Manuscript accepted on :04-04-2023
Published online on: 22-08-2024
Plagiarism Check: Yes
Reviewed by: Dr. Saumya Bipin
Second Review by: Dr. Noor Al-Huda Akram Yahya Al-Zarqi
Final Approval by: Dr. Ayush Dogra
Jay Prakash1 , Smita Shenoy2
, Smita Shenoy2 *, Krishnadas Nandakumar3
*, Krishnadas Nandakumar3 , Archana Parampalli Raghavendra1
, Archana Parampalli Raghavendra1 and Anoop Kishore3
and Anoop Kishore3
1Division of Physiology, Department of Basic Medical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, India
2Department of Pharmacology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India
3Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Corresponding Author E-mail : smita.shenoy@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2978
Abstract
Hyperlipidemia is an increase in plasma levels of lipids (cholesterol, triglycerides) and various lipoproteins. Though many drugs have been used for controlling hyperlipidemia, yet most of them have unpleasant side effects, which has stimulated the search for natural remedies. Hypolipidemic activity of hydroalcoholic extracts of Aegle marmelos (AM) leaves and Tamarindus indica (TI) seed alone and in combination on High Fat High Sugar (Fructose) Diet (HFHSD) induced hyperlipidemia in male rats was evaluated in this study. Out of 54 male Sprague Dawley rats, six received standard diet (Group I, normal control) throughout the study. The remaining 48 rats were fed orally with HFHSD for 30 days to induce hyperlipidemia (plasma cholesterol level >200 mg/dL). For the next thirty days, rats which had received HFHSD were divided into 8 groups with six animals in each. Group II- HFHSD control received standard diet, Group III- positive control (Niacin, 100 mg/kg/day), Group IV - AM25 (25 mg/kg/day), Group V - AM50 (50 mg/kg/day), Group VI - TI25 (25 mg/kg/day), Group VII - TI50 (50mg/kg/day), Groups VIII - AM25+TI25 (25 + 25 mg/kg/day) and Group IX - AM50+TI50 (50 + 50 mg/kg/day). Treatment of HFHSD fed rats with each extract alone and in combination resulted in a significant decrease in plasma cholesterol, triglycerides, LDL and VLDL and increase in HDL levels. Treatment with AM50+TI50 significantly lowered plasma cholesterol (p<0.001), triglycerides (p<0.001) and increased HDL cholesterol levels (p<0.05), in comparison to positive control. Both the extracts alone and in combination exerted hypolipidemic effect in rats.
Keywords
Antioxidant; cholesterol; herbal; metabolic syndrome; niacin
Download this article as:| Copy the following to cite this article: Prakash J, Shenoy S, Nandakumar K, Raghavendra A. P, Kishore A. Role of Hydroalcoholic Extracts of Aegle marmelos (AM) Leaves and Tamarindus indica (TI) Seeds on High Fat High Sugar Diet Induced Hyperlipidemia in Rodent Models. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Prakash J, Shenoy S, Nandakumar K, Raghavendra A. P, Kishore A. Role of Hydroalcoholic Extracts of Aegle marmelos (AM) Leaves and Tamarindus indica (TI) Seeds on High Fat High Sugar Diet Induced Hyperlipidemia in Rodent Models. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3XQ6nJ0 |
Introduction
Mortality due to cardiovascular disorders is an important health related problem in both developing and developed countries. Hyperlipidemia, a prime cause of atherosclerosis, is associated with an increase in plasma levels of cholesterol, triglycerides and various lipoproteins1. The exact cause and relation of hyperlipidemia and atherosclerosis is quite complex – may be primary (genetic) or secondary (environmental or secondary to diabetes)2. Many drugs have been used for treating hyperlipidemia. At present, five classes of hypolipidemic drugs are used for treatment of hyperlipidemia. They are HMG COA reductase (3-Hydroxy-3-methylglutaryl–coenzyme A) inhibitors, bile acid sequestrants, fibric acid derivatives (fibrates), nicotinic acid derivatives (niacin) and selective cholesterol absorption inhibitor (ezetimibe). The success of these drugs is limited on long term use as they are associated with serious side effects like myopathy, osteoporosis, renal damage, gall stones etc1,3. Hence, there is a need for drugs, which can be used effectively as hypolipidemics with minimum side effects.
Use of plants for the treatment of various diseases has been practiced since Vedic era. Currently, worldwide, around 70% of the population is dependent on herbal formulation for their health issues4. Herbal formulations have gained importance around the globe owing to their lower adverse effects and cost effectiveness. Those containing more than one plant extract are being increasingly used for the treatment of various diseases. They contain multiple phytoconstituents which may provide synergistic action for the management of diseases as compared to a single herb which may have less quantity of active principles. Such polyherbal formulations (PHF) are an important mode of therapy in Ayurveda, Chinese and Siddha system of medicine5.
Aegle marmelos (Family-Rutaceae), commonly called as ‘‘Bael’’ in Hindi and “Bilva”in Kannada, is indigenous to India. All parts of Aegle marmelos is of medicinal importance. Leaves have antidiabetic, hypolipidemic, hepatoprotective, anticancer, mild laxative, antifungal, antibacterial, cardioprotective, chemoprotective, radioprotective, anti-oxidant, anti-spermatogenic and antithyroid activities. They have been used for asthma, fever, jaundice, ophthalmia, bronchitis, ulcers, abdominal disorders, vomiting, beriberi, cholera and diarrhea6-7. Tamarindus indica (family-Caesalpiniacae), commonly called ‘‘Imli’’ in Hindi and “Hunase Hannu / Huliis”, in Kannada is found throughout India. The fruit of this tree has dark brown pods which is almost the size of a human finger, with soft sticky sour pulp containing up to 10 seeds per pod. These seeds have biologically active compounds with antidiabetic, hypolipidemic, hepatoprotective, antimalarial, antimicrobial, antioxidant, antiasthmatic, laxative, and antivenomic activities8–11.
Aegle marmelos (AM)and Tamarindus indica (TI)are tree-type plants which are abundantly found all over India. It has been reported that the aqueous and alcoholic extract of Aegle marmelos leaves and Tamarindus indica seeds have individually shown antihyperglycemic and hypolipidemic effects6,12–18. However, there are no reports on hypolipidemic effect of hydroalcoholic extract of AM leaves and TI seeds. Therefore, this study was designed to check the hypolipidemic activity of these hydroalcoholic extracts, alone and in combination, in different doses on High Fat High Sugar (Fructose) Diet (HFHSD) induced hyperlipidemia in rats.
Materials and Methods
Preparation of hydroalcoholic extract
Mature leaves of Aegle marmelos and seeds of Tamarindus indica were collected from Udupi district of Karnataka. The identification of Aegle marmelos (family Rutaceae) and Tamarindus indica (family Caesalpiniacae) was carried out by the faculty of Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal. The materials of each plant were cleaned, dried, powdered and extraction was done separately. 100 g powdered extract of each i.e Aegle marmelos leaves and Tamarindus indica seeds were subjected to maceration for 7 days at room temperature using 500 mL ethanol and 500 mL distilled water (50:50 proportion). The extracts were filtered, solvent was evaporated under vacuum and further dried on water bath following which a paste residue of Aegle marmelos leaves andpowdered residue of Tamarindus indica seeds were obtained8,19.
High-Fat and High- Sugar (Fructose) Diet (HFHSD)
High fat diet (HFD) cakes were prepared by mixing coconut oil (30 % v/w), cholesterol (2% w/w) and cholic acid (2 % w/w) with powdered standard rat feed. Each HFD cake weighed 20 g. High sugar diet consisted of 25% fructose in drinking water20.
Animals
Adult male Sprague Dawley rats (n = 54) were used for the study conducted at Central Animal Research Facility Manipal, India. The experiment was conducted following the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)21. Study approval was obtained from Institutional Animal Ethics Committee, Kasturba Medical College, Manipal; (IAEC/KMC/81/2020). The animals were maintained in conducive environment (temperature 22–240C, with 12–h light – dark cycle, 40–60% humidity, with a standard rat feed (VRK Nutritional solution, Pune, India) and water ad libitum. Well acclimatized animals were used for the experiment.
Out of 54 male Sprague Dawley rats, six were in normal control group and received standard diet consisting of protein (22.85%), fat (4.55%), carbohydrate (63%), fiber (2.70%), phosphorus (0.665), total ash (5.10%) and moisture (8.20%) (VRK Nutritional solution, Pune, India) and water ad libitum The remaining 48 rats were fed orally with HFHSD i.e., HFD as a 20 g cake combined with high sugar (25 % fructose in drinking water) for 1 month for inducing hyperlipidemia. Plasma cholesterol level above 200 mg/dL on day 30 was an indicator for the induction of hyperlipidemia in the HFHSD fed rats. Animals were weighed on 0, 10th, 20th and 30th day and HFHSD dose was adjusted according to body weight22. The study was then continued from day 31 for next thirty days and rats were treated orally as follows – Group I, normal control (n=6), received standard diet. The 48 rats which had earlier received HFHSD were divided into 8 groups (n=6 in each). Group II- HFHSD control received standard rat diet, Group III -positive control (Niacin, 100 mg/kg/day),23 Group IV – AM25 and Group V-AM50 (25 & 50mg/kg/day, respectively), Group VI TI25 and Group VII TI50 (25 and 50 mg/kg/day, respectively), Group VIII AM25+TI25 (25 mg/kg/day AM +25 mg/kg/day TI).and Group IX AM50+TI50 (50 mg/kg/day AM + 50mg/kg/day TI). Sodium carboxymethyl cellulose 1% (Na-CMC) was used as vehicle for administering the standard drug and extracts. Animals in all groups also received standard rat feed and water ad libitum.
After overnight fasting, blood samples were collected on day 61 by retro-orbital puncture from all animals and centrifuged at 4400 rpm for 15 minutes. Plasma triglycerides (TGs), total cholesterol, Low-Density Lipoprotein (LDL-cholesterol), Very Low-Density Lipoprotein (VLDL- cholesterol), High Density Lipoprotein (HDL-cholesterol) and random blood glucose were estimated as per the standard protocols given along with respective colorimetric kits (Aspen Laboratories, New Delhi, India). After collection of blood for biochemical investigations, rats were sacrificed. The liver was excised and rinsed with ice-cold saline and liver homogenates (10% w/v) were prepared in cold potassium phosphate buffer (pH 7.4). The resulting supernatant were stored at -20˚C for the estimation of catalase (CAT) activity, malondialdehyde (MDA) and reduced glutathione (GSH) levels as per the standard protocols given along with respective colorimetric kits (Aspen Laboratories, New Delhi, India).
Statistical analysis
Results were analyzed using Statistical Package for the Social Sciences (SPSS version 20.0; SPSS Inc., Chicago, USA). Normally distributed data is expressed as mean ± standard error of mean and analyzed by one-way analysis of variance (ANOVA) followed by post hoc Bonferroni test. Independent samples ‘t’ test was used to compare the body weight. A p value ≤ 0·05 was considered as statistically significant.
Results
Establishment of Hyperlipidemia Model
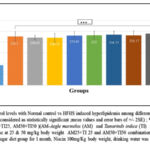 |
Figure 1: Comparison of Plasma Cholesterol levels with Normal control vs HFHS induced hyperlipidemia among different groups (One-way ANOVA followed by post hoc test – Bonferroni). |
Figure 1. Shows the establishment of hyperlipidemia model (Plasma cholesterol level >200 mg/dl), where in all groups except the normal control have significantly (p<0.0001) higher plasma cholesterol level induced by one month of HFHS diet.
Effect of HFHS diet and treatment with extracts on body weight of rats (Figure 2A and 2B)
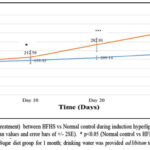 |
Figure 2A: Comparison of body weight (pretreatment) between HFHS vs NORmal control during induction Hyperlipidemia. |
 |
Figure 2B: Comparison of Body weight in HFHS inducced hyperlipidemia among different groups followed by post hoc test – Bonferroni). |
Figure 2 A and 2B: depicts a significant increase in the body weight of rats fed with HFHS diet for 30 days. There was 25.89 % increase in the body weight in normal control while HFHS fed rats showed 89.76 % increase in body weight at the end of one month (figure 2A) (p<0.001). However, there was a significant reduction in body weight (figure 2B) in rats treated with extracts (AM25, AM50, TI25, TI50, AM25+TI25, AM50+TI50) or niacin from day 31 to day 60.
Effect of treatment with extracts and niacin on lipid profile of the HFHS fed rats (Figure 3A to 3E)
Figure 3A: Plasma cholesterol level in the HFHS group was significantly high (60.61%) in comparison to the normal control (p<0.001). Following treatment with extracts (AM25, AM50, TI25, TI50, AM25+TI25, AM50+TI50) or niacin, the plasma cholesterol level was significantly reduced (35%, 30%,39%, 33%, 40%, 50% and 51% respectively) (p<0.001). In the groups which received combination of extracts (AM25+T125 and AM50+T150), the cholesterol levels were significantly lower as compared to the positive control group (Niacin, p<0.001).
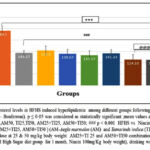 |
Figure 3A: Comparison of Plasma Cholesterol levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin(One-way ANOVA followed by post hoc test – Bonferroni). |
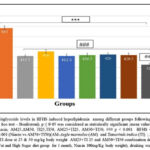 |
Figure 3B: Comparison of Plasma Triglyceride levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin(One-way ANOVA followed by post hoc test – Bonferroni). |
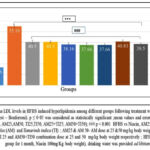 |
Figure 3C: Comparison of Plasma LDL levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin(One-way ANOVA followed by post hoc test – Bonferroni). |
 |
Figure 3D: Comparison of Plasma VLDL levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin (One-way ANOVA followed by post hoc test – Bonferroni). |
Figure. 3B to 3D: HFHS diet induced model of hyperlipidemia showed a significant increase in plasma TG levels (150.64%), LDL-cholesterol (231.09%) and VLDL- cholesterol (285%) as compared to normal control (p<0.001). However, following 30 days of treatment with extracts (AM25, AM50, TI25, TI50, AM25+TI25, AM50+TI50) or niacin, there was a significant decrease in TG, LDL and VLDL in all groups.
Rats treated with high dose of combination of extracts (AM50+T150) had significantly lower triglycerides levels when compared to the other treatment groups (51.97%) (p<0.001).
 |
Figure 3E: Comparison of Plasma HDL levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin (One-way ANOVA followed by post hoc test – Bonferroni). |
Figure 3E: There was a significant decrease in the HDL levels (56.88%) in the HFHS group as compared with that of normal control (p<0.001). All the treated groups (Niacin, AM25, AM50, TI25, TI50, AM25+TI25 and AM50+TI50), showed significant increase in HDL-cholesterol (p<0.05), however, the higher dose in combination group i.e., AM50+T150 resulted in a significant increase as compared to positive control Niacin (p<0.05).
Overall, the high dose combination of extracts (AM50+T150) significantly reduced plasma cholesterol and TGs but increased HDL levels as compared to treatment with niacin.
Effect of treatment with extracts and niacin on random blood glucose level in HFHS fed rats (Figure:4)
 |
Figure 4: Comparison of Random Blood levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin (One-way ANOVA followed by post hoc test – Bonferroni). |
Figure 4: The HFHS diet induced an increase in random blood sugar (p<0.001) level as compared to normal control group. Treatment with extracts (alone and in combination) or niacin resulted in significant reduction of blood glucose levels in comparison to HFHS diet treated animals (p<0.001).
Effect of treatment with extracts and niacin on hepatic catalase activity, GSH levels and MDA levels in HFHS fed rats (Figure 5A to 5C)
Figure 5A & 5B: There was a significant reduction in hepatic catalase level and reduced GSH levels of the HFHS group (61.39%, 43.15% respectively) when compared with the normal control group (p<0.001). All the treatment groups (Niacin, AM25, AM50, TI25, TI50, AM25+TI25 and AM50+TI50) showed significant improvement in catalase levels (p<0.001) & reduced GSH levels (p<0.05) in comparison to HFHS group.
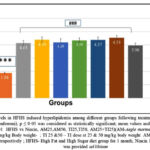 |
Figure 5A: Comparison of Catalase levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin (One-way ANOVA followed by post hoc test – Bonferroni). |
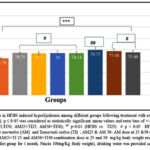 |
Figure 5B: Comparison of GSH levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin (One-way ANOVA followed by post hoc test – Bonferroni). |
Figure 5C: HFHS fed rats had a significant increase in plasma MDA levels (p value < 0.001) as compared to normal control group. A significant decrease in MDA levels in all treated groups in comparison with the HFHS group was noticed (p<0.001).
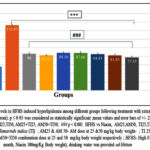 |
Figure 5C: Comparison of MDA levels in HFHS induced hyperlipidemia among different groups following treatment with extracts and Niacin (One-way ANOVA followed by post hoc test – Bonferroni). |
Discussion
Evaluation of cardiovascular risk helps to control and minimize cardiovascular diseases24. Hyperlipidemia is a cluster of symptoms, which includes high cholesterol and triglyceride levels that predispose to cardiovascular diseases. It preludes the process of atherosclerosis, coronary artery disease, myocardial infarction and ischemic stroke by inducing oxidative stress25. Further, hypertriglyceridemia can contribute to metabolic syndrome and pancreatitis 26–29. Although many drugs are prescribed for controlling hyperlipidemia, most of the drugs have unpleasant side effects on chronic use. Though statins continue to be the major anti-hyperlipidemic agents prescribed by clinicians for the management of elevated plasma cholesterol and triglycerides, yet their prolonged use is associated with severe side effects like myopathy and hepatotoxicity 30-31. Hence, the need to look for newer hypolipidemic drugs which are effective but have less adverse effects.
High fat diet increases lipid peroxidation resulting in oxidation of fatty acids in the liver leading to malondialdehyde (MDA) formation. Oxidative stress is an initial incident in the progression of hyperlipidemia32-33. The free radicals generated oxidise LDL cholesterol. This attracts the macrophages, which accumulate in the wall of the blood vessels after phagocytosing the LDL-C particles leading to atherosclerotic plaques34. Increase in serum triglycerides is consistent in coronary artery diseases35. HDL-C decreases cholesterol levels and prevents atherosclerotic plaques by transporting cholesterol and cholesterol esters from different parts of the body to liver, where they are metabolized into bile acids36. All these suggests that high levels of triglycerides, LDL-cholesterol and total cholesterol are important factors in the pathogenesis of cardiovascular diseases.
Increase in cholesterol levels alters the physical properties of cell membrane resulting in seepage of reactive oxygen species (ROS) from electron transport chain of mitochondria or NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) activation leading to lipid peroxidation and oxidation of proteins37. Lipid peroxidation damages the integrity of cell membrane, which results in inactivation of the receptors and enzymes present in cell membrane ensuing in augmented cell permeability and cell death. Further, reactive oxygen species (ROS) interact with antioxidant enzymes resulting in diminished cellular antioxidant defence38. These antioxidant enzymes are important during oxidative stress as they minimize lipid peroxidation.
There are various studies for the role of plant extracts in the treatment of hyperlipidemia. The importance of polyherbal formulations in Ayurveda literature, Sarangdhar Samhita, is well documented – a combination of herbs has better therapeutic efficacy and safety. A single active compound of an individual plant may be inadequate in producing a desirable therapeutic outcome5,39. Multiple active compounds in extracts have synergistic benefits for protection of cells. Hydroalcoholic extracts of Aegle Marmelos leaves and Tamarindus indica seeds were able to control hyperlipidemia in rodents when used individually and in combination but the effect was better with the latter. The combination in higher dose (AM50+TI50) was more effective than the positive control (Niacin) in controlling plasma triglyceride, cholesterol and improving HDL cholesterol levels. This could be due to effects of various constituents in the combination.
Studies have revealed the presence of phenolic compounds and flavonoids in the extracts of Aegle marmelos (AM) leaves and Tamarindus indica (TI) seeds act as hypolipidemic and antioxidant agents40. It has been reported that the hydroalcoholic extracts of AM leaves contain rutin, quercetin and gallic acid whereas Tamarindus indica (TI) consists of antioxidants epicatechin, taxifolin, apigenin and luteolinalong with other constituents10,17,41 Rutin, quercetin and gallic acid are known to possess antioxidant and hypolipidemic activities42-45. Rutin and quercetin suppress lipid peroxidation, protect cell membrane and prevent liver injury. Quercetin also inhibits 3-Hydroxy-3-methylglutaryl–coenzyme A (HMG CoA) reductase. Both quercetin and gallic acid decrease absorption of cholesterol, and lower plasma as well as hepatic cholesterol levels46. These compounds in the extracts could have exerted a beneficial effect on HFHS induced hyperlipidemia in the current study.
Epicatechin, present in TI seed extract, is reported to augment the levels of phospho-AMPK /total-AMPK (adenosine monophosphate-activated protein kinase) in muscle and liver. It also increases GLUT 4 (glucose transporter type 4) levels in membranes, which facilitates peripheral utilization of glucose47. This could be the one of the reasons for decrease in glucose levels in all treated groups. Taxifolin has an inhibitory effect on enzymes, α-glucosidase and α-amylase, which helps in controlling postprandial hyperglycemia. By inhibiting pancreatic lipase, taxifolin decreases the absorption of triglycerides48. Apigenin improves the lipid balance by promoting the low-density lipoprotein cholesterol absorption and increasing the conversion of hepatic cholesterol to bile acid49. Luteolin promotes the elimination of cholesterol by upregulating ABCG1 (ATP-binding cassette sub-family G member 1) transporters and SR-B1(Scavenger receptor class B type 1) receptors50-51.
The antioxidants we investigated in this study were catalase and glutathione (GSH). The present study showed a significant increase in hepatic lipid peroxidation and a decrease in catalase activity and GSH levels which, could be, associated with HFHS diet induced hyperlipidemia. Treatment with these extracts, either alone or in combination, decreased lipid peroxidation and increased antioxidant levels. This could be due to antioxidant activity of the extracts as well as inhibition of the absorption and synthesis of cholesterol and reuptake of bile acids52. The differential mode of action of various constituents in the combination could result in synergistic hypolipidemic effect. This would be an advantage of polyherbal formulations in hyperlipidemia.
Conclusion
The hydroalcoholic extracts of Aegle Marmelos leaves and Tamarindus indica seeds, alone and in combination, reduced triglycerides, total cholesterol, LDL and VLDL but increased HDL levels in serum. In combination, the higher dose (AM50+TI50) has shown better result in controlling plasma triglyceride and cholesterol while improving HDL cholesterol levels which could be due to synergistic actions of the active constituents of Aegle Marmelos leaves and Tamarindus indica seeds. This type of polyherbal formulations may help when translated to clinical scenario.
Acknowledgements
The authors would like to thank Manipal Academy of Higher Education, Manipal, India for the infrastructure provided to carry out this research.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise with this work.
Funding sources
There is no funding Sources
References
- Shattat GF. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed Pharmacol J. 2014; 7: 399–409.
CrossRef - Wouters K, Shiri-Sverdlov R, van Gorp PJ, van Bilsen M, Hofker MH. Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice. Clin Chem Lab Med. 2005; 43: 470–479.
CrossRef - Kamal F, Shahzad M, Ahmad T, et al. Antihyperlipidemic effect of Pistacia khinjuk. Biomed Pharmacother. 2017; 96: 695–699.
CrossRef - Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001; 109: 69–75.
CrossRef - Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev. 2014; 8: 73.
CrossRef - Sandeep D, Seema D. Aegle marmelos (Linn.) Correa: A potential source of Phytomedicine. J Med Plant Res. 2011; 5: 1497–1507.
- Prakhar B, Amrinder K, Megha M. Mythological and spiritual review on Aegle marmelos and its therapeutic uses. Plant Cell Biotechnol Mol Biol. 2021; 60–70.
- Bandawane D, Hivarale M, Mali A, Mhetre N. Evaluation of anti-inflammatory and analgesic activity of tamarind (Tamarindus indica L.) seeds. Int J Pharm Pharm Sci. 2013; 5:623-629.
- Isha D, Milind P. Imlii: A craze lovely. Int. Res J Pharm. 2012; 3(18):110-510
- Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. Tamarindus indica: Extent of explored potential. Pharmacogn Rev. 2011; 5: 73–81.
CrossRef - Havinga RM, Hartl A, Putscher J, Prehsler S, Buchmann C, Vogl CR. Tamarindus indica L.(Fabaceae): patterns of use in traditional African medicine. J Ethnopharmacol. 2010; 127: 573–588.
CrossRef - Sharma B, Satapathi SK, Roy P. Hypoglycemic and hypolipidemic effect of Aegle marmelos leaf extract on streptozotocin induced diabetic mice. Int J Pharmacol. 2007; 3: 444–452.
CrossRef - Vijaya C, Ramanathan M, Suresh B. Lipid lowering activity of ethanolic extract of leaves of Aegle marmelos (Linn.) in hyperlipidaemic models of Wistar albino rats. Indian J Pharmacol. 2009;47: 182-185.
- Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. 2006; 107: 374–379.
CrossRef - Maiti R, Das UK, Ghosh D. Attenuation of hyperglycemia and hyperlipidemia in streptozotocin-induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol Pharm Bull. 2005; 28: 1172–1176.
CrossRef - Hemalatha S, Shailesh Kumar KN. Phytochemical evaluation of leaf extracts of Aegle marmelos. Int J Dev Res. 2013; 3(7):029-033.
- Patel DK, Patel K, Rahman M, Chaudhary S. Therapeutic Potential of “Aegeline,” an Important Phytochemical of Aegle marmelos: Current Health Perspectives for the Treatment of Disease. In: Rahman, M, Beg S, Kumar V, Ahmad F, eds. Nanomedicine for Bioactives. Springer, Singapore; 2020 : 383 – 392. Accessed May 12, 2022. https://doi.org/10.1007/978-981-15-1664-1_14.
CrossRef - Dhankhar S, Ruhil S, Balhara M, Dhankhar S, Chhillar AK. Aegle marmelos (Linn.) Correa: A potential source of Phytomedicine. J Med Plant Res. 2011; 5: 1497–1507.
- Chockalingam V, Kadali SDVS, Gnanasambantham P. Antiproliferative and antioxidant activity of Aegle marmelos (Linn.) leaves in Dalton’s Lymphoma Ascites transplanted mice. Indian J Pharmacol. 2012; 44: 225.
CrossRef - Munshi RP, Joshi SG, Rane BN. Development of an experimental diet model in rats to study hyperlipidemia and insulin resistance, markers for coronary heart disease. Indian J Pharmacol. 2014 May;46(3):270
CrossRef - CPCSEA Guidelines For Laboratory Animal Facility 2015 in Compendium of CPCSEA 2018. Ministry of Environment, Forest and Climate Change, Government of India. Accessed February 18, 2022. https://cpcsea.nic.in/ WriteReadData/userfiles/ file/Compendium%20of%20CPCSEA.pdf
- Kumar N, Mudgal J, Parihar VK, Nayak PG, Kutty NG, Rao CM. Sesamol treatment reduces plasma cholesterol and triacylglycerol levels in mouse models of acute and chronic hyperlipidemia. Lipids. 2013;48:633-8.
CrossRef - Bolkent S, Yanardag R, Bolkent S, Döger MM. Beneficial effects of combined treatment with niacin and chromium on the liver of hyperlipemic rats. Biol Trace Elem Res. 2004 Dec;101:219-229
CrossRef - .Bhushan MS, Rao CH, Ojha SK, Vijayakumar M, Verma A. An analytical review of plants for antidiabetic activity with their phytoconstituent and mechanism of action. Int J Pharm Sci Res. 2010;1(1):29-46.
- Mishra PR, Panda PK, Apanna KC, Panigrahi S. Evaluation of acute hypolipidemic activity of different plant extracts in Triton WR-1339 induced hyperlipidemia in albino rats. Pharmacologyonline. 2011; 3: 925–934.
- Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999 May 13;83(9B):25F-29F. doi: 10.1016/s0002-9149(99)00211-8.
CrossRef - Kota SK, Kota SK, Jammula S, Krishna SV, Modi KD. Hypertriglyceridemia-induced recurrent acute pancreatitis: A case-based review. Indian J Endocrinol Metab. 2012 ;16(1):141-143.
CrossRef - Garg R, Rustagi T. Management of Hypertriglyceridemia Induced Acute Pancreatitis. Biomed Res Int. 2018; 2018:4721357. doi: 10.1155/2018/4721357.
CrossRef - Yuan G, Al-Shali KZ,Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. Can Med Assoc J. 2007;176(8):1113-1120.
CrossRef - Ravnskov U, Rosch PJ, Sutter MC, Houston M. C. Should we lower cholesterol as much as possible? Br Med J. 2006; 332: 1330–1332.
CrossRef - Gotto Jr. MG. Atherosclerosis: Evolving vascular biology and clinical implications. Circulation. 2004; 109: III–1.
- Yang RL, Shi YH, Hao G, Li W, Le GW. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J Clin Biochem Nutr. 2008; 43: 154–158.
CrossRef - Ponce-Canchihuamán JC, Pérez-Méndez O, Hernández-Muñoz R, Torres-Durán PV, Juárez-Oropeza MA. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010; 9: 1–7.
CrossRef - Ntchapda F, Djedouboum A, Talla E, et al. Hypolipidemic and anti-atherogenic effect of aqueous extract leaves of Ficus glumosa (Moraceae) in rats. Exp Gerontol. 2015; 62: 53–62.
CrossRef - Irudayaraj SS, Sunil C, Duraipandiyan V, Ignacimuthu S. In vitro antioxidant and antihyperlipidemic activities of Toddalia asiatica (L) Lam. leaves in Triton WR-1339 and high fat diet induced hyperlipidemic rats. Food Chem Toxicol. 2013; 60: 135–140.
CrossRef - Chen Q, Reis SE, Kammerer C, et al. Association of anti-oxidized LDL and candidate genes with severity of coronary stenosis in the Women’s Ischemia Syndrome Evaluation study. J Lipid Res, 2011; 52: 801–807.
CrossRef - Singh UN, Kumar S, Dhakal S. Study of Oxidative Stress in Hypercholesterolemia. Int J Contemp Med Res. 2017; 4 (5): 77.83
CrossRef - Smathers RL, Galligan JJ, Stewart BJ, Petersen DR. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem Biol Interact. 2011; 192: 107–112.
CrossRef - Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev. 2014; 8: 73.
CrossRef - Shukr MH, Ismail S, Ahmed SM. Development and optimization of ezetimibe nanoparticles with improved antihyperlipidemic activity. J Drug Deliv Sci Technol. 2019; 49: 383–395.
CrossRef - Aneesh A, George AJ, Kariyil BJ, Krishna D, Abraham MJ. Phytochemical evaluation of the leaves of Aegle marmeloes L.(L.)–an important medicinal plant. J Trop Agric. 2018; 56.
CrossRef - Jang A, Srinivasan P, Lee NY, et al. Comparison of hypolipidemic activity of synthetic gallic acid-linoleic acid ester with mixture of gallic acid and linoleic acid, gallic acid, and linoleic acid on high-fat diet induced obesity in C57BL/6 Cr Slc mice. Chem Biol Interact. 2008; 174: 109–117.
CrossRef - Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007; 98: 727–735.
CrossRef - Yan SX, Li X, Sun CD, Chen KS. Hypoglycemic and hypolipidemic effects of quercetin and its glycosides. Zhongguo. Zhongyao. Zazhi. 2015; 40: 4560–4567.
- Livingston Raja NR, Ravindran NA, Senthilpandian S, Ravi V. Hypolipidemic action of Rutin on Triton WR-1339 induced hyperlipidemia in rats. J Pre Clin Clin Res. 2021; 15: 51–55.
CrossRef - Ziaee A, Zamansoltani F, Nassiri-Asl M, Abbasi E. Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin Pharmacol Toxicol. 2009; 104: 253–258.
CrossRef - Shih CC, Wu JB, Jian JY, Lin CH, Ho HY. (−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, prevents diabetes and hyperlipidemia by regulation of glucose transporter 4 and AMP-activated protein kinase phosphorylation in high-fat-fed mice. Int J Mol Sci. 2015; 16: 24983–25001.
CrossRef - Su H, Ruan YT, Li Y, Chen JG, Yin ZP, Zhang QF. In vitro and in vivo inhibitory activity of taxifolin on three digestive enzymes. Int J Biol Macromol. 2020; 150: 31–37.
CrossRef - Zhang K, Song W, Li D, Jin X. Apigenin in the regulation of cholesterol metabolism and protection of blood vessels. Exp Ther Med. 2017; 13: 1719–1724.
CrossRef - Wong TY, Tan YQ, Lin SM, Leung LK. Apigenin and luteolin display differential hypocholesterolemic mechanisms in mice fed a high-fat diet. Biomed Pharmacother. 2017; 96: 1000–1007.
CrossRef - Dergunov AD, Savushkin EV, Dergunova LV, Litvinov DY. Significance of cholesterol-binding motifs in ABCA1, ABCG1, and SR-B1 structure. J Membr Biol. 2019; 252: 41–60.
CrossRef - Chan PT, Fong WP, Cheung YL, Huang Y, Ho WKK, Chen ZY. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999; 129: 1094–1101.
CrossRef







