Hatem Mohamed Hassan1, Eman Abo Elmaaty2, Marwa W Aboulenaga3, Ayman F Armaneous 3 Mohamed A. Shahba 4
Mohamed A. Shahba 4 and Eman R. Youness 4*
and Eman R. Youness 4*
1Departments of Reproductive Health, Medical Research and Clinical Studies Institute, National Research Center, Cairo, Egypt.
2Neonatology Department, El Galaa Teaching Hospital, Cairo, Egypt.
3Departments of Child Health, Medical Research and Clinical Studies Institute, National Research Center, Cairo, Egypt.
4Medical Biochemistry, Medical Research and Clinical Studies Institute, National Research Center, Cairo, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/3000
Abstract
Human Pegivirus (HPgV-1), which was known as hepatitis G virus (HGV) or GB virus C (GBV-C) is a single – stranded positive RNA virus belonging to the genus Pegivirus of Flaviviridae family. Its genomic organization is similar to that of HCV with which it has only 25% homology at the nucleotide level. The aim of this study is to evaluate the prevalence of HPgV-1 among high risk pregnant women (with HCV infection or history of previous blood transfusion) and normal pregnant women. In addition to detect the vertical transmission of the virus to their newborns. Thirty term high risk and thirty term normal pregnant females were screened for HPgV-1 RNA using the reverse transcription PCR technique. HPgV-1 was detected in six females among those who have HCV infection (33.3%) and in two females among recipients of blood transfusion (16.6%), also it was detected in one female of the control group (3.3%). The outcome of newborns showed three newborns with HPgV-1 infection out of six born to the females who have both HCV and HPgV-1 infection and one newborn of the infected mother of the control group, however, liver functions of the newborns were in the normal range for age requiring long term follow up.
Keywords
High risk pregnancy; Human Pegivirus; Newborn; Vertical transmission
Download this article as:| Copy the following to cite this article: Hassan H. M, Elmaaty E. A, Aboulenaga M. W, Armaneous A. F, Shahba M. A, Youness E. R. Prevalence of Human Pegivirus Infection during High Risk Pregnancy and its Vertical Transmission to the Newborn. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Hassan H. M, Elmaaty E. A, Aboulenaga M. W, Armaneous A. F, Shahba M. A, Youness E. R. Prevalence of Human Pegivirus Infection during High Risk Pregnancy and its Vertical Transmission to the Newborn. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3xUKMX8 |
Introduction
Viral infections during pregnancy may lead to adverse pregnancy outcomes, including pregnancy loss, premature labor, congenital anomalies, maternal and fetal mortality1.
One of the main causes of acute and chronic liver diseases are hepatitis viruses A-E, but these viruses do not explain all cases of viral associated liver disease. Many evidence refer to the presence of another infectious agent for example 0.4 percent of post – transfusion hepatitis is unexplained and classified as non- A, non B, non- C hepatitis 2.
Human pegivirus (HPgV-1), which was known as hepatitis G virus (HGV) or GB virus C (GBV-C) is a single – stranded positive RNA virus belonging to the genus Pegivirus of Flaviviridae family 3.
Concurrent infection with HPgV-I and HCV is common, the prevalence of HPgV-1 in patients with HCV infection undergoing liver transplant is 24% pre-transplantation and 28% post- transplantation 4.
It is documented that HPgV-1 can be transmitted parentally and sexual contact may be another important route of transmission. Vertical transmission from infected mother to her newborn has been reported. HPgV-1 can cause acute and persistent infection in human. Persistent viremia has been documented in the absence of transaminase elevation 5. HPgV-1 has been reported to be involved in the etiology of fulminant hepatic disease and others found no HPgV-1 in patient with fulminant liver disease 6.
The role of HPgV-1 is little understood. The aim of our study was to assess the prevalence of HPgV-1 in pregnant female with documented HCV and those with history of previous blood transfusion. Also, to evaluate the possibility of maternal transmission to newborns and the effect of HPgV-1 on liver functions.
Patients and Methods
Our study was done on 60 term pregnant females attending El Galaa Teaching Hospital and Medical Research Centre of Excellency, NRC. They were classified to: Group I 30 term high risk pregnant females which subdivided as:
Group I (a): Comprised 18 term pregnant females with documented HCV infection.
Group I (b): Comprised 12 term pregnant females with history of previous blood transfusion.
Group II: 30 normal pregnant females with neither history of liver disease nor blood transfusion as a control group.
Ethical Approval
The current study was carried out in accordance with the principles and regulations of the Helsinki’s declaration. Informed consent was taken from all patients before enrollment with explanation of the type of the study. Approval from the Ethical Committee of General Organization of Teaching Hospital and Institutes was obtained.
Venous blood were collected from each female of both groups for detection of HPgV-1 infection using RT-PCR technique. Serum was separated and stored at -70°C till the end of the study. Pregnant females proved to have HPgV-1 infection were subjected to complete medical, obstetric and ultrasonography examination before delivery.
Newborns of HPgV-1 infected mothers were subjected within 24 hours of delivery to thorough medical examination including birth weight and 3 CC blood were collected to detect HPgV-1 by PCR technique and serum transaminases to assess liver functions.
HPgV-1 detection
The total RNA was extracted from 100uL of serum according to the manufactures recommendations and subjected to reverse transcriptase PCR. We used 2 oligonucleotide primers from HPgV-1 5 UTR region. (Tetro cDNA Synthesis Kit (Make: Meridian Bioscience, Cat. No: BIO-65043).
The sequences of the oligonucleotides were (Oligo ACIS) AGG GTT(GC) G (AT) (AT) GGT (GC) GTAA ATCC and (Oligo 7AS) CAA GAG (AC) G (AG) CAT TGA AG (AF) GCGA. The PCR mixture was formed of MgCl2 (25mM), 10 x PCR buffer, dNTPs (100mM), Primers (10 Pmol/U1) and Taq Polymerase. The cycling condition was: 94°C for one min, 55°C for one min and 72°C for one min., to be repeated for 45 cycles. The product was visualized on 1% agarose gel electrophoresis stained with ethidium bromide. The positive cases were shown as a band at 210 bp (figure 1).
Ethical Approval
The current study was carried out in accordance with the principles and regulations of the Helsinki’s declaration. Informed consent was taken from all patients before enrollment with explanation of the type of the study. Approval from the Ethical Committee of General Organization of Teaching Hospital and Institutes was obtained.
Results
Eight pregnant females found to have HPgV-1 infection. Five of them pregnant females with HCV infection, two pregnant females with previous history of blood transfusion and one in control group.
The newborns delivered to HPgV-1 infected females (8 newborns) were tested for the presence of HPgV-1 infection within 24 hr. of delivery. HPgV-1 was detected in 3 newborns out of 5 delivered to mothers with both HCV and HPgV-1 infection (Group 1a) and in one newborn delivered to the infected mother of the control group (Group II) (Table 1).
Serum transaminases were done for the 8 newborns of HPgV-1 infected mothers and were found to be within normal rang for both HPgV-1 Positive newborns (4 newborns) and negative newborns (4 newborns).
Table 1: HPgV-1 positive frequency among pregnant female and their newborns.
|
Group |
No. of HPgV-1 +ve females |
% |
No. of HPgV-1 +ve newborns |
|
I-High risk group (n=30) |
|
|
|
|
I (a) HCV infected mothers (n=18) |
5 |
27.7% |
3 |
|
I (b) Females with previous blood transfusion (n= 12) |
2 |
16.6% |
– |
|
II control group (n=30) |
1 |
3.3% |
1 |
|
Total |
8 |
|
4 |
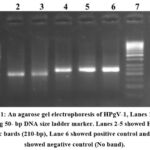 |
Figure 1: An agarose gel electrophoresis of HPgV-1, Lanes 1 and 7 showing 50- bp DNA size ladder marker. |
Discussion
The known major hepatotropic viruses (A-E) are not associated with 10% to 20% of cases of community acquired hepatitis and transfusion associated hepatitis. The detection of HPgV-1 which is about 25% identical to the HCV from patients with hepatitis, has implicated it as a cause of non A-E hepatitis 7.
In our study 5 pregnant females out of 18 (27.7%) were found to have dual infection of HPgV-1 and HCV. The risk of HPgV-1 infection seems to be increased in people who are also infected with HCV 8. Similar results were reported by Lucas who detected HPgV among 16(25%) of 63 women and 5 (8%) of 63 newborns, corresponding to vertical transmission rate of 31% 9.
This work revealed that two pregnant females out of 12 (16.6%) with previous history of blood transfusion were positive to HPgV-1.
Aniel and colleagues reported that the prevalence of HPgV infection was 12.42% in recipients of blood products and multiple blood transfusion 10. HPgV is less infectious than HCV in blood products which can be explained as HPgV is inactivated by manufacturing process or due to the presence of neutralizing antibodies 11.
The difference in frequency between HPgV-1 and HCV infection was explained as the exposed individuals are more able to clear infection with HPgV-1 due to the structural difference between the envelope glycoproteins of HCV and HPgV, which gives shielding effect preventing antibody binding to the under lying protein of HCV and by immune escape phenomenon which may prevent neutralizing antibodies. This can explain that HPgV infection is a transient infection as compared to HCV 12,13.
The prevalence of HPgV-1 infection in the normal control females in our work was 3.3%, which is similar to Jarvis who reported 3.2% prevalence in the general population in Edinburgh14. They also have noticed the paradox of the high rate of HGV infection in the general population with no history of past parenteral exposure several times that of HCV which may be attributed to different modes of transmission between both viruses 15.
Vertical transmission of HPgV could be associated with high rate of persistent infection. This may be the explanation of HPgV positively in the general population which constitutes the main reservoir of infection in communities. This is documented in HBV vertical transmission which is associated with persistent infection in around 90% of cases compared with 2-10% in adult infection 16.
Vertical HPgV-1 transmission was documented in 4 newborns out of 8 infected mothers, however, this infection did not affect liver functions of the newborns. This mother to baby transmission was also reported by Lucas and colleagues in 2017 9.
Conclusion and Recommendations
HPgV infection is relatively common especially in patients with HCV infection and its does not seem to contribute to clinical or biochemical liver disease. However, follow up of individuals especially newborns identified as HPgV PCR positive is essential to detect the persistence of infection and its impact on liver functions.
Acknowledgments
All appreciations to all participants
Conflicts of Interest
There is no conflict of interest.
Funding Sources
There are no funding Sources
References
- Yu W, Hu X, Cae B, Shi D. Viral Infections During pregnancy. The Big Challenge Threatening Maternal and Fetal health. Maternal – Fetal Medicine. 2022; 4 (1): 72-86.
CrossRef - Norah A; Miriam T; Ka Wang, and Gonzague J. Viral hepatitis and pregnancy. Nature Reviews Gastroenterology and Hepatology. 2021; 18: 117-130.
CrossRef - Yaqi Y, Zhenzhou W, Jian H, Xiang U Y, Chiyu Z. Review of Human Pegivirus: Prevalence, transmission, pathogenesis and clinical implication. Virulence 2022; 13(1): 324-341.
CrossRef - Berenguer M, Terrult NA, Piatak M. Hepatitis G. Virus infection in patients with hepatitis C virus infection undergoing Liver transplantation. Gastroenterology. 1996; III: 1569-75.
CrossRef - Chan MY and Smith MA. Infections in Pregnancy. Comprehensive Toxicology. 2018; 232-249.
CrossRef - Mortada HF, Naglaa M, Engy A, Mona A, Mohamed F. Perinatal Transmission of Hepatitis C Virus: an update, Arch Med Sci. 2020; 16 (6): 1360-1369.
CrossRef - Kayesh MEH, Kohara M, Tsukiyama K. Epidemiology and Risk factors for acute Viral Hepatitis in Bangladesh: An over view. Microorganisms. 2022; 15; 10 (11): 2266.
CrossRef - DaMota LD, Nishiya AS, Finger – Jardim F, Barral MF, Silva CM, Nader MM et al., prevalence of human pegivirus infection in Patients carrying HIV- I C or non C in Southern Brazil. J. Med Virol. 2016; 88 (12): 2016-2114.
CrossRef - Lucas M, Ruben C;, Maria F;, Carla V;, Vanusa P, Ana MB. Prevalence and Vertical transmission of human pegivirus among pregnant women infected with HIV. Int J Gynaecol Obstet. 2017; 138 (1): 113-118.
CrossRef - de Sarom A, Clayton P, Rafael R, Pedro V, Patricia D, Leticia L. et al. Human Pegivirus (HPgV, GBV-C) RNA in Volunteer blood donors from a public hemotherapy service in Northern Brazil. Virol J. 2020; 14; 17 (1) 153.
CrossRef - Michelle S, Ingrid C, Karen R, Ja- Young K, Paula A, Gil M. Viral infections during pregnancy Am J Reprod. Immunol 2015; 73 (3): 199-213.
CrossRef - Jack T. Human pegivirus Type 1: A common Human Virus that is Beneficial in Immune – Mediated Disease. Front Immunol. 2022; 30: 13-88.
CrossRef - Victor N and Justin C. Viral Hepatitis in Pregnancy. Eur J Obstet. Gynecol Reprod BioL. 2021; 256: 287-296.
CrossRef - Jarvis L M, Davidson F, Hanley JP. Infection with hepatitis G Virus among recipients of plasma products. Lancet, 1996; 348: 1352-55.
CrossRef - Vasiliy I, Tatiana I, ljudmila U. Hepatitis G Virus. World J. Gastroenterol. 2008; 14 (30): 4725-4734.
CrossRef - Yao H and Hui Y. Prevention Strategies of mother to child transmission of hepatitis B Virus infection. Pediatr Investig. 2020;4 (2): 133-137.
CrossRef








