Manuscript accepted on :06-05-2024
Published online on: 11-07-2024
Plagiarism Check: Yes
Reviewed by: Dr. Moumita Hazra
Second Review by: Dr Zakir Hussain
Final Approval by: Dr. Patorn Promchai
Marisca Evalina Gondokesumo1* and Faisal Akhmal Muslikh2,3
and Faisal Akhmal Muslikh2,3
1Faculty of Pharmacy, University of Surabaya, Surabaya, Indonesia. Jl. Raya Kali Rungkut (Tenggilis), Surabaya, Indonesia
2Faculty of Pharmacy, Hang Tuah University, Surabaya, Indonesia. Jl. Arif Rahman Hakim No.150, Keputih, Sukolilo, Surabaya, East Java, Indonesia
3The Indonesian Society for Bioinformatics and Biodiversity (ISBB). Jl. Raya Arjuna Utara No. 9, Kebon Jeruk, Jakarta, Indonesia.
Corresponding Author E-mail: marisca@staff.ubaya.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2972
Abstract
Stunting is a chronic nutritional problem that occurs during the first 1000 days of life which is considered a golden window of opportunity. Indonesia has the highest prevalence compared to other middle-income countries. Keluwih (Artocarpus camansi) is known to have various compounds that are beneficial for the body such as anti-inflammatory and antioxidant. This study was conducted to determine the potential of the ethanol extract of Keluwih leaves (Artocarpus camansi) zebrafish stunting model against inflammatory markers, growth factors and body size. Artocarpus camansi leaves were extracted using the maceration method for 3x24 hours with 96% ethanol solvent. Zebrafish larvae were obtained from male and female broodstock (2:1), then induced using rotenone and ethanol extract of Artocarpus camansi leaves, then immunohistochemical staining was performed using growh factor (VEGF and TGF-β), inflammation (IL-6 and TNF-α) and body length measurements on day 9 dpf. The results showed that rotenone can provide a picture of stunting in zebrafish larvae from observations of growth factors, inflammation and body length, by administering ethanol extract of Artocarpus camansi leaves this can improve stunting conditions due to administration of rotenone, the concentration of ethanol extract of Artocarpus camansi leaves 2.5 ppm is the optimal concentration in improve stunting conditions. The ethanol extract of Artocarpus camansi leaves can improve stunting conditions by increasing the expression of growth factors, decreasing pro-inflammatory cytokines and improving body length in zebrafish larvae.
Keywords
Artocarpus camansi; Stunting, Growth factors; Malnutrition; pro-inflammatory cytokines
Download this article as:| Copy the following to cite this article: Gondokesumo M. E, Muslikh F. A. The Effect of Keluwih (Artocarpus camansi) Leaves Extract On Pro-Inflammatory Expression, Growth Factors and Bodies in Zebrafish Larvae (Danio rerio) Stunting Model. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Gondokesumo M. E, Muslikh F. A. The Effect of Keluwih (Artocarpus camansi) Leaves Extract On Pro-Inflammatory Expression, Growth Factors and Bodies in Zebrafish Larvae (Danio rerio) Stunting Model. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3xUxJ86 |
Introduction
Stunting represents a significant nutritional challenge encountered by developing nations 1. Stunting is a persistent nutritional issue that manifests during the initial 1000 days of life, recognized as a critical window of opportunity 2. Indonesia has one of the highest rates of stunting prevalence among middle-income countries 3.
Stunting can be caused by several factors, including carrying out malnutrition care and assessing the insufficient awareness of health and nutrition among mothers prior to pregnancy, during pregnancy, and after the mother gives birth 3. Stunting causes growth disorders, which are characterized by height that does not match age 4. Children who undergo stunting may exhibit suboptimal intelligence levels, rendering them more vulnerable to diseases and, in the long term, exposing them to potential declines in productivity 5. In addition, stunting also has an impact on cognitive development disorders and delays in motor development, most of which are irreversible 6.
The prevention of stunting is crucial to safeguard the quality of the next generation. By nurturing a superior next generation, Indonesia can enhance its competitiveness on the global stage and effectively tackle future challenges 7. Family assistance teams have been deployed across all regions of Indonesia with the aim of decreasing the stunting rate to 14% by 2024. According to data from the 2021 Indonesian Toddler Nutrition Status Survey, the current prevalence of stunting stands at 24.4% 8.
Traditional medicine using herbal plants is still an option that can be used for treatment 9. When compared to chemical drugs, traditional medicine has slower performance, but the use of herbal plants as the main raw material makes traditional medicine have milder side effects 10. Keluwih (Artocarpus camansi) is a plant that is widely distributed in tropical and subtropical parts of Asia. Keluwih comes from Papua New Guinea, Indonesia, and the Philippines. One of the distribution areas of keluwih in Indonesia is Maluku, keluwih plants are also found in lowland areas 11. Based on the results of phytochemical screening, simplicia and ethanol extracts of keluwih leaves contain alkaloids, flavonoids, tannins, glycosides, anthraquinone glycosides, and steroids/triterpenoids 10,11. The rich content of compounds in keluwih leaves is expected to provide benefits to the Indonesian people, especially in existing stunting conditions. It is known that this keluwih has potential as an anti-inflammatory, antioxidant, antifungal, and antibacterial 12,13. Therefore, this study was undertaken to assess the potential of keluwih leaf extract against zebrafish stunting models against inflammatory markers, growth factors, and body size. Zebrafish induced by rotenone 12.5 ppb can be used as a stunting model in previous studies 14.
Methods
Animal Care
Adult male and female zebrafish sourced from the wild were identified at the reproduction laboratory of the Faculty of Fisheries and Marine Sciences, Brawijaya University. The zebrafish are housed in semi-static 60 L tanks, with temperature in water maintained between 24-26.5°C and a light cycle of 14:10 (dark:light) 15. The fish received three daily feedings using Tetra Color ® Tropical Flakes from Blacksburg at Germany 16,17.
Embryos from zebrafish are collected after male and female fertilization in a 2:1 ratio. These embryos, aged 0-2 hours post-fertilization, are selected based on being round, clear, fertile, and free from mold. A total of 100 larvae are used in the study. The research is approved by the University of Brawijaya’s Medical Faculty Ethics Committee (No. 149-KEP-UB-2023). The embryos are categorized into five different groups, including a normal control, a negative control with 12.5 ppb rotenone, and three experimental groups with varying levels of EEKL added to 12.5 ppb rotenone.
Embryo Media
The medium for embryo was prepared at a concentration of 10x, composed of 0.15 grams of CaCl, 0.15 grams of KCl, 5 grams of NaCl, 0.815 grams of MgSO4, and 500 milliliters of distilled water 14.
Extraction of Keluwih Leaves (Artocarpus camansi)
Artocarpus camansi has obtained certification from UPT (Unit Pelaksana Teknis) Materia Medica, with number 074/124/102.20-A/2022. The extraction procedure followed the maceration method, utilizing 96% ethanol (at a ratio of 1:10) and conducted over a period of 3 cycles, each lasting 24 hours. The extract obtained was evaporated until a concentrated ethanol extract of Keluwih Leaves (EEKL) was obtained.
Rotenone and EEKL Administration
Rotenone (R8875) purchased at sigma aldrich, with a purity of 95%, were dissolve in 1% DMSO to produce a stock solution. Rotenone was administered at a concentration of 12.5 ppb 14, and the EEKL concentration varied 2.5; 5; 10 ppm.
Body Length Measurement
On the 9th day post-fertilization (dpf), measurements of zebrafish larvae’s body length were conducted. The larvae were examined utilizing an Olympus SZ61 stereomicroscope and subsequently quantified using calibrated Image J software. The body’s length is determined by measuring from the nose’s tip (snout) to the tail fin’s base 18.
Growth factor (VEGF and TGF-β) and inflammation (IL-6 and TNF-α) measurements
Zebrafish larvae aged 9 dpf were euthanized based on the NIH protocol. Whole zebrafish larvae were placed in a microtube in ice water for at least 5 minutes and confirmed that there was no movement. Then rinsed and fixed with cold methanol 20 °C for 3-5 minutes, followed by inactivation using peroxide blocking solution at 25 oC for 10 minutes and under running water for 5 minutes. Whereupon incubated in prediluent blocking solution for 10 minutes at room temperature.
The next stage was by incubating 100µL of commercial monoclonal primary antibody per preparation in the refrigerator for 24 hours, then washing it using PBS for 5 minutes. Then added biotinylated universal secondary antibody for each IL-6 observation (100µL at 1:50 dilution, Sigma Aldrich, HPA035283); TNF-α (1-2µg/mL, Sigma Aldrich, SAB1404480); VEGF (at 1:20 to 1:100 dilution, Sigma Aldrich, AB1876-I); TGF-β (with 1:100 dilution, Termofisher, MA5-16949) was then incubated at 25 oC for 10 minutes. The incubated preparations were washed PBS (5 minutes) and again incubated using streptavidin/peroxide complex reagent (10 minutes) and washed PBS (5 minutes).
The next step is to detect the reaction by incubating with peroxide substrate solution (DAB) 100µL per preparation for 2-10 minutes, washing with running water and adding 100µL of Mayer’s hemoxylin (counterstrain) reagent per preparation and incubating for 1-3 minutes then washing under water flow. The final stage is dehydration and mouting as usual. Afterward, it was scrutinized using a Nikon Eclipse type Ei light microscope, aided by an Optilab Microscope Camera that was connected to a computer.
Statistic analysis
Statistical evaluation was conducted through IBM ANOVA SPSS version 23.0, followed by the LSD post hoc test at a 95% confidence interval. Shapiro-Wilk was used for the normality assessment and the Levene test was used for checking homogeneity.
Results and Discussion
 |
Table 1: Visualization of positive cells containing observed proteins |
The use of pesticides as an environmental factor can trigger stunting. Rotenone is a pesticide with a concentration of 12.5 ppb, which can induce stunting 19. Rotenone works to inhibit mitochondrial complex I and inhibit the process of ATP synthesis so that the amount of ATP decreases and induces an elevation in ROS (reactive oxygen species), leading to potential cell death, cellular damage, and other oxidative risks 20.
Giving rotenone with a concentration of 12.5 ppb to zebrafish larvae affected growth factors and inflammatory cytokines, which manifested in the body length of the larvae. Rotenone administration can increase levels of inflammatory cytokines (IL6 and TNFa) and decrease growth factors (TGF-β and VEGF). This can be seen from the many brown images in Figure 1, which show a positive immunohistochemical reactivity. Rotenone administration also showed a shorter body length in zebrafish larvae compared to the normal group (Table 2) 14.
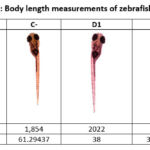 |
Table 2: Body length measurements of zebrafish larvae |
The images obtained were measured quantitatively to determine the persentage positive area in immunohistochemical (immunoreactive) observations. The results of the quantification values obtained can be seen in Figure 1.
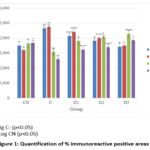 |
Figure 1: Quantification of % immunoreactive positive areas |
Giving EEKL can improve the condition of the stunting model in zebrafish. It has been proven to reduce inflammatory cytokine levels, increase growth factor levels, and improve body length in zebrafish stunting larvae. The optimal dose of EEKL is 2.5 ppm, because this is the lowest dose that has an effect on improving stunting conditions in zebrafish larvae.
Poor nutrition and ongoing inflammation driven by pro-inflammatory cytokines play a role in causing growth delays. At the initial stage, the levels of several highly inflammation mediators such as TNF-α, IL-6, and IL-12 were observed to be reduced in stunted children when compared to those in the normal control group 21. Inflammatory cytokines observed in this study were IL6 and TNF-α. TNF consists of two related proteins mainly produced by mononuclear lymphocytes (TNF-β) and phagocytes (TNF-α). Assessing TNF-α levels in malnourished kids is crucial as low TNF-α can weaken the immune system, while high levels can worsen nutrition by causing anorexia and cachexia. IL-6 is key in triggering acute phase protein synthesis in liver cells. Excessive IL-6 in children can cause chronic inflammation and contribute to growth issues, including stunting. It negatively affects liver IGF-I gene activity and facilitates the reduction of IGFBP-3 (insulin-like growth factor-binding protein-3).
Sederquist and colleagues discovered that multiple inflammatory cytokines like TNF-α and IL-6 can singly or jointly impact child growth. These cytokines can operate through overall systemic pathways or specifically target the growth speed of long bones 22,23.
The cytokine Transforming Growth Factor β (TGF-β) holds a pivotal role in regulating cell growth and differentiation across diverse tissues. Additionally, it is involved in processes such as inflammation, autoimmunity, and tumor development 24. Under normal circumstances, local sources maintain tissue homeostasis by preserving baseline levels of TGF-β signaling. Following tissue damage, TGF-β is extensively secreted by blood platelets and other stromal elements to aid in tissue repair, wound healing, and reducing inflammation. The interaction between TGF-β signaling and reactive oxygen species (ROS) metabolites is crucial 25.
Reduced ROS levels play crucial roles in determining cell fate and cellular responses affecting cell proliferation, differentiation, and death 26, similar to TGF-β signaling. If ROS levels surpass the body’s antioxidant defenses, this imbalance leads to oxidative stress, harming proteins, nucleic acids, and lipids either directly or indirectly 27. TGF-β is abundant in bones and cartilage. It promotes the growth, differentiation, and formation of osteoblasts from osteoprogenitor cells 28.
Under normal circumstances, increased ROS also causes hypoxia, which stimulates VEGF expression. Hypoxia boosts VEGF through enhanced mRNA transcription and stability, triggering blood vessel formation to sustain oxygen levels. Excessive ROS from oxidative stress damages cells by mutating VEGF via intricate signaling routes. Chronic hypoxia lowers VEGF expression, impairs tissue vascularization, and causes endothelial dysfunction. Such dysfunction disrupts angiogenesis, regulated by VEGF’s interaction with VEGFR-2, affecting cell proliferation and growth 29.
Conclusion
Artocarpus camansi leaves ethanol extract (EEKL) can improve stunting conditions through increasing growth factor expression, decreasing pro-inflammatory cytokines and increasing body length in zebrafish larvae induced by rotenone of 12.5 μg/mL significantly, optimal concentration of Artocarpus camansi leaves ethanol extract in overcoming stunting conditions it’s 2.5 ppb.
Acknowledgment
Thank you to all the teaching staff, especially laboratory staff, who helped with this research.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
No external funding for this study.
References
- WHO. Guideline updates on the management of severe acute malnutrition in infants and children. Update 2013; cited 21, 2023. available from: www.who.int
- Shofiya D, Sumarmi S, Sulistyono A, Suyanto B. Determinants of successful exclusive breastfeeding on primiparas mothers. Journal of Public Health in Africa. 2023 14(s2):2614. https://doi.org/10.4081/ jphia.2023.2614
CrossRef - Zahrah SN, Damayanti NA. The relationship between religious leaders and the knowledge of mothers in reducing stunting: a literature review. Journal of Public Health in Africa. 2023 14(s2):2622. https://doi.org/10.4081/jphia.2023.2622
CrossRef - Nasution IS, Susilawati S. Analysis of factors causing stunting in toddlers aged 0-59 months. FLORONA: Jurnal Ilmiah Kesehatan. 2022 1(2), 82-87. https://doi.org/10.55904/florona.v1i2.313
CrossRef - Astuti DK, Sumarmi S. Dietary Diversity among Stunting Toddlers in Rural and Urban Areas of Probolinggo Regency. Media Gizi Indonesia. 2020 15(1), 14-21. https://doi.org/10.204736/mgi.v15i1
CrossRef - Maulina R, Qomaruddin MB, Kurniawan AW, Fernandes A, Astuti E. Prevalence and predictor stunting, wasting and underweight in Timor Leste children under five years: An analysis of DHS data in 2016. Journal of Public Health in Africa. 2022 13(2).2116. https://doi.org/10.4081/jphia.2022.2116
CrossRef - Fadmi FR, Kuntoro K, Otok BW, Melaniani S. Stunting incident prevention: a systematic literature review. Journal of Public Health in Africa. 2023 14(s2):2547. https://doi.org/10.4081/jphia.2023.2547
CrossRef - Putri DUP, Mahmudiono T, Indriani D, Lisatriana B. The relationship between the competence and performance of family planning instructors in family assistance at risk of stunting in Lampung province. Journal of Public Health in Africa. 2023 14(s2):2544. https://doi.org/10.4081/jphia.2023.2544
CrossRef - Yiğit D. Antifungal activity of Lawsonia inermis L.(Henna) against clinical Candida isolates. Erzincan University Journal of Science and Technology. 2017 10(2), 196-202. ttps://doi.org/10.18185/erzifbed.328754
- Amelia S, Sogandi S. Antibacterial Potency from Ethanol Extract Leaves of Kluwih (Artocarpus camansi Blanco) against Shigella dysenteriae and Bacillus subtilis. J Ilmu Dasar. 2020 21, 105-114. https://doi.org/10.19184/jid.v21i2.11568
CrossRef - Eryuda F, Soleha TU. Kluwih (Artocarpus camansi) Leaf Extract in Lowering Blood Glucose Levels in Diabetes Mellitus Patients. Jurnal Majority. 2016 5(4), 71-75.
- Sikarwar MS, Hui BJ, Subramaniam K, Valeisamy BD, Yean LK, Balaji K. A review on Artocarpus altilis (Parkinson) Fosberg (breadfruit). Journal of Applied Pharmaceutical Science. 2014 4(8), 091-097. https://doi.org/10.7324/JAPS.2014.40818
CrossRef - Alcon CLM, Barrion ASA, Nguyen-Orca MF. Proximate Composition, Antioxidant Capacity and Functional Properties of Breadnut Seed Flour (Artocarpus camansi). Turkish Journal of Agriculture-Food Science and Technology. 2021 9(8), 1495-1499. https://doi.org/ 10.24925/turjaf.v9i8.1495-1499.4319
CrossRef - Zahara E, Nuraenah E, Yuliyani T, Darwitri D, Khotimah H, Kalsum U, et al. Ethanolic e xtract of Centella asiatica increase bone ossification and increase the body length and in zebrafish (Danio rario) larvae Stunting Model at 9 day post fertilization. AcTion: Aceh Nutrition Journal. 2018 3(2), 95-102. https://doi.org/ 10.30867/action.v3i2.87
CrossRef - Ma’arif B, Muslikh FA, Saidah NL, Fihuda DAP, Khotimah H, Taek MM, Agil M. The effect of ethanol extract of Marsilea crenata Presl. leaves on rotenone-induced zebrafish locomotor activity. Jurnal Farmasi Sains dan Komunitas (JFSK). 2022 19(2), 87-92. https://doi.org/ 10.24071/jpsc.004576
CrossRef - Khotimah H, Yuliyani T, Nuraenah E, Zahara E, Umi KN. Centella asiatica increased the body length through the modulation of antioxidant in rotenone-induced zebrafish larvae. Biomedical and Pharmacology Journal. 2018 11(2), 827-833. https://dx.doi.org/ 10.13005/bpj/1438
CrossRef - Ma’arif B, Maimunah S, Muslikh FA, Saidah NL, Fihuda DA, Khotimah H, Agil M. The Effect of Marsilea crenata Presl. Leaves Extracton Zebrafish Locomotor Activity. FARMASIS: Jurnal Sains Farmasi. 2022 3(1), 18-24. https://doi.org/10.36456/farmasis.v3i1.5389
CrossRef - Primaditya V, Cory’ah FAN, Ariati LIP, Zakiah Z, Wardani DWKK, Yuningsih Y, et al. Effect of Centella asiatica to the glucose transporter 4 and osteocalcin on the rotenonee-induced zebrafish larvae (Danio rerio) stunting model. In AIP Conference Proceedings 2020 2231(1). https://doi.org/10.1063/5.0002607
CrossRef - Primihastuti D, Ali MM & Kalsum U. The Effect of Ethanol Extract of Gotu Kola (Centella Asiatica) on Bone Ossification and Osteoclastogenesis in Rotenone-Induced Zebrafish (Danio Rerio) Larval Stunting Model. Thesis. Universitas Brawijaya. Malang. 2017
- Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends in molecular medicine. 2009 15(10), 468-477. https://doi.org/1016/j.molmed.2009.08.004
CrossRef - Hossain M, Nahar B, Haque MA, Mondal D, Mahfuz M, Naila NN, et al. Serum adipokines, growth factors, and cytokines are independently associated with stunting in Bangladeshi children. Nutrients. 2019 11(8), 1827. https://doi.org/10.3390/nu11081827
CrossRef - Sederquist B, Fernandez-Vojvodich P, Zaman F, Sävendahl L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. Journal of molecular endocrinology. 2014 53(1), T35-T44. https://doi.org/10.1530/JME-14-0006
CrossRef - Abd El-Maksoud AM, Khairy SA, Sharada HM, Abdalla MS, Ahmed NF. Evaluation of pro-inflammatory cytokines in nutritionally stunted Egyptian children. Egyptian Pediatric Association Gazette. 2017 65(3), 80-84. https://doi.org/10.1016/j.epag.2017.04.003
CrossRef - Batlle E, Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity. 2019 50(4):924–40. https://doi.org/10.1016/j.immuni.2019.03.024
CrossRef - Chang CH, Pauklin S. ROS and TGFβ: from pancreatic tumour growth to metastasis. Journal of Experimental & Clinical Cancer Research. 2021 40(1), 1-11. https://doi.org/10.1186/s13046-021-01960-4
CrossRef - Durand N, Storz P. Targeting reactive oxygen species in development and progression of pancreatic cancer. Expert Rev Anticancer Ther. 2017 17(1):19–31. https://doi.org/10.1080/14737140.2017.1261017
CrossRef - Ogrunc M, Di Micco R, Liontos M, Bombardelli L, Mione M, Fumagalli M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014 21(6):998–1012. https://doi.org/10.1038/cdd.2014.16
CrossRef - Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, et al. Expression of osteoprotegerin, receptor activator of NF‐κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. Journal of Bone and Mineral Research. 2001 16(6), 1004-1014. https://doi.org/10.1359/jbmr.2001.16.6.1004
CrossRef - Kusumo WDWK, Mulyohadi A, Husnul K, Wibi R, Dianita P, Puspita ALI, Vanda P. The effect of Centella asiatica to the vascular endothelial growth factor and vascular endothelial growth factor receptor-2 on the rotenone induced zebrafish larvae (Danio rerio) stunting model. GSC Biological and Pharmaceutical Sciences. 2018 5(2). https://doi.org/10.30574/gscbps.2018.5.2.0117
CrossRef







