Rana Mohammad Al-awadhi1 , Heba Mohammed Ahmed Abdelrazek2
, Heba Mohammed Ahmed Abdelrazek2 *, Eman Mohammed Abouelhassan3
*, Eman Mohammed Abouelhassan3 , Marwa Samir Kamel4
, Marwa Samir Kamel4 , Nayrouz Ali Attia5
, Nayrouz Ali Attia5 , Asem Abdelmonem Awad5
, Asem Abdelmonem Awad5 , Mohamed Refaat Saad5
, Mohamed Refaat Saad5
1Department of Science, The Public Authority for Applied Education and Training (PAAET), Collage of Basic Education, Kuwait.
2Department of Physiology, Faculty of Veterinary Medicine, Suez Canal University, Egypt.
3Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Egypt.
4Department of Plant Protection, Faculty of Agriculture, Suez Canal University, Ismailia, Egypt.
5Pharmacology program, Faculty of Veterinary Medicine, Suez Canal University, Egypt.
Corresponding Author E-mail: hebaabdelrazekvet@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2957
Abstract
The neem plant (Azadirachta indica) is one of the most important medicinal plants in Asia and Africa. This study aimed to investigate the possible anti-fertility potential of dietary neem leaves' extract in female Wistar albino rats as. This model could be further applied to stray dogs. In this experiment 24 adult female albino Wister rats were divided into two groups: control and treated with neem leave extract, they were tested for estrous regularity by vaginal smears, progesterone, and estrogen hormone were measured. Ovarian caspase-3 expression and histopathological inspection were examined. The results have revealed reducing sex steroids, increment in ovarian caspase-3 expression, and histological deteriorations in the ovary and uterus. The study concluded that neem flower extract can be used as an antifertility agent in animals.
Keywords
Antifertility; Female; Neem; Reproduction
Download this article as:| Copy the following to cite this article: Al-awadhi R. M, Abdelrazek H. M. A, Abouelhassan E. M, Kamel M. S, Attia N. A, Awad A. A, Saad M. R. Neem Leaves Extract Reduces Sex Steroids and Gonadal Function in Female Wistar Albino Rats. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Al-awadhi R. M, Abdelrazek H. M. A, Abouelhassan E. M, Kamel M. S, Attia N. A, Awad A. A, Saad M. R. Neem Leaves Extract Reduces Sex Steroids and Gonadal Function in Female Wistar Albino Rats. Biomed Pharmacol J 2024;17(3).Available from: https://bit.ly/4duAiwf |
Introduction
One of the most serious global problems is stray dogs overpopulation, which has an adverse impact on public health, the environment, and communities, various zoonoses have developed and become endemic as a result of this problem 1. Thus, it is important to search for a new effective method with low side effects to solve this problem, such as medicinal plants with antifertility effects. Moreover, the human population explosion is considered a major cause of human beings’ suffering and environmental squalor all over the world 2. Thus, it is important to search for a new effective method with low side effects to solve this problem. Synthetic contraceptives possess an effective role in solving such problems however, they are not safe as they may cause allergies or heart attack3.
Since ancient times, herbs have been reported for their useful remedial influences against various disease conditions owing to their pharmacological belongings4. Therefore, plants have provided humans with new medicinal solutions for thousands of years, acting as a basis for traditional medical systems worldwide5. Herbs could be used as an alternative to drugs of synthetic origin and they have gained a recent popularity among developing countries due to their affordability, availability and abridged side/toxicity influences 6,7. A variety of medicinal plants that were previously proved to have antifertility action could be industrialized into contraceptives for both females and males8. They can cause estrous cycle disruption, anti-estrogenic effects, anti-implantation influence, or even abortion9.
One of the most significant medicinal plants found in Asia and Africa is neem (Azadirachta indica), which is rich in proteins and trace minerals, has Anti-inflammatory and Antioxidant effects, and can be used to treat a variety of animal parasites, bacteria, and viruses10. Additionally, fresh neem leaves contain polyphenolic flavonoids with antibacterial and antifungal effects, Furthermore, neem seeds contain beneficial components like azadirachtin and gedunin11. Gbotolorun, Osinubi, Noronha and Okanlawon12 detected that the antifertility effects of the neem flower extract on adult female rats, causing disruption of the estrous cycle and a partial blockage in the ovulation. Patil Patil, Shirahatti, VB, Ramu and Prasad13 detected that the consumption of A. indica does not cause any harm to the entire reproductive system, nevertheless, has the ability to be used as a temporary or reversible contraceptive. This study aimed to investigate the possible anti-fertility potential of dietary neem leaves’ extract in female Wister albino rats as a model to be further applied to stray dogs.
Materials and Methods
Plant Extract
Creation of Crude Aqueous Neem Extract
According to Mamoon-ur-Rashid, Abdullah and Hussain. 14, the Azadirachta indica tree leaves were dried, then grinding and homogenized with distilled water in an electric blender, and triple-folded gauze was used to filtrate the homogenate. Before usage, a rotary vacuum evaporator was used to evaporate the solvent, then 70% dilation of the extracts was prepared.
Experimental Animals
A total of 24 mature female rats (5.5–6 months; 180–200 g) of Wistar strain were castoff in this study. Animals were acquired from Laboratory Animal House, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. They were housed in polyethylene cages; four females per cage at room temperature (25°C ± 1°C) and natural daylight cycles. Water and feed were offered ad libitum. Rats were kept 1 week to adapt. The experimental producers adhered to the ethical rules for the utilization of animals in laboratory settings at the Faculty of Veterinary Medicine, Suez Canal University, Egypt (SCU-VET 2024032).
Reproductive Procedures
After 1 week of adaptation, daily cytological inspection of vaginal smears was done to detect the estrous cyclicity progression and regularity. Females that revealed two successive regular cycles were designated for the current study and others were excluded.
Design of the Experiment
Twenty-four regular cyclic females were split equally into two groups; G (I): control group (n = 12) was fed a basal diet misted with 53 mL distilled water and G (II): neem extract group (n = 12) that was fed basal diet misted with 3 mL neem leaves extract mixed with 50 mL distilled water with a dose 10 mL neem leaves extract / kg diet. A total of 200 g diet was offered / cage. The experimental diet was offered daily for 30 days.
Estrous Regularity
After 15 days from the start of the experiment, vaginal smears were smeared from individual rats every 12 hours to detect the average duration/hours for each estrous cycle phase for two consecutive cycles.
Sampling
At the end of 30 days of treatment, 3 females representing each stage of the cycle were sacrificed. Blood was drawn from retro-orbital venous vessels allowed to clot then centrifugated at 3000 rpm to obtain serum. Serum was kept at -70oC. The uterus and ovaries of each female were dissected and weighed.
Feed Intake and Relative Sex Organs Weights
Final body weights were recorded. The feed intake for rat each experimental animal was calculated. The food remaining was subtracted from the offered food then the obtained value was divided by the number of rats per cage. The dissected ovaries and uteri were weighed, and their relative weights were obtained as follows (organ weight/body weightX100).
Sex Steroids Levels
Serum levels of 17-β estradiol of females in the follicular phase of the cycle (proestrus and estrus phases) and serum progesterone levels in the luteal phase of the cycle (metestrus and diestrus) were determined using Kamiya Biomedical Company, ELISA kits (USA). Both analyses were performed according to the enclosed pamphlet instructions.
Histopathology
Part of the dissected uteri and ovaries were put in a 10% formalin solution. Afterward, they were immersed into paraffin wax and stained by hematoxylin and eosin15.
Ovarian Caspase-3 Expression
One ovary/ animal was kept at -80oC until RNA extraction has proceeded. The frozen ovaries were subjected to RNA extraction using a total RNA extraction kit (QIAGEN, Maryland, USA), as mentioned in pamphlet of the manufacturer. The complementary DNA was synthesized using (Thermo Fisher Scientific Inc., Lithuania) kit according to manufacturer’s instruction. Gene expression of ovarian caspase-3 against the housekeeping gene β-actin following He, Sun and Huang 16 primer sequence and methodology. Primer for caspase-3 was Forward: 5′-GTGGAACTGACGATGATATGGC-3′ and reverse: 5′-CGCAAAGTGACTGGATGAACC-3′. Primers for β-actin were Forward: 5′-AAGATCCTGACCGAGCGTGG-3′ and reverse: 5′-CAGCACTGTGTTG GCATAGAGG-3′. Fold change 2-ΔΔCt was implemented to estimate the levels of gene expression of caspase-3.
Statistical Analysis
The results obtained in the present work were calculated by student t-test. Then the data were expressed as a mean ± standard error (mean ± SE), where p ≤ 0.05 is considered statistically significant. All of the analyses were carried out using the R programming language17.
Results
The Shapiro-Wilk test for univariate normality showed that the data were normally distributed (p>0.05).
Estrous Regularity
Experimental rats had significantly (p<0.05) longer diestrus phases in the neem leaves extract administered group compared to controls. While these rats had no significant differences in the duration of proestrus, estrous, and metestrus phases (Table 1).
Table 1: Effect of neem leaves extract on estrous cycle duration of female albino rats.
|
Group |
Proestrus (h) |
Estrous (h) |
Metestrus (h) |
Diestrus (h) |
|
Neem extract treated group |
0.93 |
0.95 |
0.50 |
3.43 |
|
Control group |
11 0.52 |
19.67 1.20 |
10.33 0.33 |
63.67 3.88 |
Data was expressed as a mean ± standard error (mean ± SE) then data was analyzed by student t-test using analyses carried out using the R programming language. NS means that neem leaves extract group was non significantly varied (p0.05) than the control group.* means there was a significant difference at p<0.05. Control group (n = 12) was fed a basal diet misted with 53 mL distilled water. Neem extract group (n = 12) was fed a basal diet misted with 3 mL neem leaves extract mixed with 50 mL distilled water with a dose 10 mL neem leaves extract / kg diet.
Feed Intake and Relative Sex Organs Weights
Female rats treated with neem extract had a mean rat feed intake of 24.42 (g/day), compared to 24.23 (g/day) in the control group. The level of rat feed intake in treated female rats is approximately the same and non-significantly (p>0.05) altered as matched to controls (Figure 1). Concerning ovarian and uterine relative weights, there were non-significant (p>0.05) differences observed between neem leaves extract group and the control one either in follicular or luteal phases of the cycle (Table 2).
 |
Figure 1: Effect of neem leaves extract on rats’ feed intake. Data is expressed as a mean ± standard error (mean ± SE) then data was analyzed by student t-test using analyses carried out using the R programming language. |
Table 2: Influence of neem leaves extract on rats’ relative ovarian and uterine weights.
|
|
Ovarian relative weight (%) |
Uterine relative weight (%) |
||
|
Follicular phase |
Luteal phase |
Follicular phase |
Luteal phase |
|
|
Neem Extract treated group |
0.003 |
0.004 |
0.018 |
0.011 |
|
Control group |
0.066 0 0.02 |
0.004 |
0.518 0.026 |
0.485 0.006 |
Data is expressed as a mean ± standard error (mean ± SE) then data was analyzed by student t-test using analyses were carried out using the R programming language NS means that Neem leaves extract group was non significantly varied (p>0.05) than control group. Control group (n = 12) was fed a basal diet misted with 53 mL distilled water. Neem extract group (n = 12) was fed a basal diet misted with 3 mL neem leaves extract mixed with 50 mL distilled water with a dose 10 mL Neem leaves extract / kg diet.
Sex Steroids Levels
Female rats treated with neem leaves extract had a mean serum estradiol content of 16.45 (pg/mL), compared to 25.26 (pg/mL) in the control group. The level of estradiol in treated female rats is approximately 34.88% lower and significant (p<0.05) as compared to controls (Table 3).
Female rats treated with neem leaves extract had a mean serum progesterone content of 7.83 (ng/mL), compared to 12.70 (ng/mL) in the control females. In comparison to controls, the level of progesterone in treated female rats is approximately 38.35% lower and significant (p<0.05) (Table 2).
Table 3: Effect of neem leaves extract on serum levels of sex hormones in female albino rats.
|
Group |
Serum estradiol (pg/mL) |
Serum progesterone (ng/mL) |
|
Neem Extract treated group |
1.39 |
1.09 |
|
Control group |
25.26 2.30 |
12.70 1.43 |
Data is expressed as a mean ± standard error (mean ± SE) then data was analyzed by student t-test using analyses carried out using the R programming language. * means there was significant difference at p<0.05. Control group (n = 12) was fed a basal diet misted with 53 mL of distilled water. Neem extract group (n = 12) was fed a basal diet misted with 3 mL neem leaves extract mixed with 50 mL distilled water with a dose 10 mL of neem leaves extract / kg diet.
Histopathology
Microscopically, the ovary of control rats in estrus (CE) was covered externally by a single cuboidal cells layer, germinal epithelium. Additionally, a thick layer of connective tissue rich in fibroblasts; tunica albuginea, was observed beneath the covering epithelium. The cortical region revealed various follicular stages along with stromal cells interspersed in between. The most numerous types of ovarian follicles were primordial follicles that mainly formed by a primary oocyte covered by a monolayer of squamous granulosa cells. Within the ovarian cortical tissue, the primary follicles were recorded. It was larger in size than the primordial one. It was formed by a larger primary oocyte covered by a monolayer of cuboidal granulosa cells. Late and early stages of secondary follicles were noted, whereas the largest primary oocytes were bounded by numerous layers of granulosa cells that are polyhedral in shape with the various fluid-filled places among the granulosa cells that are covered outwardly by theca cells. Those multiple spaces were merged with each other founding a single big antrum (Figure 3). On the opposite, the ovarian tissue of females fed neem extract in estrus (NE) displayed the same histoarchitecture as that of CE with some differences; the cortical tissue was composed mainly of dense irregular connective tissue rich in fibroblasts and other connective tissue cells that surrounds the ovarian follicles, the follicular developmental stages were declined than that of CE, and finally, great numbers of blood capillaries were evident within the ovarian medulla (Figure 3).
Uterine tissue sections that stained with H&E of CE showed inner folded endometrial mucosa (simple columnal epithelium and lamina propria-submucosa contained uterine glands), myometrial middle (outer longitudinal bundles and inner circular of smooth muscles along with stratum vascular separating) and outermost perimetrium (contained simple squamous epithelium surrounding a connective tissue layer) (Figure 3). The uterus of NE displayed the same histological structures as CE, meanwhile the most prominent uterine findings in NE were the uterine epithelial stratification, few or no uterine glands were recorded in the lamina propria-submucosa and proliferation of blood capillaries was observed in perimetrium (Figure 3).
During the luteal phase, the ovarian sections of control females during metestrus (CM) demonstrated highly active and well-developed corpora lutea with abundant granulosa lutein cells enclosed by a well-vascularized connective tissue capsule (Figure 4).However, the ovarian tissue of the neem group (NM was intermingled and characterized by rudimentary ovarian follicles, a huge amount of connective tissue with connective tissue cells and the proliferation of blood capillaries (Figure 4). It was interesting to note that the ovary of both CM and NM groups was covered externally by a single layer of flat cells, germinal epithelium.
The uterine tissue sections of CM and NM showed the same histo-architecture as follows; inner endometrium (simple columnal epithelium and lamina propria-submucosa contained uterine glands), middle myometrial layer (longitudinal bundles and inner circular outer of smooth muscles along with stratum vascular separating) and outer perimetrium (contained simple squamous epithelium surrounding a connective tissue layer) (Figure 4).
Caspase-3-fold change expression
Figure (2) revealed a significant increase (p<0.05) in the fold change mRNA of ovarian caspase-3 in the neem leaves extract group as compared to control during the luteal and follicular phases of the estrous cycle.
 |
Figure 2: Effect of neem leaves extract on fold change expression of ovarian caspase-3 in female albino rats. |
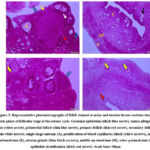 |
Figure 3: Representative photomicrographs of H&E-stained ovarian and uterine tissues sections during estrus phase of follicular stage of the estrous cycle. |
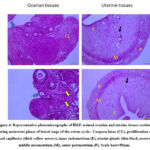 |
Figure 4: Representative photomicrographs of H&E-stained ovarian and uterine tissues sections during metestrus phase of luteal stage of the estrus cycle. |
Discussion
Neem (Azadirachta indica) is recognized as a highly potent natural contraceptive 18. Employing A. indica as a contraceptive offers several benefits due to its safety, effectiveness, and reversibility in both genders 19. The contraceptive properties of A. indica are linked to its capacity to interfere with various aspects of the female reproductive system, such as altering hormonal balance 20, causing irregularities in the estrous cycle 21, and inducing apoptosis in ovarian cells 7, while having no adversative properties on productive performance 22-24.
The usage of chemical or synthetic contraceptives could also alter the hormonal balance and reproductive cycle that impede ovarian and uterine functions 25. Among these effects; are the suppression of folliculogenesis, follicle development and suppression of ovulation. Moreover, most of them are hormones that interact with hypothalamic endocrine neurons to suppress pituitary gonadotropins 26. The usage of these contraceptives could produce myriads of side effects such as hypertension, breast cancer susceptibility, dyslipidemia and susceptibility to thrombosis 27 and cardiovascular diseases 28 that made herbal contraceptives a safe alternative.
Our treated rats showed that A. indica extract significantly prolongs the diestrus phase without altering the durations of the proestrus, estrus, and metestrus phases. Such a result aligned with that of Mamoon-ur-Rashid, Abdullah and Hussain 12 who declared prolonged diestrus in female rats that were given 1 g/kg body weight of alcoholic neem flower extract with a total 80% estrous irregularity percentage. Auta and Hassan 29 found that neem extract administration at doses 5, 50 and 100 mg/kg neem wood aqueous extract for 20 days in mice produced a significant prolongation in the diestrus phase in a dose dependent pattern. Sitasiwi, Isdadiyanto and Mardiati 19, also reported that ethanolic A. indica leaves at levels 8.4, 11.2, and 14 mg/mice/day for 21 days can disrupt the regularity of the estrous cycle and prolong diestrus in Swiss Webster mice. Despite the significant physiological disruptions within the estrous cycle, A. indica extract did not significantly affect mean daily feed intake, or the relative weights of the ovaries and uterus as shown in herein study and in parallel with El-Zaiat, Elshafie, Al-Marzooqi and Dughaishi 30 as well as Biswas, Chattopadhyay, Banerjee and Bandyopadhyay 31. This implies that the contraceptive effects of A. indica extract are not due to changes in overall nutrition, the weight of reproductive organs or body conditions 19,21,32. So, the extension of the diestrus phase in neem-treated rats suggests that A. indica extract disrupts the hormonal feedback mechanisms essential for the progression of the estrous cycle. Where the estrous cyclicity is completely governed by neuroendocrine feedback that is completely controlled by anterior pituitary gonadotropins secretion and gonadal steroids production 33-35 that seemed to be reduced in the current study (noticed by 17-β estradiol and progesterone reductions).
Normally, an increase in 17-β estradiol signals the end of the diestrus phase and the start of a new cycle 36. However, in neem-treated rats, 17-β estradiol levels were about 34.88% lesser in females those in the control group during the follicular phase. The former result was aligning with observations mentioned by Sitasiwi, Isdadiyanto and Mardiati who reported that ethanolic A. indica in leaves 8.4, 11.2, and 14 mg/mice/day for 21 days can reduce 17-β estradiol in mice 19. Also, Shaikh, Naqvi and Khani 37 demonstrated that neem oil administration to female albino rats at doses 0.6 and 1.2 mL/animal significantly reduced 17-β estradiol in the blood. Moreover, Tripathi, Shrivastav and Chaube 38 detected lower 17-β estradiol in follicular lysate after administration 50 mg/day aqueous neem extract to immature female rats for 10 days. This reduction suggests that the hormonal signal, needed to end diestrus and trigger follicular development and maturation (proestrus) 39, is impaired. As a result, the hormonal threshold for transitioning from diestrus might not be reached, leading to an extended diestrus phase. These decreased 17-β estradiol levels imply that A. indica extract exhibits strong anti-estrogenic effects19.
Our findings show that serum progesterone levels were significantly reduced, approximately 38.35% lower than in controls, which in line with the study by Moravati, Mahmoudi, Ghazi-Khansari, Aria and Jabbari 40 who found that gavage of neem methanolic extract at a dose of 15 mg/ kg for 6 days significantly abridged blood progesterone level in female rats that support the strong anti-progestational effects exerted by A. indica extract. Moreover, Physiologically, progesterone plays a crucial role in maintaining the diestrus phase, with its decline signalling the end of this phase 39. Progesterone upregulation signals neuroendocrine reflex through the hypothalamus to promote pituitary gonadotropin production to establish a new cycle. The reduction of progesterone level in the herein study led to prolongation of diestrus due to delay of its feedback on hypothalamic releasing hormones that delay pituitary gonadotropins secretion thus hindering initiation of new cycle 41. This interference might involve altering progesterone receptor sensitivity, acting as a progesterone receptor blocker, or disrupting the signalling pathways that process these hormone levels, resulting in a delayed transition despite low progesterone 42. The signalling pathways that process progesterone involve dimerization to receptors where there are different progesterone receptor (PR) subtypes related to reproductive function (PR-A, PR-B and PR-C) as well as G-protein coupled membrane receptors. PR-A limits transcription of target genes referring to the active inhibitory domain that prohibits further translation into new proteins. However, PR-B receptors stimulate DNA transcription and further translation to new proteins specifically nuclear receptor coactivator that has a close association with maintenance of wet uterine weight 43. PR-C lacks DNA binding affinity as PR-A or PR-B but it can dimerize receptors as a heterodimer that is not as effective as a homodimer43. The G protein coupled PR exerts its regularity function in ovaries and hypothalamus via inhibition of cAMP 44. The ablation of PR function by neem could contribute to altering of neuroendocrine regulation exerted by such receptors in reproductive cycle regulation.
Beyond their role in the progression of the estrous cycle, 17-β estradiol and progesterone have protective, anti-apoptotic effects on ovarian cells, supporting the proper development and maintenance of follicles and the corpus luteum 45. When these hormone levels decline, ovarian caspase-3 activity increases, as demonstrated in our results. The increase in ovarian caspase-3 expression may be due to neem suppression to catalase activity that promotes H2O2 induction to BAX and P53 that hasten caspase-3 expression46. Caspase-3 is a key executioner of apoptosis 47. Its activation triggers the cleavage of cellular proteins, leading to programmed cell death. This process constitutes a crucial part in the follicular atresia of non-dominant follicles during the follicular phase and the relapse of the corpus luteum during the luteal phase if pregnancy does not occur 48. Consequently, increased caspase-3 activity leads to heightened apoptosis, disrupted ovarian function, and increased follicular atresia. This results in fewer follicles maturing and ovulating, accompanied by increased connective tissue in the ovary compared to control groups, as observed in the present histological examination. These findings are consistent with studies by Akpantah, Ekong, Obeten, Akpaso and Ekanem 49 in female rats and Swamy and Mohan 50 in freshwater fish.
Additionally, insufficient endometrial proliferation and glandular development are consequences of reduced levels of 17-β estradiol in A. indica-treated rats 51. This explains the observed epithelial stratification and the scarcity of uterine glands in our histological sections, which align with the finding of Auta and Hassan 29 who found that neem extract administration doses of 5, 50, and 100 mg/kg neem wood extract for 20 days to mice produced uterine epithelial stratification especially in 50 mg and 100 mg doses . Furthermore, the low progesterone levels in these treated rats impair secretory functions, leading to diminished glandular activity and compromised structural integrity of the endometrium 29. The absence of proper glandular structures in the endometrium reflects impaired uterine preparation for potential implantation, which is consistent with the contraceptive properties of neem extract 29,51.
Conclusion
Neem leaves extract produced antifertility potential in female Albino rats through Reducing sex steroids; estrogen in the follicular phase and progesterone in the luteal phase. That led to the prolongation of estrous cycle duration especially the diestrus phase. Moreover, the reduced sex steroids by neem leaves extract promoted an increment in the ovarian caspase-3 mRNA expression that reflected follicular apoptosis and atrasia. The previous events provoked histological deteriorations in the ovary such as a reduced number of developing follicles and connective tissue proliferation beside uterine epithelial stratification with diminished uterine glands that reflect their incompetence for ovulation and implantation.
Acknowledgement
The authors would like to acknowledge Haneen Mohammed, undergraduate student, Faculty of Veterinary Medicine, Suez Canal University for her help during experimental procedures.
Conflict of Interest
The authors do not have any conflict of interest
Funding sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
Authors’ Contribution
Conceptualization, E.M.A., Heba M.A. Abdelrazek;
Methodology, Heba M.A. Abdelrazek, Asem A. Awad, Nayrouz A. Attia, Mohamed R. Saad, Marwa S. Kamel
Formal analysis, Rana M. Al-awadhi., Heba M.A. Abdelrazek., Eman M. Abouelhassan,
Investigation, Rana M. Al-awadhi., Heba M.A. Abdelrazek., Nayrouz A. Attia., Marwa S. Kamel.;
Resources, Rana M. Al-awadhi, Heba M.A. Abdelrazek., Mohamed R. Saad, Asem A. Awad.;
Writing- original draft preparation, Nayrouz A. Attia, Mohamed R. Saad., Asem A. Awad;
Writing-review and Editing, Rana M. Al-awadhi., Heba M.A. Abdelrazek., Eman M. Abouelhassan., Marwa S. Kamel
All authors have read and agreed to the published version of the manuscript.
References
- Seimenis A., Tabbaa D. Stray animal populations and public health in the South Mediterranean and the Middle East regions. Vet Ital. 2014;50(2):131-136.
- Besculides M., Laraque F. Unintended pregnancy among the urban poor. J Urban Health : bulletin of the New York Academy of Medicine. 2004;81(3):340-8.
CrossRef - Anand A. K., Prasad V., Alam M. Herbal or modern methods of contraception! Choice is yours. Int J Rep Cont Obst Gynaecol 2015;4(4):947-953.
CrossRef - Ogbuewu I. P., Unamba-Oparah I. C., Odoemenam V. U., Etuk I. F., Okoli I. C. The potentiality of medicinal plants as the source of new contraceptive principles in males. N Am J Med Sci. 2011;3(6):255-63.
CrossRef - Camp D., Davis R. A., Campitelli M., Ebdon J., Quinn R. J. Drug-like properties: guiding principles for the design of natural product libraries. J Nat Prod. 2012;75(1):72-81.
CrossRef - Oyedemi B. O., Oyedemi S. O., Chibuzor J. V., Ijeh, II, Coopoosamy R. M., Aiyegoro A. O. Pharmacological Evaluation of Selected Medicinal Plants Used in the Management of Oral and Skin Infections in Ebem-Ohafia District, Abia State, Nigeria. Sci World J. 2018;2018:4757458.
CrossRef - Njoga U. J., Jaja I. F., Onwuka O. S., Ilo S. U., Eke I. G., Abah K. O., Oguejiofor C. F., Ochiogu I. S. Reproductive effects of medicinal plant (Azadirachta indica) used as forage and for ethnoveterinary practices: New insights from animal models. Challenges. 2022;13(2):40.
CrossRef - Joshi S. C., Sharma A., Chaturvedi M. Antifertility potential of some medicinal plants in males: An overview. Int J Pharm Pharm Sci. 2011;3(5):204-217.
- Sharma R., Goyal A., Bhat R. Antifertility activity of plants extracts on female reproduction: A review. Int J Pharm Biol Sci. 2013;3(3):493-514.
- Abo-Elhassan E. M. Plant extracts: An Eco-friendly Approach to Parasite Management in Rabbit production. Egypt Vet Med Soc Parasitol J. 2024;20(1):44-57.
CrossRef - Alzohairy M. A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. J Evid Based Complementary Altern Med 2016;2016(1):7382506.
CrossRef - Gbotolorun S., Osinubi A., Noronha C., Okanlawon A. Antifertility potential of neem flower extract on adult female Sprague-Dawley rats. Afr Health Sci. 2008;8(3):168-173.
- Patil S. M., Shirahatti P. S., VB C. K., Ramu R., Prasad N. Azadirachta indica A. Juss (neem) as a contraceptive: An evidence-based review on its pharmacological efficiency. Phytomedicine. 2021;88:153596.
CrossRef - Mamoon-ur-Rashid M., Abdullah M., Hussain S. Toxic and residual activities of selected insecticides and neem oil against cotton mealybug, Phenacoccus solenopsis Tinsley (Sternorrhyncha: Pseudococcidae) under laboratory and field conditions. Mortality. 2011;10:100.
- Bancroft J. D., Gamble M. Theory and practice of histological techniques. Elsevier health sciences; 2008.
- He X., Sun J., Huang X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Experimental and therapeutic medicine. Jan 2018;15(1):873-877.
CrossRef - Team R. C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021.
- Idu M., Ogedegbe-George S., Oriarewo P. E., Gabriel B. O. Spermicidal, Antifertility and Contraceptive Effect of Azadirachta indica A. Juss. Seed Extract in Female and Male Wistar Rats. Biol Med Natural Prod Chem. 2023;12(1):79-88.
CrossRef - Sitasiwi A. J., Isdadiyanto S., Mardiati S. M. The estradiol 17-β concentration in mice after treated with ethanolic leaf extract of Azadirachta indica (neem). In: AIP Conference Proceedings; AIP Publishing, 2017; 1844(1).
CrossRef - Tiwari M., Gupta A., Prasad S., Tripathi A., Yadav P., Pandey A. N., Premkumar V., Pandey A., Shrivastav T., Chaube S.Neem (Azadirachta Indica L.) and Oocyte Quality. J Assist Reprod Genet. 2017;28(10):877-878.
- Mishra R., Singh S. K. Effect of aqueous leaf extract of Azadirachta indica on the reproductive organs of male mice. Indian J Exp Biol. 2005;43(11):1093-1103.
- Aruwayo A., Maigandi S. A., Malami B. S., Daneji A. I. Haematological and biochemical parameters of Uda lambs fed graded levels of alkali-treated neem kernel cake. Nig J Basic Appl Sci. 2011;19(2):277-284.
- Rao S. B. N., Jash S., Dineshkumar D., Krishnamoorthy P., Elangovan A. V., Sivaram M., Parthipan S., Selvaraju S. Influence of detoxified neem seed cake on diet digestibility, body weight change, hormonal profiles, immune response and testicular gene expression in male sheep. Anim Feed Sci Technol. 2016; 211: 41-49.
CrossRef - Cordero-Mora J. L., Martínez-Aispuro J. A., Martínez-García J. A., Mendoza-Martínez G. D., Sánchez-Torres M. T., Figueroa-Velasco J. L. Neem (Azadirachta indica A. Juss) leaves as growth promoter in lambs’ diets. Agro Productividad. 2024; 17(2):35.
CrossRef - Alhaj W. T., Moharram B. A., Al-Maqtari T., Al-Mahbashi H. M., Al-Doaiss A. A. The Potential of Jatropha variegata Fruits as a Natural Contraceptive: Antifertility Activity and Phytochemical Analysis. Evid Based Complement Alternat Med: eCAM. 2022;2022:1365526.
CrossRef - Baird D. T., Glasier A. F. Hormonal contraception. N Engl J Med. 1993;328(21):1543-9.
CrossRef - Nylander M. C., Clausen H. V. [Serious adverse effect of combined oral contraceptive pills among teenagers]. Ugeskrift for laeger. 2014;176(26):V05130336.
- Roach R. E., Helmerhorst F. M., Lijfering W. M., Stijnen T., Algra A., Dekkers O. M. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;2015(8):Cd011054.
CrossRef - Auta T., Hassan A. T. Alteration in oestrus cycle and implantation in Mus musculus administered aqueous wood ash extract of Azadirachta indica (neem). Asian Pac J Reprod. 2016;5(3):188-192.
CrossRef - El-Zaiat H. M., Elshafie E. I., Al-Marzooqi W., Dughaishi K. A. Effects of neem (Azadirachta indica) leaf powder supplementation on rumen fermentation, feed intake, apparent digestibility and performance in Omani sheep. Animals. 2022;12(22):3146.
CrossRef - Biswas K., Chattopadhyay I., Banerjee R. K., Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci. 2002;82(11):1336-1345.
- Ndodo N. The Effects Of Neem (Azadirachta Indica) Leaves Extracts, On Some Haematological Indices Of Wistar Rats. IIOSR J Pharm. 2013; 3(8):24-28.
CrossRef - Deshmukh V., Zade V. Antifertility effect of aqueous, ether and chloroform extract of Caesalpinia pulcherrima on female albino rats. Int J Res Stud Biosci. 2014;2(11):13-20.
- Marcondes F., Bianchi F., Tanno A. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609-614.
CrossRef - Romero V., Cruz C. D., Pereira O. Reproductive and toxicological effects of isoflavones on female offspring of rats exposed during pregnancy. Anim Reprod. 2018;5(3):83-89.
- Wall E. G., Desai R., Khant Aung Z., Yeo S. H., Grattan D. R., Handelsman D. J., Herbison A. E. Unexpected plasma gonadal steroid and prolactin levels across the mouse estrous cycle. Endocrinol. 2023;164(6):bqad070.
- Shaikh M., Naqvi S., Khani Z. Effect of Neem oil on the structure and function of the mature female albino rat ovaries. Einstein. 2009;7(1 Pt 1):28-34.
CrossRef - Tripathi A., Shrivastav T. G., Chaube S. K. An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. International journal of applied & basic medical research. 2013;3(1):27-36.
CrossRef - Aritonang T. R., Rahayu S., Sirait L. I., Karo M. B., Simanjuntak T. P., Natzir R., Sinrang A. W., Massi M. N., Hatta M., Kamelia E.The role of FSH, LH, estradiol and progesterone hormone on estrus cycle of female rats. International Journal of Sciences: Basic and Applied Research (IJSBAR). 2017;35(1):92-100.
- Moravati M., Mahmoudi M., Ghazi-Khansari M., Aria A. K., Jabbari L. Sterility and abortive effects of the commercial neem (Azadirachta indica a. juss.) extract neemazal- t/s® on female rat (Rattus norvegicus). Turk J Zool. 2008;32(2):155-162.
CrossRef - Marques P., Skorupskaite K., Rozario K. S., Anderson R. A., George J. T. Physiology of GNRH and gonadotropin secretion. Endotext [internet]. 2022;
- Jeelani I., Bhosale M., Qadir T., Sharma P. K., Nawaz A., Sharif A., Amin A., Sheikh A., Ahmad S., Kukreja V. Pharmaceutical Potential of Constituents from Azadirachta indica and their Specific Role as Anti-cancer Agents. Curr Bioact Compd. 2023;19(3):94-103.
CrossRef - Wetendorf M., DeMayo F. J. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int. J. Dev. Biol. 2014;58(2-4):95-106.
CrossRef - Sleiter N., Pang Y., Park C., Horton T. H., Dong J., Thomas P., Levine J. E. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinol. 2009;150(8):3833-44.
CrossRef - Zhang F.-L., Kong L., Zhao A.-H., Ge W., Yan Z.-H., Li L., De Felici M., Shen W. Inflammatory cytokines as key players of apoptosis induced by environmental estrogens in the ovary. Environ Res. 2021;198:111225.
CrossRef - Chaube S. K., Shrivastav T. G., Tiwari M., Prasad S., Tripathi A., Pandey A. K. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus. 2014;3:464.
CrossRef - Zaręba K., Dorf J., Cummings K., Pryczynicz A., Guzińska-Ustymowicz K., Kędra B. Survivin and caspase-3 and PanIN. Disorders of apoptosis in the process of pancreatic cancer formation. Medical Studies/Studia Medyczne. 2024;40(1):1-7.
CrossRef - Hussein M. R. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11(2):162-178.
CrossRef - Akpantah A. O., Ekong M. B., Obeten K. E., Akpaso M. I., Ekanem T. B. Hormonal and histomorphologic effects of Azadirachta indica leaf extract on the pars anterior of wistar rats. Int J Morphol. 2011;29(2):441-445.
CrossRef - Swamy S. Y., Mohan M. R. Impact of Neem Oil on Ovarian Changes in the Fresh Water Fish Glossogobius Giuris. Asian J Exp Biol Sci. 2010;1(3):677-680.
- Moura K. K. V. d. O., Ribeiro Júnior C. L., Guillo L. A. Estrogen signaling in the proliferative endometrium: implications in endometriosis. Rev Assoc Med Bras. 2016;62(1):72-77.
CrossRef








