Rashmi Mallya* and Vasanti Suvarna
and Vasanti Suvarna
SVKM’s Dr.Bhanuben Nanavati College of Pharmacy, Gate No:1 Mithibai College Campus, Vile Parle (W), Mumbai, India.
Corresponding Author E-mail:rashmi.mallya@bncp.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2962
Abstract
Zanthoxylum rhetsa is extensively utilized in the realm of gastronomy and therapeutics. However, existing literature lacks any documentation concerning the systematic assessment of the toxicity characteristics of the foliage and produce of Z.rhetsa. Consequently, the present inquiry was executed to appraise the immediate toxicity examinations of the methanol extract derived from the leaves and fruits in mice, in accordance with the regulations delineated by the Organization for Economic Cooperation and Development (OECD 420, 2001). In this investigation, a solitary dosage of 5000 mg/kg body mass of the specimen was administered to female mice, as stipulated by the OECD guidelines. Daily surveillance ensued for a total of fourteen days, with the objective of detecting any indications of toxicity or fatality. Upon the culmination of this two-week period, a comprehensive analysis involving hematological, biochemical, and histopathological evaluations was conducted. The outcomes of the immediate toxicity investigations evinced that neither the leaves nor the fruits extract exhibited any morbidity, conspicuous alterations in corporeal mass, nor any discernible impairment in behavioral or motor neural activities in the mice. The haematological and serum biochemical parameters did not exhibit significant disparities from the control groups. These findings were corroborated by histopathological investigations, wherein no abnormalities or pathological alterations were detected in the vital organs. The LD 50 (oral lethal dose) of the samples was determined to exceed 5000 mg/kg body weight. Consequently, the methanol extract of leaf and fruit extract of Z.rhetsa can be regarded as non-toxic and demonstrated a broad margin of safety for its therapeutic application.
Keywords
Acute toxicity; Fruit; Leaf; OECD guidelines; Zanthoxylum rhetsa
Download this article as:| Copy the following to cite this article: Mallya R, Suvarna V. Phytochemical and Toxicity Evaluation of Methanol Extracts of Leaves and Fruits of Zanthoxylum rhetsa (Rutaceae). Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Mallya R, Suvarna V. Phytochemical and Toxicity Evaluation of Methanol Extracts of Leaves and Fruits of Zanthoxylum rhetsa (Rutaceae). Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3LIf7eS |
Introduction
Herbal drugs, have been used since time immemorial as food and medicine. Traditional herbal remedies generally use drugs in their natural form with minimal processing and are used to treat diseases that occur locally. In last few years there is a huge surge of interest in these traditionally used drugs because of the belief that they are safe and promote healthier living. Due to the limitations observed with synthetic drugs, the pharmaceutical industry also has turned to plant sources for their drug discovery programmes. Even though there is tremendous increase in use of herbal drugs there is a huge concern about potential toxicities resulting from use of these drugs. Generally, toxicities or adverse reactions observed by use of herbs can be due to its inherent toxic nature or their irrational usage and it is very crucial to evaluate the safety of these traditionally used drugs, when they are used in newer forms. Therefore, toxicity studies on plants for medicine or formulations made from them have to be performed before they are widely used1-4.
Bangladesh, Burma, India, and the Himalayan region are home to Zanthoxylum rhetsa, a species of the Rutaceae family, also known as Z. budrunga and Z. limonella.. It can be found in India’s northeast and along the eastern and western Ghats5.The fruits are consumed as a spice and the leaves as vegetables in India’s northern region. Plants have long been used to treat illnesses like urinary tract infections and intestinal worms. In addition, it is used as an analgesic and to treat bronchitis, asthma, and cardiac problems. Fruit-derived volatile oil called mullimam has been utilised as a lipid-lowering, antiseptic, anti-inflammatory, and mosquito-repelling agent. The leaf decoction is used as a pesticide and to cure intestinal worm diseases by the Naga tribe of northeastern India. Many studies are reported in literature that reveal the presence of various classes of secondary metabolites with good biological and pharmacological activities. Zanthoxylum rhetsa is widely used in food and therapeutics 6,7. However, there are no reports in literature on the systematic evaluation of the toxicity profile of the leaves and fruits of Z.rhetsa. Thus, the present study was conducted to evaluate acute toxicity studies of the methanol extract of leaves and fruits in mice according to Organization for Economic Cooperation and Development (OECD) 425 guidelines.
Materials and Methods
Collection of plant material and preparation of methanol extract
Leaves and fruits were collected from Karkala, Udupi district, Karnataka, India. Dr. Rajendra D. Shinde of Blatters Hebarium at St. Xaviers College in Mumbai validated the authenticity of the materials. Fresh fruit and leaves were shade-dried, pulverised, A typical maceration procedure was used to prepare the methanol extract. To remove the solvent, the extract was filtered, concentrated using a distillation unit, and evaporated over an electric water bath set at 40°C.
Acute toxicity studies
Total methanol extract of leaf and fruit Z.rhetsa was subjected to acute toxicity studies as per Organization for Economic Co-operation and Development guideline for testing of chemicals (OECD 420, 2001). To assess the safety of the extracts when taken orally, a single dose acute oral toxicity trial involving three rats of the same sex was conducted.
Animal procurement and acclimatization
The Institutional Animal Ethical Committee of SVKM’s Dr. Bhanuben Nanavati College of Pharmacy, Vile Parle(W), Mumbai, granted approval for the study. (IAEC approval Number: CPCSEA/IAEC/P-78/2017) The acute toxicity was conducted on 8-12 weeks old female Swiss albino mice weighing 20-25 gm. Nine animals were procured from National Institute of Biosciences, Pune. The cages made of polypropylene held the animals. Three mice per cage in a 12:12 light:dark lighting cycle, with controlled temperature (25±2oC) and relative humidity (55±5%). The animals were maintained as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) India. They were randomly selected and marked on the tail for identification and assigned to three groups. Before beginning the experiment, they had a week to get used to the lab environment.
Procedure of limit test
Animals were abstained from food overnight prior to dosing but was provided unrestricted access to water. Following the fasting duration, the mice’s body weight was determined and the dosages were calculated. The dosages were freshly prepared and were administered via an oral feeding tube as a suspension using 0.05% of CMC as a solvent at a volume of 1.0 mL/kg as exemplified below.
Control group (n=3) administered 0.05% CMC
Treatment group A (n=3) administered 5000 mg/kg body weight of methanol extract of foliage
Treatment group B (n=3) administered 5000 mg/kg body weight of methanol extract of fruit
Food was provided after 1 hour of treatment. Throughout the study, all animals were kept under constant observation to assess their health and look for any abnormalities. The animals were evaluated for gross behavioural abnormalities, clinical symptoms (tremors, convulsions, lethargy, and coma), and mortality at 30 minutes, 1 hour, 2 hours, and 24 hours. These observations were conducted for 14 days. When two of the three animals showed signs of toxicity or fatality, the dose given proved toxic, and a lower dosage was given to the animals in the following group. To confirm the results, the same dosage was administered again in the event that any one of the three animals showed signs of toxicity or death. The dosage was regarded as safe if there was no fatality. On days 0, 7, and 14, the mean body weights of the animals in the control and test groups were measured. At the end of the experiment, blood was collected by retro orbital route into non heparinized and EDTA containing tubes for detailed biochemical and hematological analysis. The animals were killed by cervical dislocation, after which the liver, kidney, lung, heart, and spleen were removed and kept for histological analysis in a fixation medium containing a 10% solution of buffered formalin.
Results and Discussion
When no other toxicological information is available, acute toxicity testing can offer an initial assessment of a material’s toxicity. The median lethal dosage, or LD50, is a useful tool for estimating a chemical’s possible risk to humans. those having an LD50 of less than 5 mg/kg are considered very toxic, whilst those having an LD50 of more than 5,000 mg/kg are considered basically non-toxic9.
According to OECD rules, methanol extracts and vehicle were administered to the mice in the treatment and control groups of this investigation at a dose of 5000 mg/kg body weight. For fourteen days straight, they were observed for any indications of toxicity or death.10
Evaluation of mortality, physical and behavioural pattern:
As seen in Table 1. In the mice, there was no mortality noted. Throughout the study period, the mice’s body weight rose (Fig. 1), and their skin, fur, and eyes characteristics did not alter (Table 2). These findings suggest that the administration of the crude extract had a small amount of toxicity on the animals’ growth. No gross changes in behaviour, nor excessive salivation, lethargy, sleep, coma or tremors were observed. This showed that treatment with the leaf and fruit extracts did not cause any changes in the growth pattern and metabolism of the mice.11
Table 1: Toxicity of crude extracts of leaves and fruits in mice.
|
|
Control |
Total methanol extract of leaf |
Total methanol extract of fruit |
|
Number of dead mice/ Number of mice used per extract |
0/3 |
0/3 |
0/3 |
Table 2: Effect of treatment of methanol extract of leaf and fruits on physical appearance and behavioural pattern in mice
|
Observation |
Groups |
30 min |
1 hour |
2 hours |
3 hours |
24 hours |
7 days |
14 days |
|
Skin and Fur |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Eyes |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Mucous membrane |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Behaviour pattern |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Salivation |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Lethargy |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Sleep |
Control |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Leaf |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Fruit |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
Diarrhoea |
Control |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Leaf |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Fruit |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Coma |
Control |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Leaf |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Fruit |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Tremors |
Control |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Leaf |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Fruit |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 = Normal and 0= not observed
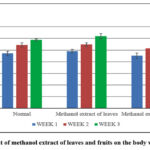 |
Figure 1: Effect of methanol extract of leaves and fruits on the body weight of mice |
Evaluation of haematological parameters
Evaluation of haematological parameters is very important to check the toxicity of plant extract because the blood cells are very susceptible to toxic compounds. Evaluating the impact of the sample on the hematopoietic system is also beneficial. Generally, decrease in haemoglobin content, RBC count and packed cell volume (PCV) is correlated to anaemia and defective haematopoiesis, On the other hand, anaemia is indicated by a rise in mean corpuscular volume (MCV) and a fall in mean corpuscular haemoglobin content (MCHC)12. Results tabulated in Table 3. revealed that although treatment of both leaves and fruit extract in mice caused changes in the haemoglobin, RBC and PCV content, all values were within CPCSEA specified normal range for mice 13. Mice treated with extracts exhibited a decrease in MCV value and increase in MCHC content in comparison to control showing that the extract did not cause anaemia. WBC content and differential count (count of neutrophils, eosinophil, lymphocyte and monocytes ) in extract treated groups and control group were well within the CPCSEA specified normal range normal range thereby exhibiting that the treatment of extracts did not change the haematological parameters of the mice. The content of platelets in extract treated groups decreased in comparison to control and it could be due to the thrombolytic nature of the extracts14. Thus, the haematological evaluation showed that the leaves and fruits exhibited minimum changes the parameters indicating the nontoxic nature of extracts.
Table 3: Effect of Z.rhetsa leaf and fruit extract on haematological parameters in acute oral toxicity study.
|
Parameter |
Units |
Control |
Leaf |
Fruit |
Normal parameters |
|
Hemoglobin |
g/dL |
9.2 ± 0.14 |
12.2 ± 0.21 |
8.06 ± 0.16 |
10.2 -16.6 |
|
Red Blood Cell |
million/ cmm |
6.16 ± 0.02 |
7.53 ± 0.35 |
5.55 ± 0.25 |
7 -12.5 |
|
White Blood Cell |
thousands/ cmm |
11.7 ±0.18 |
9.4 ±0.25 |
6.6 ± 0.39 |
6 -15 |
|
Platelets |
lakh /cmm |
945.6 ± 39.71 |
561 ± 46.54 |
649 ± 37.69 |
160 – 410 |
|
Packed Cell Volume |
% |
30.46 ± 0.355 |
34.6 ± 0.68 |
33.5 ± 0.35 |
39 – 49 |
|
Mean Corpuscular Volume |
fL |
49.7± 0.64 |
46.33 ± 1.08 |
42.5 ± 0.93 |
– |
|
Mean Corpuscular Hemoglobin Concentration |
gm / dL |
30.23 ± 2.18 |
35.2 ± 1.77 |
34.0 ±3.08 |
– |
|
Mean Corpuscular Hemoglobin |
pg |
15 ± 0.70 |
16.2 ± 0.34 |
14.4 ± 0.28 |
– |
|
Neutrophils |
% |
27.06 ±1.41 |
35.06 ± 2.19 |
29 ± 1.41 |
10-40 |
|
Eosinophils |
% |
1 ± 0.0 |
1 ± 0.0 |
0.0 |
0-4 |
|
Lymphocytes |
% |
72.15 ± 4.3 |
63.0 1 ± 4.6 |
71.0 ± 3.93 |
55-95 |
|
Monocytes |
% |
1 ± 0 |
1 ± 0 |
0.0 |
0.1-3.5 |
Evaluation of serum biochemical parameters
Drug toxicity evaluations involve the assessment of serum biochemical markers, the results of which are summarised in Table 4. Serum biochemical indicators of the liver function test include alkaline phosphatase, aspartate amino transferase, and alanine amino transferase (ALT, AST). Elevated levels of ALT in serum indicate tissue damage and hypertrophy in living organisms. An increase in AST levels is indicative of malfunction in the liver, muscles, and heart. Alkaline phosphatase is a measure of the bile duct’s condition. Alkaline phosphatase, ALT, and AST levels are hence elevated in liver disorders and hepatotoxicity15. The values of ALT, AST, and alkaline phosphatase in the extract treated groups did not vary to a much in comparison to the control. Further measurement of total protein and albumin showed that it was within CPCSEA specified range. This exhibited that the methanol extracts of the leaves and fruits did not cause hepatotoxicity.
Renal function test was evaluated by measurements of urea and creatinine16. It showed that nephrotoxicity was not observed on treatment of extracts. These findings were confirmed by the histopathological observation of the liver and kidney where no gross morphological changes suggesting lesions were observed. Measurement of serum cholesterol and inorganic ions showed that the extracts did not cause much difference comparison to control.
Table 4: Effect of Z.rhetsa leaf and fruit extract on biochemical parameters in acute oral toxicity study.
|
Parameter |
Units |
Control |
Leaf |
Fruit |
Normal parameters |
|
Total Serum Bilirubin |
mg/dL |
0.6 ± 0.16 |
0.4 ± 0.11 |
0.5 ± 0.04 |
0.1-0.9 |
|
SGOT / AST |
mg/dL |
120 ± 6.5 |
131 ±7.11 |
128.6 ± 15.5 |
– |
|
SGPT/ ALT |
IU / L |
62 ± 2.44 |
59 ± 4.08 |
74.3 ± 2.0 |
– |
|
Serum alkaline phosphatase |
IU / L |
231.3±15.2 |
181 ± 20.7 |
234 ± 11.5 |
– |
|
Serum total proteins |
IU / L |
7.1 ± 0.43 |
6.2 ± 0.14 |
6.6 ± 0.29 |
3.5-7.2 |
|
Serum total albumin |
g / dL |
3.4 ± 0.28 |
4.2 ± 0.09 |
3.9 ± 0.57 |
2.5-4.8 |
|
Blood urea nitrogen |
g / dL |
14.1 ± 0.66 |
18.3 ± 1.2 |
12.46 ± 1.3 |
12-28 |
|
Serum creatinine |
mg / dL |
0.4 ± 0.14 |
0.7 ± 0.08 |
0.4 ± 0.21 |
0.3-1 |
|
Random blood sugar |
mg / dL |
98.33 ±2.4 |
107 ± 2.16 |
88 ±0.81 |
62-175 |
|
Serum cholesterol |
mg /dL |
78.33 ±2.05 |
72.0 ±2.94 |
75 ± 4.08 |
26-82 |
|
Serum calcium |
mg/dL |
7.9 ± 0.29 |
8.2 ± 0.24 |
8.1 ± 0.28 |
– |
|
Serum phosphorous |
mg/dL |
4.2 ± 0.32 |
3.9 ± 0.69 |
4.8 ± 0.77 |
– |
|
Serum sodium ion |
mEq/L |
140.3 ± 3.68 |
138.76±1.8 |
140.5 ± 0.41 |
– |
|
Serum potassium ion |
mEq/L |
3.4 ±0.21 |
4 ± 0.71 |
4.13 ± 0.24 |
– |
|
Serum chloride ion |
mEq/L |
102 ± 2.9 |
104.8±3.02 |
103.7 ± 4.00 |
– |
Histological evaluation
Histological evaluation helps in identification of toxicity of compound at the organ or tissue level. As observed under light microscope (Fig.2) treatment with extracts did not cause any abnormalities or lesions in the morphology of the tissues of the major organs such as heart, kidney, lung, liver and spleen. No hypertrophy was found in the tested organs. Hypertrophy is an indication of toxicity in the organs 17.
During the 14-day study, the animals did not appear any mortality or change in body weight. There were no changes in the behavioural or motor neural functions of the body. The haematological and serum biochemical parameters along with the histopathological studies also did not exhibit any abnormalities or pathological changes in the major organs. Consequently, it was discovered that the leaf and fruit extract’s LD50 (oral fatal dose) was greater than 5000 mg/kg body weight.
The findings of acute toxicity tests executed on Z. rhetsa leaves and fruits matched those of toxicity tests done on other Zanthoxylum species. In the past, it has been found that Z.armatum fruit and leaf extracts were safe at doses up to 2000 mg/kg body weight 18. Similarly, it has been found that a methanol extract of F. zanthoxyloides root bark was nontoxic up to 5.0 g/kg of rats’ body weight19.
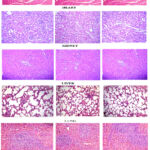 |
Figure 2: Histopathology of the lung, spleen, heart, liver and kidney of mice in acute toxicity test at 40x magnification (haematoxylin-eosin stain) |
Conclusion
Zanthoxylum rhetsa leaves and fruits are widely used in food and therapeutics. Therefore, toxicity tests must be carried out in order to evaluate the safety profile of Z. rhetsa leaves and fruits. In compliance with OECD necessities, research on the acute toxicity of methanol extract of leaves and fruits has been carried out. From the results obtained, it can be concluded that Z.rhetsa did not exhibit any toxic effects even when used at a very high concentration. Thus, the leaves and fruits can be safely explored for its use in therapeutics and drug discovery research. Further genotoxicty and mutagenicity studies can be conducted in vivo to establish complete safety profile of the drug.
Acknowledgements
None
Conflict of Interest
There is no conflict of interest
Funding Source
There are no funding sources
References
- Tilburt J.C., Kaptchuk T.J. Herbal medicine research and global health: an ethical analysis. Bull World Health Organ. 2008; 86:594–599.
CrossRef - Yuan H., Ma Q., Ye L., Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016; 21:559. doi:10.3390/molecules21050559
CrossRef - Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology. 2014; 4:177.
CrossRef - Ping K.Y., Darah I., Chen Y., Sreeramanan S., and Sasidharan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats, BioMed Research International. 2013; 2:182064·
CrossRef - Mallya R. and Bhitre M.J. Pharmacognostic standardisation and chromatographic fingerprinting of leaves and fruits of Zanthoxylum rhetsa. International journal of pharmacy and pharmaceutical sciences. 2018; 10:101-104.
CrossRef - Anonymous. The Wealth of India: Raw Materials and Industrial Products. Publication and Information Directorate, CSIR, New Delhi, Vol. XI, 1956, pp.22-23.
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology, CRC Press, India;2012, pp.3650.
- OECD. OECD Guideline for Testing of Chemicals: Acute Oral Toxicity-Fixed Dose 17 Procedure (No.420). OECD, Paris;2001
- Erhirhie E.O, Ihekwereme C.P., Ilodigwe E.E. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdisciplinary toxicology. 2018;11:5–12. doi:10.2478/intox-2018-0001
CrossRef - Jothy S.L., Zakaria Z., Chen Y., Lau Y.L., Latha L.Y., Sasidharan S. Acute Oral Toxicity of Methanolic Seed Extract of Cassia fistula in Mice. Molecules. 2011; 16:5268.
CrossRef - Sreeramanan S., Sasidharan S.. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Biomedical Research International. 2013; 2013:182064.
CrossRef - Chanda S., Parekh J., Vaghasiya Y., Dave R., Baravalia Y. and Nai R: Medicinal Plants – From Traditional Use to Toxicity Assessment. A Review International Journal of Pharmacetical Sciences and Research. 2015; 6:2652-2670.
- CPCSEA. Compendium of CPCSEA. New Delhi. 2018. http://thsti.res.in/ pdf/Compendium_CPCSEA_2018-I.pdf. Accessed June 5, 2019.
- Afolayan A.J., Wintola O.A., Fouche G. Acute and Subacute toxicological evaluation of the aerial extract of Monsonia angustifolia E. Mey. ex. A. rich in Wistar Rats. Evidence Based Complementary and Alternative Medicine. 2016; 2016:4952485.
CrossRef - El Kabbaoui M., Chda A., El-Akhal J. Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. Journal of Ethnopharmacology. 2017; 209:147-156.
CrossRef - Kuatsienu L.E., Ansah C., Adinortey M.B. Toxicological evaluation and protective effect of ethanolic leaf extract of Launaea taraxacifolia on gentamicin induced rat kidney injury. Asian Pacific Journal of Tropical Biomedicine. 2017; 7: 640-646.
CrossRef - Mawoza T., Tagwireyi D. and Nhachi C: Acute and sub-chronic toxicity studies of an aqueous stem bark extract of Sclerocarya birrea using a rat model. International Journal of Pharmaceutical Sciences and Research. 2016; 7:9-17.
- Barkatullah, Muhammad I., Muhammad N. Evaluation of Zanthoxylum armatum DC for in-vitro and in-vivo pharmacological screening. African Journal of Pharmacy and Pharmacology. 2011; 5:1718-1723.
CrossRef - Ogwal-Okeng J.W., Obua C., Anokbonggo W.W: Acute toxicity effects of the methanolic extract of Fagara zanthoxyloides (Lam.) root-bark. African Health Science. 2003; 3:124-126.








