Agnis Pondineka Ria Aditama1* , Dewi Taurisiawati Rahayu2
, Dewi Taurisiawati Rahayu2 , Brivian Florentis Yustanta2
, Brivian Florentis Yustanta2 , Wuri Widi Astuti2
, Wuri Widi Astuti2 , Wahyu Wijayati2
, Wahyu Wijayati2 , Estin Gita Maringga2
, Estin Gita Maringga2  and Fitri Yuniarti2
and Fitri Yuniarti2
1Health Polytechnic of Jember, Jember, Indonesia.
2Department of Midwifery, STIKES Karya Husada Kediri, Kediri, Indonesia.
Corresponding Author E-mail:agnisaditama@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2980
Abstract
Women over the age of 40 often experience a decrease in bone mass due to a lack of estrogen, which can increase the risk of osteoporosis. Previous studies have shown that the leaves of Green Clover (Marsilea crenata C. Presl.) contains phytoestrogen compounds which can potentially replace the function of estrogen in the body. In this context, this study aims to evaluate the ability of water fractions from M. crenata leaves in increasing the expression of osterix (Osx) and osteocalcin (Ocn), which are important indicators of increased bone formation, specificially on differentiation and maturation of osteoblast cell. In this study, the water fraction with a dose of 62.5; 125; and 250 µg/L, as well as Genistein 2.5 µg/ml, were given to human fetal osteoblast (hFOB) 1.19 cells that had reached the confluence point. The analysis method uses immunocytochemical techniques with confocal laser scanning microscopy (CLSM) to identify changes in the bone formation process. All dosages of water fraction considerably enhanced the bone formation in hFOB 1.19 cells, as indicated by the results. The results of this study provide confirmation that the water fraction derived from M. crenata leaves has promise as a possible agent for combating osteoporosis. This is achieved by enhancing differentiation and maturation of osteoblast cell through the upregulation of Osx and Ocn genes.
Keywords
Differentiation in vitro; Maturation; Marsilea crenata C. Presl.; Osteoblast; Osteocalcin; Osterix
Download this article as:| Copy the following to cite this article: Aditama A. P. R, Rahayu D. T, Yustanta B. F, Astuti W. W, Wijayati W, Maringga E. G, Yuniarti F. The Effect of Water Fraction Derived from Green Clover (Marsilea crenata C. Presl.) Leaves on Differentiation and Maturation of Human Osteoblast Cell. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Aditama A. P. R, Rahayu D. T, Yustanta B. F, Astuti W. W, Wijayati W, Maringga E. G, Yuniarti F. The Effect of Water Fraction Derived from Green Clover (Marsilea crenata C. Presl.) Leaves on Differentiation and Maturation of Human Osteoblast Cell. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3SsWO0M |
Introduction
It is common for women over the age of 40 to suffer reduced bone mass as a result of estrogen shortage, which in turn increases the chance of developing osteoporosis. Data from the Central Bureau of Statistics of Indonesia 2020 indicate that the number of older individuals in Indonesia increased by 9.92% to around 26 million. The proportion of elderly women in Indonesia is higher than that of elderly males (10.43% vs 9.42 in Indonesia), 19.7% of the elderly population, which accounts for 3.6 million people, suffer from osteoporosis. In 2005, there were 18 million elderly individuals in Indonesia, and this number is projected to increase to 33 million by 2020, with a life expectancy of 70 years. According to predictions, 30% of women who are 50 years old or older will have osteoporosis, while 37-54% are expected to have osteopenia, and 54% are at risk of experiencing issues related to fractures1. It is projected that by the year 2050, Asia, including Indonesia, would account for almost 50% of global osteoporotic fractures2. According to Gallagher and Sai (2010)3, the incidence of osteoporosis is twice as commonly seen in females as it is in males.
Hormones including estrogen, testosterone, and parathyroid hormone (PTH) have a significant role in the creation and preservation of bones. During menopause in women and in older men, a drop in hormones causes a gradual decline in bone mass4. This phenomenon is observed in both types of individuals. It is common for women to experience menopause after the age of 40, which is defined by the cessation of menstruation and a decline in ovarian activity5. Menopause often occurs around the age of 40, and when weman become older, estrogen levels drop, which raises the likelihood that we may develop osteoporosis5,6.
Bones go through a process of ongoing production and repair while the remodeling process is taking place. During this process, estrogen plays a significant role by controlling the equilibrium between bone-forming cells (osteoblasts) and bone-destroying cells (osteoclasts)7. Additionally, estrogen has a function in the formation of bone. Activation of the transcription factor osterix (Osx) is necessary for osteoblast differentiation and maturation8. Bone formation is dependent on osteoblast differentiation and maturation9. Important proteins such as osteocalcin (Ocn), osteopontin (OPN), collagen type I (COL-I), and bone sialoprotein (BSP) are also altered by Osx10.
There are phytoestrogens present in Marsilea crenata C. Presl. as well11. According to Putra and Laswati (2011)12, the amounts of phytoestrogens that are present in these leaves are relatively high13. It has been demonstrated that the ethyl acetate fraction of M. crenata have antiosteoporosis activity, which in turn has been shown to enhance bone density in female osteoporotic mice14. This plant, belonging to the Marsileaceae group, is found in Laos, Kalimantan, Java, the Lesser Sunda Islands, New South Wales, Malaya, New Guinea, the Philippines, South Australia, Queensland, Thailand, the Northern Territory, Victoria, Vietnam, and Western Australia15,16.
So, the purpose of this research is to determine the water fraction of M. crenata leaves could increase the expression of Osx and Ocn, which would indicate an increase in bone production. hFOB 1.19 cells were given water fractions in varying dosages, with genistein serving as a positive control.
Material and methods
Materials
The leaves of M. crenata were collected from Surabaya, East Java, Indonesia. The hFOB 1.19 cell line (CRL-11372) was purchased from ATCC, Virginia, USA. Genistein, paraformaldehyde (PFA), dimethyl sulfoxide (DMSO), phosphate-buffered saline (PBS), and bovine serum albumin (BSA) were purchased from Sigma Aldrich, Missouri, USA. The anti-mice osteocalcin and anti-rabbit osterix antibodies were obtained from Abcam, Cambridge, UK. Penicillin-streptomycin antibiotics, and fetal bovine serum (FBS), G418, dulbecco’s modified eagle’s medium (DMEM), were supplied by Aretha, Bandung, Indonesia. Tween-80, paraformaldehyde, fluorescein isothiocyanate (FITC), rhodamine, and an anti-mouse and anti-rabbit secondary antibody were purchased from Universitas Brawijaya, Malang, Indonesia.
Method
Extraction, Fractionation, and Preparation of Water Fraction
Using ultrasonic-assisted extraction, 1.5 kg of M. crenata leaves was extracted with 96% ethanol solvent to get 60 g of extract17. Liquid-liquid extraction were used in the fractionation process. The extract was suspended in 700 ml of water and fractionated at a 1:1 ratio using n-hexane. After that, the resulting aqueous phase was separated and further fractionated using n-butanol and ethyl acetate. A Heidolph Hei-VAP ML/G3 rotary evaporator was then used to evaporate the water phase.
The samples were obtained by mixing 50 mg of water fraction with 0.5% Tween-80 in 0.5% DMSO (w/v), and prepared at doses of 62.5, 125, and 250 µg/L.
Cell Culture
Complete medium formulated from DMEM, penicillin and streptomycin 1%, G418, and FBS 10%, were used to cultivate hFOB 1.19 cells in a culture flask. The cells were then incubated for six days at 37°C in an incubator with 5% CO2. Every 24 hours, the progress of the cells was monitored, and the medium was changed out until it had 80–90% content. Following that, the cells were moved to a microplate with 24 wells.
Osx and Ocn Measurement
The hFOB 1.19 cells, which had reached confluence, were given a water fraction at doses of 62.5, 125, and 250 µg/L, as well as 2.5 µg/ml genistein as a positive control. Following the cultivation of cells to 80% confluence in a 24-well microplate, the administration of 10 ng/ml TNF-α was performed. The cells were subjected to a 48-hour treatment with samples and genistein. After a subsequent washing with PBS, the cells were subjected to fixation using a 4% PFA solution, Triton X-100, BSA, and Osx and Ocn primary antibodies for immunochemistry. The cells were then incubated at 4 °C overnight. The cells then received the secondary antibodies rhodamine and FITC, respectively. The samples were then subjected to analysis utilizing CLSM (Fluoview Olympus FV1000) at wavelengths of 488 nm and 543 nm. The immunofluorescence of the markers was analyzed using Olympus Fluoview Ver.4.2a software to determine the expression levels of Osx and Ocn18.
Data analysis
Following the acquisition of data through CLSM, the subsequent procedure involves quantification to derive numerical data. This quantification will be performed through one-way ANOVA and followed with post-hoc LSD analysis utilizing Statistical Product and Service Solutions (SPSS) statistical software, with a significance threshold set at p<0.05.
Results and Discussion
A water fraction of 70 g was produced from the extraction process using 1.6 kg of M. crenata leaves powder using 96% ethanol solvent. After that, extract 96% ethanol from the leaves of M. crenata separated using liquid-liquid extraction and produced 27 g water fraction.
Figure 1 and Figure 2 show that after each dose was given, the amount of Osx immunofluorescence in hFOB 1.19 cells increased in all groups. The positive control group exhibited the highest intensity, followed by the treatment groups based on the dose level, while the negative control group displayed the lowest intensity. The 250 µg/ml dose group had the best results. They significantly increased Osx expression compared to the negative control (p = 0.000), but not significantly compared to the genistein 2.5 µg/ml dose group (p = 0.125). At the dose of 62.5 µg/ml, Osx expression was higher compared to the negative control (p = 0.000) and genistein 2.5 µg/ml (p = 0.000). It was also higher at the dose of 125 µg/ml compared to the negative control (p = 0.000) and genistein 2.5 µg/ml (p = 0.000).
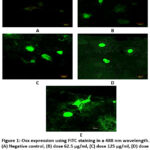 |
Figure 1: Osx expression using FITC staining in a 488 nm wavelength. (A) Negative control, (B) dose 62.5 µg/ml, (C) dose 125 µg/ml, (D) dose 250 µg/ml, (E) genistein 2.5 µg/ml. |
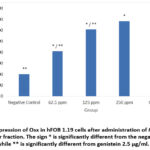 |
Figure 2: Expression of Osx in hFOB 1.19 cells after administration of M. crenata leaves water fraction. |
A similar pattern was seen in Ocn observations, as depicted in Figure 3 and Figure 4, which show Ocn immunofluorescence in hFOB 1.19 cells in all groups. The positive control group showed the highest intensity, followed by the treatment group based on the dose level, and the negative control group showed the lowest intensity. In terms of increasing Ocn expression, the 250 µg/ml dose group did the best. It did this significantly more than the negative control (p = 0.000) and also significantly more than genistein 2.5 µg/ml (p = 0.000). The increase in Ocn expression at a dose of 62.5 µg/ml was also significantly different from the negative control (p = 0.000) and genistein 2.5 µg/ml (p = 0.000), as well as at a dose of 125 µg/ml, which was significant compared to the negative control (p = 0.000) and genistein 2.5 µg/ml (p = 0.000).
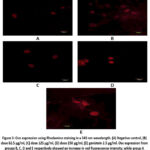 |
Figure 3: Ocn expression using Rhodamine staining in a 543 nm wavelength. (A) Negative control, (B) dose 62.5 µg/ml, (C) dose 125 µg/ml, (D) dose 250 µg/ml, (E) genistein 2.5 µg/ml. |
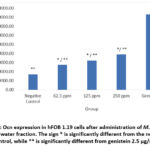 |
Figure 4: Ocn expression in hFOB 1.19 cells after administration of M. crenata leaves water fraction. |
The results showed that the water fraction from the leaves of M. crenata at a dose of 62.5; 125; and 250 µg/ml significantly and linearly increased the expression of Osx and Ocn in hFOB 1.19 cells compared with the negative control. Osx is a key transcription factor that plays an important role in the process of osteoblast cell differentiation, activating the SP7 promoter gene during the maturation of osteoblasts into mature osteoblasts and osteocytes. Inactivation of Osx can inhibit the expression of osteocalcin, bone sialoprotein, and osteopontin, thereby reducing the activity of forming new bone cells19,20. The function of Osx is to regulate the production of bone formation proteins, including Ocn, which is the main non-collagen protein produced by mature osteoblast cells.
The Ocn protein plays a crucial role in the bone mineralization process and maintaining calcium ion homeostasis. There are two forms of Ocn, the carboxylated form which has a high affinity for calcium and hydroxyapatite crystals, so they are in the bone matrix during the mineralization process with the help of γ-carboxyglutamic acid (Gla) which helps absorption into hydroxyapatite, which is a crucial step in bone mineralization. Uncarboxylated osteocalcin has a low affinity for bone matrix and is likely to enter the bloodstream to reach other organs20,21. The formation of the osteocalcin matrix plays an important role in increasing bone mineralization20,21,22.
The administration of a water fraction derived from M. crenata leaves resulted in an upregulation of Osx and Ocn expression. This observation serves as evidence supporting the involvement of this fraction in enhancing the process of bone growth. The observed phenomenon might perhaps be attributed to the regulatory mechanism of phytoestrogens present in the aqueous portion of M. crenata leaves, which has resemblance to the mode of action of estrogen. Phytoestrogens are botanical substances exhibiting a structural resemblance to 17β-estradiol, hence enabling them to serve as substitutes for estrogen. Phytoestrogen substances encompass a variety of chemicals, including flavonoids like kaempferol and quercetin, isoflavonoids such as genistein and daidzein, as well as triterpenoids and steroids23,24,25. Aditama et al., 2021 also conducted similar research. The ethyl acetate fraction of M. crenata leaves can increase osterix with an optimal dose of 250 ppm26, and 96% ethanol extract of M. crenata leaves can increase osteocalcin expression with an optimal dose of 125 ppm27.
Conclusion
The administration of water fractions derived from M. crenata leaves has the ability to enhance the expression of Osx and Ocn in hFOB 1.19 cells, the best dose that can increase Osx and Ocn is 250 µg/ml. This suggests that it has the potential to serve as a natural source for promoting differentiation and maturation of osteoblast through the upregulation of the Osx and Ocn genes.
Acknowledgment
None
Conflict of Interest
The authors declare there is no conflict of interest.
Funding Source
No funding agency in the governmental, commercial, private, or not-for-profit sectors provided a particular grant for this research.
References
- Widarsa I. K. T, Darwata I. W, Sarmadi M, Rachmanu M. J, Juwita D. A. P. R, Pradnyawati I. G, Sukmawati N. M. H. (Association between osteoporosis and age, physical activity, and obesity in elderly of Tulikup village, Gianyar. WMJ (Warmadewa Medical Journal), 2018; 3(2): 33-42.
- Chandran M, Brind’Amour K, Fujiwara S, Ha Y. C, Tang H, Hwang J. S, … & Eisman J. A. Prevalence of osteoporosis and incidence of related fractures in developed economies in the Asia Pacific region: a systematic review. Osteoporosis International, 2023; 34(6): 1037-1053.
CrossRef - Gallagher J. C, and Sai A. J. Molecular biology of bone remodeling: Implications for new therapeutic targets for osteoporosis. Maturitas, 2010; 65: 301-307.
CrossRef - Noh J. Y, Yang Y, Jung H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. International Journal of Molecular Sciences, 2020; 21(20): 7623. doi:10.3390/ijms21207623
CrossRef - Mozhgan M. Investigating the prevalence of menopausal complications and its related factors in women referred to Shahroud Health Centers in 2014. Revista Latinoamericana De Hipertention. 2020; 15(2). 10.5281/zenodo.4074633.
- Stevenson J, and Marsh M. 2007. An Atlas of Osteoporosis, Third Edition. doi:10.3109/9780203090848.
CrossRef - Khalid A. B, Krum S. A. Estrogen receptors alpha and beta in bone. Bone, 2016; 87: 130–135. doi:10.1016/j.bone.2016.03.016.
CrossRef - Lu X, Gilbert L, He X, Rubin J, Nanes M. S. Transcriptional Regulation of the Osterix (Osx, Sp7) Promoter by Tumor Necrosis Factor Identifies Disparate Effects of Mitogen-activated Protein Kinase and NFκB Pathways. Journal of Biological Chemistry, 2006; 281(10): 6297–6306. 10.1074/jbc.m507804200.
CrossRef - Zhang C, Tang W, Li Y, Yang F, Dowd D. R, MacDonald P. N. Osteoblast-Specific Transcription Factor Osterix Increases Vitamin D Receptor Gene Expression in Osteoblasts. PLoS ONE, 2011; 6(10): e26504.
CrossRef - Tu Q, Valverde P, Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun. 2006; 341: 1257–65. [PubMed: 16466699].
CrossRef - Ma’arif, B., Anwar, M. F., Hidayatullah, H., Muslikh, F. A., Suryadinata, A., Sugihantoro, H., … & Taek, M. M. (2024). Effect of polar fractions of Marsilea crenata C. Presl. leaves in zebrafish locomotor activity. Journal of Advanced Pharmaceutical Technology & Research, 15(2), 125-129.
CrossRef - Putra L. M, Laswati H. Phytoestrogen in Several Fruits and Leaves. Indonesian Journal of Clinical Pathology and Medical Laboratory, 2011; 18(1): 43-47.
CrossRef - Aditama A. P, Muslikh F. A, Shirvi I. N, Islamiyah F. R, Putra K. H, Inayatilah F. R, … & Rahayu A. Induction Effect of Proliferation of Osteoblas Cells Trabecular Bone Male Mice by 96% Ethanol Extract of Semanggi Leaves (Marsilea crenata Presl.). Jurnal Sains dan Kesehatan, 2021; 3(4): 429-435.
CrossRef - Agil M, Laswati H, Purwitasari N, Adityara R. A, Widiasari F. A. Effect of Ethanol, normal Hexane, and Ethyl Acetate Extracts of Marsilea crenata Leaves on ERß Expressions of Neurons in Estrogen-Deficient Female Mice. International Journal of Pharmaceutical Research. 2021; 12(2): 3109-3115.
CrossRef - Rahayu, S., Annisa, R., Anzila, I., Christina, Y. I., Soewondo, A., Marhendra, A. P. W., & Djati, M. S. (2021). Marsilea crenata ethanol extract prevents monosodium glutamate adverse effects on the serum levels of reproductive hormones, sperm quality, and testis histology in male rats. Veterinary world, 14(6), 1529.
CrossRef - Royal botanic garden. https://powo.science.kew.org/taxon/ urn:lsid:ipni.org:names:17274610-1. accessed on 02 July 2024
- Ma’arif B, Aditama A. P, Muslikh F. A, Sari D. P, Purbosari, I, Laswati H, Agil M. Inhibitory Effect of Free-ERβ Expression by n-Butanol Fraction of Semanggi (Marsilea crenata Presl.) Leaves on hFOB 1.19 Cells. Jurnal Sains dan Kesehatan, 2021; 3(4): 475-481.
CrossRef - Aditama A. P. R, Ma’arif B, Muslikh F. A. Effect of Osterix and Osteocalcin Enhancement By Quercetin (3,3’,4’,5,7-Pentahydroxyflavone) on Osteoblast hFOB 1.19 Cell line. International Journal of Applied Pharmaceutics, 2022; 14(1): 32-35.
- Zhou X, Zhang Z, Feng J. Q, Dusevich V. M, Sinha K, Zhang H. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA, 2010; 107: 12919-12924.
CrossRef - Tang W, Yang F, de Crombrugghe B, Jiao H, Xiao G, Zhang C. Transcriptional regulation of vascular endothelial factor (VEGF) by osteoblast-specific transcription factor Osterix (Osx) in osteoblasts. J Biol Chem, 2012; 287: 1671-1678.
CrossRef - Aonuma H, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku Journal of Experimental Medicine, 2009; 218: 201–205.
CrossRef - Laswati H, Subadi I, Widyowati R, Agil M, Pangkahila J. A. Spilanthes acmella and physical exercise increased testosterone levels and osteoblast cells in glucocorticoid-induced osteoporosis male mice. Bali Medical Journal, 2015; 4(2): 76-81.
CrossRef - Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck A. J. Phytoestrogens: recent developments. Planta Med. 2003; 69(7): 589-99.
CrossRef - Ososki A. L, Kennelly E. J. Phytoestrogens: a Review of the Present State of Research. Phytotherapy Research, 2003; 17: 845-869.
CrossRef - Sirotkin A. V, Harrath A. H. Phytoestrogens and their effects. European Journal of Pharmacology, 2014; 741: 230–236. doi:10.1016/j.ejphar. 2014.07.057.
CrossRef - Aditama A. P, Ma’arif B, Laswati H, Agil M. The effect of ethyl acetate fraction of Marsilea crenata presl. Leaves in increasing osterix expression in hFOB 1.19 cells. International Journal of Health Sciences, 2022; 6(2): 789-796.
CrossRef - Aditama A. P, Ma’arif B, Laswati H, Agil M. In vitro and in silico analysis of phytochemical compounds of 96% ethanol extract of semanggi (Marsilea crenata Presl.) leaves as a bone formation agent. Journal of Basic and Clinical Physiology and Pharmacology, 2021; 32(4): 881-887. http://dx.doi.org/10.1515/jbcpp-2020-0515.
CrossRef








