Najam Siddiqi1 , Faisal Moin2*
, Faisal Moin2* and Mohammad A. Al Kindi3
and Mohammad A. Al Kindi3
1Department of Anatomy and Neurobiology, College of Medicine and Health Sciences, National University of Sciences and Technology, Sohar, Oman.
2Department of Medicine, College of Medicine and Health Sciences, National University of Sciences and Technology, Sohar, Oman.
3Department of Pathology, College of Medicine and Health Sciences, Sultab Qaboos University, Muscat, Alkoudh, Oman.
Corresponding Author E-mail: drfaisalmoin@nu.edu.om
DOI : https://dx.doi.org/10.13005//bpj/2970
Abstract
The electromagnetic environment surrounding us has dramatically evolved over the past decade, with the proliferation of Wi-Fi, Bluetooth, and other wireless technologies becoming commonplace in our daily lives. Mobile phones emit non-ionizing low-frequency electromagnetic waves (EW). To examine the effects of EW on living cells, this study aims to explore the impact of cell phone EW on the developing brain of chick embryos. The fertilized eggs were allowed to develop under exposure to electromagnetic waves emitted by cell mobile. A cell phone was placed inside the incubator with 20 eggs and was called from outside on a precise schedule. The same number of fertilized eggs were placed in another incubator without a mobile phone and served as the control. Embryos were sacrificed on days 10 and 15, and the cerebral cortex and cerebellum were removed and sent for electron microscopy. In the control group, cerebral neurons appeared healthy, with a large, centrally placed nucleus, visible oligodendrocytes, and a less dense extracellular matrix. In contrast, neurons from the exposed group were smaller, fewer in number, with unclear nuclear margins, signs of shrinkage, and apoptosis and a dense extracellular matrix. In the cerebellum, the exposed group revealed a reduced number of Purkinje neurons and noticeable mitochondrial swelling. The blood-brain barrier remained intact in the control group but was compromised in the exposed group. We conclude that electromagnetic waves emitted by cell phones adversely affect the normal development of the brain in chick embryos.
Keywords
Blood Brain Barrier; Electromagnetic Waves; Mobile Phone; Neuron
Download this article as:| Copy the following to cite this article: Siddiqi N, Moin F, Al-Kindi M. The Impact of Mobile Phone Electromagnetic Waves on the Neurons and Blood Brain Barrier Integrity in the Chick Embryo. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Siddiqi N, Moin F, Al-Kindi M. The Impact of Mobile Phone Electromagnetic Waves on the Neurons and Blood Brain Barrier Integrity in the Chick Embryo. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3N7HDHl |
Introduction
Mobile phones utilize non-ionizing low electromagnetic waves (EW), causing rapid environmental pollution due to exposure to radio waves at frequencies of 800-900 Mega Hz. Excessive exposure to these EW may increase the production of Radical Oxygen Species (ROS). Excessive use of mobile phones by teenagers and pregnant mothers raises concerns due to their increased radiosensitivity. Teenagers developing bodies and the rapid division of embryonic cells in fetuses potentially heighten their vulnerability to electromagnetic waves. Cell phone exposure to prenatal and postnatal children resulted in behavioral problems 1. These electromagnetic waves affect the brain and memory 2. Xu demonstrated mt DNA and levels of mitochondrial RNA in the neuron was affected by the electromagnetic fields 3-4. High levels of electromagnetic waves in mother’s bedroom during pregnancy may cause autism in the baby 5.
The effect of radio waves in the adult brain was also a point of interest for investigators due to its proximity to the brain and ears while talking on mobile phones. Reports mention increased headache, fatigue, irritability, stress, sleepiness, concentration difficulties, nausea, lack of appetite, blurred vision, depression 6-12. Disturbances in sleep and alteration of synaptic plasticity, neurotransmitter release and life cycle of nerve cells because of the use of cellular phone 13-14. Neuropsychiatric changes such as depression, somatization, obsessive compulsivity, phobic anxiety, paranoid ideation, sleeping disturbances were also reported in literature 14-18. The increasing incidence of depression and increasing use of mobile phones seems to directly relate to each other suggests memory disfunction, attention dysfunction, decreased motor function, decelerated reaction time and lowered neuromuscular strength 19. Asad reported a positive relationship between anxiety and depression with smartphone addiction in South Korea 20. In another study, Siddiqi also mentioned the excessive use of smartphones by medical students 21.
Salford reported that prenatal and postnatal exposure of radio waves stimulate neural cell death and prevents stem cells to change into adult neurons 4. It is now understood that many neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis may be due to damage to mitochondria of the neurons 22-28. Furthermore, Down syndrome 29-30, Huntington’s disease 31, Friedreich ataxia; oxidative damage of mitochondria is most likely playing a major role in the pathogenesis of these diseases 32-34.
Not much research has been done on the negative side effects of EW on the nervous system. The study revealed more profound knowledge on the effects of EW exposure on the neurons, neuroglia, blood brain barrier and brain intercellular matric.
Hypothesis
The radio waves affect the developing neurons and the neuroglia in the developing chick embryo.
Objectives
To expose the developing chick embryo with EW for 10 and 15 days starting from day zero.
To examine the morphological changes in the cerebral cortex and cerebellum at days 10 and 15 of embryo development in the control and exposed groups.
Observe the blood brain barrier in the exposed and control groups.
Materials and Methods
Chicken fertilized eggs (‘Cobb’ Gallus gallus domesticus) were received from Sohar poultry. This animal model has been used extensively for research purposes 35.
An incubator (Model EH-35, Sino-PFE Company, China) with a capacity of holding 30 eggs at a time and with computerized control of temperature, humidity and air ventilation was utilized (Fig.1). Eggs were rotated ten times per day. Forty zero-day fertilized eggs were divided equally at random into control and exposed groups. In each group, 20 eggs were placed inside the incubator and the temperature was set to 37 degrees and humidity 50-60% by the control panel.
In the exposed groups, the eggs were subjected to EW released by a cell phone placed inside the incubator, while in the control group the eggs were unexposed to EW. A specific branded cell phone with a common cellular service provider was used, operating at 1800 MHz, with a power output of 0.47 W/kg and SAR of 1.10 W/kg (head). The intensity of the EW during the experiment was measured using a Tri Field Meter, model 100XE. The cell phone was activated for 5 minutes at a time, ten times daily, by calling from another cell phone outside the incubator, with no exposure periods in between. Calls were not made during the night. This resulted in a total daily exposure duration of 50 minutes, starting from day zero. The chick embryos were sacrificed on day 10 (500 minutes of exposure) and day 15 (750 minutes of exposure). The egg fully develops in 21 days, day 10 and 15 of development is a reasonably good interval to observe the effects on cells and had been used in other studies35. The eggshell was carefully served by scissors, the membranes were removed around the embryo, the chest wall then opened with sharp micro scissors, and the heartbeat was observed. The brain of the embryo was dissected and removed after opening the bones of the skull. The cerebral cortex and cerebellum of the brain were dissected, and specimens were removed for fixed for EM studies (Fig.2).
 |
Figure 1: A 30-egg incubator
|
 |
Figure 2: Day 10 a) Removal of the embryo on day 10 b) brain at day 10. c) Opening of the skull of chick embryo on day 15 d) brain at 15.
|
Control Group
20 eggs were incubated under the same environment except that the cell phone was placed without a battery to make sure that there is no EW emitted. The embryos were dissected at day 10 and 15, brain was removed and specimens of cerebral cortex and the cerebellum were removed and fixed for EM study.
Electron microscopy (EM)
Brain specimens were fixed in glutaraldehyde for 5 hours, followed by washing in buffer solution. Further processing for the EM was done at EM Laboratory at Sultan Qaboos University, Oman. Same number of specimens of cerebral cortex and cerebellum in both the groups were taken to the EM lab for processing. Processing of the samples were done based on a protocol by Bozzola and Russell with a few changes. All samples were immediately placed on vials containing EM fixative (2.5% Glutaraldehyde) and left for 2 hours. Tissues were then washed twice using cacodylate buffer (PH 7.2-7.4) 10 minutes each, post fixation was carried using osmium tetroxide for one hour and dehydration was done using series of acetone starting from washing with distilled water, 25% acetone, 75% acetone, 95% acetone and 99.9% acetone. Every step was carried for 10 minutes. Tissues then were placed in 1:1 acetone to pure epoxy resin for one hour then in 1:3 acetone to pure epoxy resin for half an hour. Next it was placed in pure epoxy resin for one hour followed by fresh pure resin for half an hour. Embedding was carried out in size 00 embedding beam capsules (Agar) and polymerized in 70°C oven for 12 hours.
All tissue blocks were cut using Lieca ultramicrotome for semi thin sectioning (0.5 um) with glass knives to allocate the areas of interest using light microscopy then were again cut to have ultrathin sections (60-90 nm thick) with diamond knives and picked up on 300 mesh cupper grids. All sections were stained using uranyl acetate and lead citrate. Sections were then observed using JEOL JEM-1230 EX Japan transmission electron microscope and EM photos were subsequently analyzed.
Results
Brain: 1) TEM of Cerebral cortex: Control group
Day 10: Cerebral cortex of the chick embryo showing large well-developed neurons (pyramidal neurons and oligodendrocytes). The nerve fibers can be seen occupying the space in between the neurons; however, many empty spaces could be seen in between the neurons (Fig.3a.b).
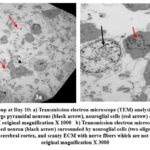 |
Figure 3: Control group at Day 10: a) Transmission electron microscope (TEM) analysis shows multiple well developed healthy large pyramidal neurons (black arrow), neuroglial cells (red arrow) and thin extracellular matric (ECM). |
Day 15: Cerebral cortex of the chick embryo shows large well-developed neurons (pyramidal neurons) and oligodendrocytes and clear nucleus. The nerve fibers are well developed and organized regularly with small gaps in between. (Fig.4 a, b).
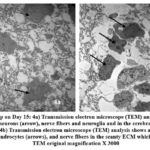 |
Figure 4: Control group on Day 15: 4a) Transmission electron microscope (TEM) analysis shows many well-developed pyramidal neurons (arrow), nerve fibers and neuroglia and in the cerebral cortex. |
Brain: 2) TEM of Cerebral cortex: Exposed group
Day 10: The neurons showing changes of apoptosis, chromatin condensation, cellular membrane not visible as compared to the control group. Neurons become shrunken and appear smaller than healthy neurons, with condensed cytoplasm and reduced organelle density. The loss of cytoplasmic volume is often accompanied by a distorted cellular shape and irregular contours. The extracellular matrix (ECM) becomes dense and dark and much fewer white gaps visible thus filling most of the matrix. Axons in the matrix are not clearly seen (Fig.5a).
Day 15: Shrunken and atrophied neurons with chromatin condensation and degenerative alteration were observed in the pyramidal neurons; nucleus membrane blebbing was also apparent. The extracellular matrix (ECM) became very dense and disorganized when compared to the control group. (Fig 5b,c).
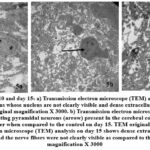 |
Figure 5: Exposed day 10 and day 15: a) Transmission electron microscope (TEM) analysis on day 10 shows multiple cortical neurons whose nucleus are not clearly visible and dense extracellular matrix as compared to the control. |
Brain: 3) TEM of Cerebellum: Control
Day 10: cerebellum shows multiple large neurons and patchy extracellular matrix with nerve fibres. These large neurons are most likely Purkinje cells. The extracellular matrix is less dense with empty spaces in between the neurons. (Fig.6a).
Day 15: Multiple large neurons with clear margins and prominent nucleoli can be seen. The extracellular matrix density is increased than day 10 (Fig 6b). The mitochondria in the neurons were oval or slender in shape (Fig.8a). A normal blood brain barrier can be seen with tight junctions between the capillary endothelia (Fig.9a.).
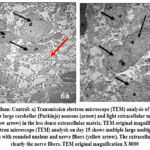 |
Figure 6: Cerebellum: Control: a) Transmission electron microscope (TEM) analysis of control group on
|
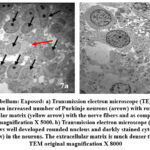 |
Figure 7: Cerebellum: Exposed: a) Transmission electron microscope (TEM) analysis on day 10 shows an increased number of Purkinje neurons (arrow) with rounded nucleus, dense extracellular matrix (yellow arrow) with the nerve fibers and as compared to control.
|
Brain: 4) TEM of Cerebellum: exposed group
Day 10: Multiple large neurons were seen and nucleus showing chromatin condensation. The extracellular matrix is much denser as compared to the control and many black apoptotic granules in the extracellular matrix were observed (Fig 7a).
Day 15: The neurons were shrunk, and chromatin condensation was apparent. The neurons show multiple rounded swollen mitochondria. (Fig.7b, 8b) At higher magnification the mitochondria can be seen swollen and irregular in shape and rupture of the inner and outer membranes was noted (Fig 8c). A blood brain barrier shows disruption of the tight junctions and damage of capillary endothelium (Fig.9b.).
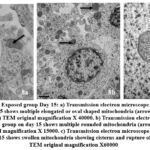 |
Figure 8: Cerebellum: Exposed group Day 15: a) Transmission electron microscope (TEM) analysis in the control group on day 15 shows multiple elongated or oval shaped mitochondria (arrow) with cisterns present in the neurons (arrow) TEM original magnification X 40000.
|
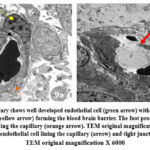 |
Figure 9: a) The capillary shows well developed endothelial cell (green arrow) with tight junction (black arrow) and pericyte (yellow arrow) forming the blood brain barrier. The foot process of astrocytes can also be seen surrounding the capillary (orange arrow).
|
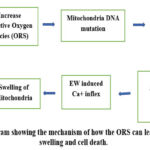 |
Figure 10: The diagram showing the mechanism of how the ORS can lead to mitochondrial swelling and cell death.
|
Discussion
EW adverse effects were studied in the chick embryo brain tissues on days 10 and 15 of its fetal development. The effects of the electromagnetic waves are classified as thermal and non-thermal. Thermal effects are now controlled by strict regulation on Specific Absorption Rate (SAR); this is a measure of the rate at which energy is absorbed per unit mass by the body when exposed to EW. Regulatory bodies like the FCC set limits on SAR to ensure safety (e.g., 1.6 W/kg in the U.S.). Non-thermal effects such as oxidative stress, damage to mitochondria, cell wall, DNA etc. need further research. This study explores the effects of EM on the neurons, neuroglia and extracellular matrix in a developing chick embryo. In the control groups, neurons were well developed surrounded by oligodendroglia cells and the nerve fibers were showing increasing density from days 10 to 15. In the exposed groups, the neurons showed shrunken morphology with degenerative alteration, nucleus not clearly visible, extracellular matrix became very dense and disorganized as compared to the control. Oligodendrocytes were also not clearly visible.
Sadequl Islam reported darkly stained nuclei in degenerated neurons and decrease in healthy neurons when the chick embryo was exposed with electromagnetic waves 36. On day 14 of chick development, the number of degenerated neurons with hyperchromatic deeply stained nucleus were significantly more in the group exposed than the healthy neurons in the control group (p=0.05).
Hasan 37 observed similar results and found fewer neurons in the exposed embryo. Apoptosis in a few neurons were also observed in the exposed group 38-41. Radiation causes an increase in superoxide dismutase, catalase and malondialdehyde levels in the embryo.
Eser reported that radio waves caused morphological alterations in the cerebral cortex, brain stem and cerebellum in albino male rats 42. They observed marked degenerative changes, decrease in cytoplasm and dark pyknotic nuclei and a smaller number of neurons in the EM exposed group versus the control group.
Further reported in literature that radio waves of 900 MHz have caused pyknotic neurons in the hippocampus, deeply stained granules in cerebellum 43-45. This study also observed deeply stained granules in the cerebellum which are stress granules (SRs). These stress granules inhibit stress-induced apoptosis and help the cell to repair.
Romeo did a meta-analysis on the fact that electromagnetic waves exposure causes apoptosis and found a mixed result; however, many studied reported apoptosis as a result of electromagnetic exposure 46.
Islam also reported an increase of VEGF-A expression in the exposed group which mean inflammation and hypoxia leading to oxidative stress induced by the radio waves 36. Vahid reported the electromagnetic radiation induced the entry of calcium via TEPV1 channel in hippocampus and dorsal root ganglia in rats resulting in apoptosis 47. Absence of mitochondrial function, rise in amyloid beta expression and stimulation of apoptotic factors e.g. caspase-9 and -3 in the hippocampus after EMR-2450 MHz exposure was reported 48.
Blood brain barrier is another structure which can be affected by electromagnetic waves. Persson reported that radiation from cell phones can damage the blood brain barrier in rats allowing albumin to enter the brain 49. This study observed very dense extracellular matrix due to which the nerve fibers cannot be identified. It seems that rupture of the blood brain barrier in the exposed group on day 15, resulted in leakage of albumin in the extracellular matrix giving it a dense matrix appearance. (Fig.7a, b).
Salford, L. G investigated the impact of microwave exposure from GSM mobile phones on rat brains, specifically looking at the blood-brain barrier and neuron damage and reported significant damage of the neurons of the cortex, hippocampus and basal ganglia because of albumin leakage through blood-brain barrier 50. Stam reported damage of blood brain barrier by radio waves exposure in animal model however stated that in humans the evidence of damage is still not reported 51. Leszczynski in his research focused on how mobile phone radiation can non-thermally activate hsp27 stress pathways in human endothelial cells, thereby increasing permeability of blood-brain barrier 52. Nittby also reported the permeability changes in blood-brain barrier of rats after exposure to GSM-900 mobile phone radiation and found significant correlation of albumin excavation and exposure level 53. Eberhardt, J. L. in his research provides an overview of how microwave radiation affects blood-brain barrier permeability, leakage of albumin into the extracellular matrix and resulting in neuron damage in rats54. Shabani also reported increased permeability of the blood-brain barrier in rats55. Gao examined albumin immunohistochemistry and Evans blue staining after exposure of rat’s brain to electromagnetic pulses. Zonula occludens were evaluated using western blotting, results revealed increased permeability of blood brain barrier 56. This research revealed that the blood brain barrier in the cerebellum was damaged, and leakage of albumin was evident in the extracellular matrix of the exposed group.
This study also reported the degenerative changes in the cerebral cortical and cerebellum neurons after exposure to radio waves. This exposure also affected the mitochondria in the cerebellum neurons and observed deformity of the mitochondria which became rounded instead of oval or elongated. This agrees with other reports mentioned in literature. Mitochondrial DNA damage due to oxidative stress in primary cultured neurons were observed 3. Increased permeability to calcium due to ROS production leads to mitochondrial swelling. Vicious cycle causing further increase in ROS triggers apoptosis (Fig.10). First sign of mitochondria cell membrane damage is its swelling, which increase with further increase production of ROS, and the mitochondria will become round. Oxidative stress cause mitochondrial DNA mutation, damage mitochondrial respiratory chain, alter membrane permeability and effect ca+ homeostasis and mitochondrial defense system 57. Mitochondria became swollen and vacuolated in motor neurons in mice 58. In this study, mitochondria were observed to be swollen in the cerebellum in the exposed group versus the control where they were oval and elongated. Permeability of mitochondrial membrane depends on communication between ca2+ and ROS system. A major cause of EM-induced calcium influx into the mitochondria is increased sympathetic activity 59. ROS when stimulated will produce free O +2 on the mitochondrial inner surface. This free O+ 2 will invade thiol protein to open up the transition pores, increases membrane permeability and causes mitochondria to swell 60. Mitochondria is the most sensitive organelles to oxidative stress and swells under such conditions 61.
To carry out highly specialized functions the neurons require more energy as compared to other cells. Mitochondria generate energy, and its dysfunction thus plays a major role in different neurodegenerative diseases. It is now understood that abnormal mitochondrial dynamics pertinent to neuronal synaptic loss and cell death may be a cause of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease 48. Abnormal mitochondrial morphology was reported in mutants of Parkinson’s disease-related genes.
Mitochondria are unique organelles within cells, distinct from the nucleus, as they contain their own DNA known as mitochondrial DNA. They are capable of synthesizing their own RNA and proteins, and has the mitochondrial respiratory chain on its inner membrane 62. This chain comprises five complexes—namely I, II, III, IV, and V. The first four complexes are accountable for converting adenosine diphosphate (ADP) into adenosine triphosphate (ATP)63.
1-5% oxygen is converted into ROS under normal conditions; mitochondria is primary source of intracellular ROS 64. Complex III is the major site of ROS production under typical metabolic conditions 65. Elevated ROS levels can damage these complexes and other mitochondrial macromolecules, including lipids, proteins, and DNA 66. Damage to mitochondrial DNA exacerbates oxidative stress, creating a harmful cycle of ROS generation and eventually leading to apoptosis, which can disrupt mitochondrial energy production 67,68.
Increased permeability of the mitochondrial inner membrane disrupts mitochondrial calcium homeostasis 60. Elevated calcium levels enhance the production of superoxide radicals, propagating a cycle of damage. Excessive calcium accumulation leads to osmotic swelling and damage of the outer mitochondrial membrane 69. Increased ROS production further compromises membrane integrity, boosts calcium uptake, and triggers apoptosis and cell death 46, 70.
Calcium ions must enter the neuron’s cytosol for neurotransmitter release 5. Neuronal signaling relies on the release of neurotransmitters, which is initiated by a brief influx of calcium into the cytosol. Exposure to radio waves can cause membrane leaks, elevating intracellular calcium concentrations. This heightened calcium level causes the cell to be overly responsive, releasing more neurotransmitters and leading to increased brain activity 71-74. Consequently, the brain can become overloaded with excessive signals, potentially resulting in concentration issues and attention deficit hyperactivity disorder (ADHD).
Conclusion
We concluded that electromagnetic waves have produced damage to the cerebral and cerebellar neurons and shown increased density in the extracellular matrix of the developing chick embryo because of damage to the blood-brain barrier. Brain blood-barrier damage and leakage of albumin are the most probable causes of the increased density in the extracellular matrix and neuronal damage. Swelling of the mitochondria and damage to its membrane might be due to an increase in oxidative stress caused by electromagnetic waves. To bridge our findings with broader health implications and an increase in the neurodegenerative disease in old age, further research is needed to understand how the observed cellular changes found in this study due to exposure of electromagnetic waves could translate into the development of neuro degenerative diseases.
Acknowledgements
Deeply acknowledge the Department of Pathology at SQU for processing the specimens for EM and allowing the use of the electron microscope. I am also thankful to our Dean, Prof. Mohammed Al Shafaee, whose tremendous encouragement is always stimulating us to continue research and allow us to visit SQU. 11, 16
Conflicts of Interest
The author(s) do not have any conflict of interest
Funding Sources
The study was supported by Oman Ministry of Higher Education (RG) grant no: MOHERI/BFP/RGP/18/161
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and with the main author. The raw data that support this study is available at the EM laboratory at Sultan Qaboos University, Oman, which are available from the 1st author upon reasonable request.
Ethics Statement
This article was approved by the research committee of the college of medicine and health sciences, National university of science and technology
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Author’s contribution
Prof. Najam Siddiqi: Writing the proposal, doing the experiment, collecting the data, analyzing the data, writing the paper
Dr Faisal Moin: Analyzing the data, writing the paper, collection the references, and putting them in order
Mr. Mohammed Al Kindi: Processing the EM mesh for EM, Taking the pictures and analyzing the data
References
- Divan HA, Kheifets L, Obel C, Olsen J. Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology. 2008 1;19(6):S94-5.
- Chaturvedi CM, Singh VP, Singh P, Basu P, Singaravel M, Shukla RK, Dhawan A, Pati AK, Gangwar RK, Singh S. 2.45 GHz (CW) microwave irradiation alters circadian organization, spatial memory, DNA structure in the brain cells and blood cell counts of male mice, mus musculus. Progress In Electromagnetics Research B. 2011; 29:23-42.
- Xu S, Zhou Z, Zhang L, Yu Z, Zhang W, Wang Y, Wang X, Li M, Chen Y, Chen C, He M. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain research. 2010 22; 1311: 189-96.
- Salford LG, Brun AE, Eberhardt JL, Malmgren L, Persson BR. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environmental health perspectives. 2003;111(7):881-3.
- Klinghardt, Dietrich, MD. Pilot Study. Does high radiation exposure during pregnancy cause autism. 2018: https://www.dqindia.com/high-radiation-exposure-pregnancy-cause-autism/
- Frey AH. Headaches from cellular telephones: are they real and what are the implications? Environmental health perspectives. 1998;106(3):101-3.
- Huber R, Treyer V, Borbely AA, Schuderer J, Gottselig JM, Landolt HP, Werth E, Berthold T, Kuster N, Buck A, Achermann P. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. Journal of sleep research. 2002;11(4):289-95.
- Bortkiewicz A, Zmyślony M, Szyjkowska A, Gadzicka E. Subjective symptoms reported by people living in the vicinity of cellular phone base stations: review. Medycyna pracy. 2004 1;55(4):345-51.
- Chu MK, Song HG, Kim C, Lee BC. Clinical features of headache associated with mobile phone use: a cross-sectional study in university students. BMC neurology. 2011;11:1-7.
- Dasdag S, Akdag MZ, Ulukaya E, Uzunlar AK, Ocak AR. Effect of mobile phone exposure on apoptotic glial cells and status of oxidative stress in rat brain. Electromagnetic biology and medicine. 2009 1;28(4):342-54.
- Dwyer MJ, Leeper DB. A Current Literature Report on the Carcinogenic Properties of Ionizing and Non-: Ionizing Radiation. 1978.
- Salama OE, Abou El Naga RM. Cellular phones: are they detrimental? The Journal of the Egyptian Public Health Association. 2004 1;79(3-4):197-223.
- Wagner P, Röschke J, Mann K, Hiller W, Frank C. Human sleep under the influence of pulsed radiofrequency electromagnetic fields: a polysomnographic study using standardized conditions. Bioelectromagnetics: Journal of the Bioelectromagnetics Society, The Society for Physical Regulation in Biology and Medicine, The European Bioelectromagnetics Association. 1998;19(3):199-202.
- Lemola S, Perkinson-Gloor N, Brand S, Dewald-Kaufmann JF, Grob A. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. Journal of youth and adolescence. 2015 ;44(2):405-18.
- Oto R, Akdaǧ Z, Daşdaǧ S, Celik Y. Evaluation of psychologic parameters in people occupationally exposed to radiofrequencies and microwave. Biotechnology & Biotechnological Equipment. 1994 J 1;8(4):71-4.
- Eger H, Jahn M. Specific symptoms and radiation from mobile basis stations in Selbitz, Bavaria, Germany: evidence for a dose-effect relationship (original article in German). Umwelt Med. Ges. 2010; 23:130-9.
- Abdel-Rassoul G, Abou El-Fateh O, Abou Salem M, Michael A, Farahat F, El-Batanouny M, Salem E. Neurobehavioral effects among inhabitants around mobile phone base stations. Neurotoxicology. 2007 1;28(2):434-40.
- Conrad RH. Smart Meter Health Effects Survey and Report 2013. https://www.mainecoalitiontostopsmartmeters.org/wp-content/uploads/2013/01/Exhibit-10-Smart-Meter-Health-Effects-Report-Survey2.pdf
- Kolodynski AA, Kolodynska VV. Motor and psychological functions of school children living in the area of the Skrunda Radio Location Station in Latvia. Science of the Total Environment. 1996 2;180(1):87-93.
- Shahjehan A, Afridi SA, Haider M, Iqbal A, Aziz S, Mansehra P. Smartphone addiction and behavioral outcomes in South Korea: A systematic review and meta-analysis. Humanities & Social Sciences Reviews. 2021; 9 (2): 358-369
- Siddiqi N, Jahan F, Moin F, Al-Shehhi F, Al-Balushi F. Excessive use of mobile phones by medical students: should precautions be taken? Biomedical and Pharmacology Journal. 2017 21;10(4):1631-8.
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006 19;443(7113):787-95.
- Schüz J, Waldemar G, Olsen JH, Johansen C. Risks for central nervous system diseases among mobile phone subscribers: a Danish retrospective cohort study. PLoS One. 2009 5;4(2): e4389.
- Chinnery PF, Taylor GA, Howell N, Brown DT, Parsons TJ, Turnbull DM. Point mutations of the mtDNA control region in normal and neurodegenerative human brains. The American Journal of Human Genetics. 2001 1;68(2):529-32.
- Gao J, Wang L, Liu J, Xie F, Su B, Wang X. Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants. 2017 5;6(2):25.
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S. Oxidative damage is the earliest event in Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 2001 1;60(8):759-67.
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature genetics. 2006 1;38(5):515-7.
- Alqahtani T, Deore SL, Kide AA, Shende BA, Sharma R, Chakole RD, Nemade LS, Kale NK, Borah S, Deokar SS, Behera A. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis-an updated review. Mitochondrion. 2023 1;71:83-92.
- Valenti D, de Bari L, De Filippis B, Henrion-Caude A, Vacca RA. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: an overview of Down syndrome, autism, Fragile X and Rett syndrome. Neuroscience & Biobehavioral Reviews. 2014 1;46:202-17.
- Helguera P, Seiglie J, Rodriguez J, Hanna M, Helguera G, Busciglio J. Adaptive downregulation of mitochondrial function in down syndrome. Cell metabolism. 2013 8;17(1):132-40.
- Siddiqui A, Rivera-Sánchez S, Castro MD, Acevedo-Torres K, Rane A, Torres-Ramos CA, Nicholls DG, Andersen JK, Ayala-Torres S. Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free Radical Biology and Medicine. 2012 1;53(7):1478-88.
- Marobbio CM, Pisano I, Porcelli V, Lasorsa FM, Palmieri L. Rapamycin reduces oxidative stress in frataxin-deficient yeast cells. Mitochondrion. 2012 1;12(1):156-61.
- Xia H, Cao Y, Dai X, Marelja Z, Zhou D, Mo R, Al-Mahdawi S, Pook MA, Leimkühler S, Rouault TA, Li K. Novel frataxin isoforms may contribute to the pathological mechanism of Friedreich ataxia. PLoS One. 2012;7(10):e47847.
- Calabrese V, Lodi R, Tonon C, D’Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AG, Butterfield DA. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. Journal of the neurological sciences. 2005 15;233(1-2):145-62.
- Siddiqi N, Norrish M, Heming T. Growth Retardation of Chick Embryo Exposed To A Low Dose Of Electromagnetic Waves. Journal of Ayub Medical College Abbottabad. 2016 1;28(2):224-8.
- Islam MS, Islam MM, Rahman MM, Islam K. 4G mobile phone radiation alters some immunogenic and vascular gene expressions, and gross and microscopic and biochemical parameters in the chick embryo model. Veterinary Medicine and Science. 2023 ;9(6):2648-59.
- Hasan I, Jahan MR, Islam MN, Islam MR. Effect of 2400 MHz mobile phone radiation exposure on the behavior and hippocampus morphology in Swiss mouse model. Saudi Journal of Biological Sciences. 2022 1;29(1):102-10.
- Dasdag SÜ, Bilgin HM, Akdag MZ, Celik H, Aksen F. Effect of long-term mobile phone exposure on oxidative-antioxidative processes and nitric oxide in rats. Biotechnology & Biotechnological Equipment. 2008 1;22(4):992-7.
- Kesari KK, Behari J, Kumar S. Mutagenic response of 2.45 GHz radiation exposure on rat brain. International Journal of Radiation Biology. 2010 1;86(4):334-43.
- Misa-Agustiño MJ, Leiro-Vidal JM, Gomez-Amoza JL, Jorge-Mora MT, Jorge-Barreiro FJ, Salas-Sánchez AA, Ares-Pena FJ, López-Martín E. EMF radiation at 2450 MHz triggers changes in the morphology and expression of heat shock proteins and glucocorticoid receptors in rat thymus. Life sciences. 2015 15;127:1-1.
- Ozguner F, Altinbas A, Ozaydin M, Dogan A, Vural H, Kisioglu AN, Cesur G, Yildirim NG. Mobile phone-induced myocardial oxidative stress: protection by a novel antioxidant agent caffeic acid phenethyl ester. Toxicology and industrial health. 2005 ;21(7-8):223-30.
- Eser O, Songur A, Aktaş C, Karavelioğlu E, Çağlar V, Aylak F, Kanter M. The effect of electromagnetic radiation on the rat brain: an experimental study. Turkish neurosurgery. 2013.
- Bas O, SÖNMEZ O, Aslan A, Ikinci A, Hanci H, Yildirim M, Kaya H, Akça M, Odaci E. Pyramidal cell loss in the cornu ammonis of 32-day-old female rats following exposure to a 900 Megahertz electromagnetic field during prenatal days 13-21. NeuroQuantology. 2013;11(4).
- Odaci E, Bas O, Kaplan S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: a stereological and histopathological study. Brain research. 2008 31;1238:224-9.
- Şahin A, Aslan A, Baş O, İkinci A, Özyılmaz C, Sönmez OF, Çolakoğlu S, Odacı E. Deleterious impacts of a 900-MHz electromagnetic field on hippocampal pyramidal neurons of 8-week-old Sprague Dawley male rats. Brain Research. 2015 22; 1624:232-8.
- Romeo S, Zeni O, Scarfì MR, Poeta L, Lioi MB, Sannino A. Radiofrequency electromagnetic field exposure and apoptosis: A scoping review of in vitro studies on mammalian cells. International Journal of Molecular Sciences. 2022 19;23(4):2322.
- Ghazizadeh V, Nazıroğlu M. Electromagnetic radiation (Wi-Fi) and epilepsy induce calcium entry and apoptosis through activation of TRPV1 channel in hippocampus and dorsal root ganglion of rats. Metabolic brain disease. 2014 ; 29:787-99.
- Gupta SK, Mesharam MK, Krishnamurthy S. Electromagnetic radiation 2450 MHz exposure causes cognition deficit with mitochondrial dysfunction and activation of intrinsic pathway of apoptosis in rats. Journal of biosciences. 2018 ; 43:263-76.
- Persson BR, Salford LG, Brun A. Blood‐brain barrier permeability in rats exposed to electromagnetic fields used in wireless communication. Wireless Networks. 1997 ; 3:455-61.
- Salford LG, Brun AE, Eberhardt JL, Malmgren L, Persson BR. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environmental health perspectives. 2003 ;111(7):881-3.
- Stam R. Electromagnetic fields and the blood–brain barrier. Brain research reviews. 2010 5;65(1):80-97.
- Leszczynski D, Joenväärä S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer-and blood-brain barrier-related effects. Differentiation. 2002 1;70(2-3):120-9.
- Nittby H, Brun A, Eberhardt J, Malmgren L, Persson BR, Salford LG. Increased blood–brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology. 2009 1;16(2-3):103-12.
- Eberhardt JL, Persson BR, Brun AE, Salford LG, Malmgren LO. Blood-brain barrier permeability and nerve cell damage in rat brain 14 and 28 days after exposure to microwaves from GSM mobile phones. Electromagnetic biology and medicine. 2008 1;27(3):215-29.
- Shabani F , Jadidi M, Esmaili M H , Sameni H R, Nazari H . Detrimental Effect of Mobile Phone Electromagnetic Field on Permeability of Blood-Brain Barrier. Middle East J Rehabil Health Stud. 2020;7(3):e103714.
- Gao P, Chen Q, Hu J, Lin Y, Lin J, Guo Q, Yue H, Zhou Y, Zeng L, Li J, Li J, et al: Effect of ultra‑wide‑band electromagnetic pulses on blood‑brain barrier permeability in rats. Mol Med Rep 2020; 22: 2775-2782.
- Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cellular and Molecular Life Sciences. 2010 ; 67:3435-47.
- Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural regeneration research. 2013 25;8(21):2003-14.
- Sasaki S, Warita H, Murakami T, Shibata N, Komori T, Abe K, Kobayashi M, Iwata M. Ultrastructural study of aggregates in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta neuropathologica. 2005 ; 109:247-55.
- Lahijani MS, Tehrani DM, Sabouri E. Histopathological and ultrastructural studies on the effects of electromagnetic fields on the liver of preincubated white Leghorn chicken embryo. Electromagnetic biology and medicine. 2009 1;28(4):391-413.
- Siddiqi N, Al Nazwani N. Role of radio-frequency electromagnetic waves in causing oxidative stress. In Fundamental Principles of Oxidative Stress in Metabolism and Reproduction 2024 1 (pp. 77-91). Academic Press.
- Douarre C, Sourbier C, Dalla Rosa I, Brata Das B, Redon CE, Zhang H, Neckers L, Pommier Y. Mitochondrial topoisomerase I is critical for mitochondrial integrity and cellular energy metabolism. PloS one. 2012 20;7(7): e41094.
- Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. Journal of the neurological sciences. 2007 15;257(1-2):221-39.
- Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2005 30;1746(1):1-7.
- Wei YH, Lu CY, Wei CY, Ma YS, Lee HC. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chinese Journal of Physiology. 2001 31;44(1):1-2.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. nature. 2000 9;408(6809):239-47.
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Archives of general psychiatry. 2010 1;67(4):360-8.
- Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA repair. 2006 3;5(2):145-52.
- Joshi G, Sultana R, Perluigi M, Butterfield DA. In vivo protection of synaptosomes from oxidative stress mediated by Fe2+/H2O2 or 2, 2-azobis-(2-amidinopropane) dihydrochloride by the glutathione mimetic tricyclodecan-9-yl-xanthogenate. Free Radical Biology and Medicine. 2005 15;38(8):1023-31.
- Ghezzi D, Zeviani M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Mitochondrial Oxidative Phosphorylation: Nuclear-Encoded Genes, Enzyme Regulation, and Pathophysiology. 2012 11:65-106.
- Ross WN. Understanding calcium waves and sparks in central neurons. Nature Reviews Neuroscience. 2012 ;13(3):157-68.
- Beason RC, Semm P. Responses of neurons to an amplitude modulated microwave stimulus. Neuroscience Letters. 2002 29;333(3):175-8.
- Krey JF, Dolmetsch RE. Molecular mechanisms of autism: a possible role for Ca2+ signaling. Current opinion in neurobiology. 2007 1;17(1):112-9.
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011 13;108(37):15037-42.








