., Aboufatima R., Zaid Y., Limami Y., Duval R. E., Laadraoui J., Belbachir A., Chait A., Bagri A. Antioxidant and polyphenol-rich ethanolic extract of Rubia tinctorum L. prevents urolithiasis in an ethylene glycol experimental model in rats. Molecules. 2021; 26(4): 1005.
CrossRef - Khushvaqova M. A., Islomov A. X., Jalmurodova D. D., Ishmuratova A. S. Determination of Macro and Micro Elements in Raisbased Bioactive Additives and Content. European Scholar Journal. 2021; 2(4): 305-311.
- Ishmuratova A. S., Ibragimov B. T., Toraev A. S., Ashurov J. M., Baratov Q. R., Khajibaev T. A., Islamov A. Painter royan obtain dry extract of the root of the plant and determination of acute toxicity. American Journal of Applied Science and Technology. 2023; 3(08): 28-37.
- Khushvaqova M. A., Islomov A. X., Jalmurodova D. D., Ishmuratova A. S. Determination of Macro and Micro Elements in Raisbased Bioactive Additives and Content. European Scholar Journal. 2021; 2(4): 305-311.
- Ishmuratova A. S., Islomov A. X., Abdimalikov I. I. Rubia tinctorum L. o ‘simligini ildizining mineral elementlari va tabobatda qo‘llanilishi. Academic research in educational sciences. 2022; 3(5): 1214-1220.
- Fan J., Glass M. A., Chandhoke P. S. Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Scanning Microsc. 1999; 13(2-3): 299-306.
- Bouanani S., Henchiri C., Migianu-Griffoni E., Aouf N., Lecouvey M. Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. Journal of ethnopharmacology. 2010; 129(1): 38-45.
CrossRef - Abbagani S., Gundimeda S.D., Varre S., Ponnala D., Mundluru HP. Kidney stone disease: etiology and evaluation. Int J Appl Biol Pharmaceut Technol. 2010; 1:175–82.
- Baheti D. G., Kadam S. S. Antiurolithiatic activity of a polyherbal formulation against calcium oxalate induced urolithiasis in rats. Journal of advanced pharmacy education and research. 2013; 3(1): 31-41.







