Manuscript accepted on :30-08-2024
Published online on: 24-09-2024
Plagiarism Check: Yes
Reviewed by: Dr. Gayathri M Rao and Dr. Anjaneyulu Konuri
Second Review by: Dr. Dito Anurogo and Dr. Moumita Hazra
Final Approval by: Dr. Mariia Shanaida
Yash Garg1 , Jaseem T2*
, Jaseem T2* and Kavita Rasalkar1
and Kavita Rasalkar1
1Department of Biochemistry, Manipal TATA Medical College, Manipal Academy of Higher Education, Manipal, India.
2Department of Biochemistry, Dr. Moopen's Medical College, Wayanad, Kerala, India.
Corresponding Author E-mail: jaseem.t@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2969
Abstract
The link between sleep-related disorders and inflammation is well-known, but the association between inflammatory indices and sleep deprivation is still unclear. In our study, we aimed to investigate the relationship between irregular sleep patterns and systemic inflammation using Hemogram-Based Inflammatory Indices. We collected demographic information from 90 undergraduate medical students through a confidential questionnaire. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). Blood samples were obtained for complete blood count analysis, with platelet (P), lymphocyte (L), and neutrophil (N) counts measured. These values were then used to calculate hemogram-based inflammatory indices, including the Systemic Immune-Inflammation Index (SII), Platelet-to-Lymphocyte Ratio (PLR), and Neutrophil-to-Lymphocyte Ratio (NLR). A high prevalence of inconsistent sleep was observed among medical students, with 53% reporting a PSQI score greater than 5(mean score 5.9±2.9). Subjects with poor sleep quality had elevated SII values. Additionally, female participants who experienced poor sleep quality demonstrated a significant positive correlation with SII (r=0.322; p<0.049). Irregular sleep patterns are associated with greater systemic inflammation milieu specifically with SII compared to NLR and PLR. This effect was more pronounced in female participants, suggesting a potential gender-specific influence.
Keywords
Medical students; Neutrophil-to-Lymphocyte Ratio (NLR); Pittsburgh Sleep Quality Index (PSQI); Platelet-to-Lymphocyte Ratio (PLR); Sleep quality; Systemic Immune-Inflammation Index (SII)
Download this article as:| Copy the following to cite this article: Garg Y, Jaseem T, Rasalkar K. Impact of Sleep Quality on Hemogram-Derived Inflammatory Indices in Medical Undergraduates: A Cross-Sectional Study. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Garg Y, Jaseem T, Rasalkar K. Impact of Sleep Quality on Hemogram-Derived Inflammatory Indices in Medical Undergraduates: A Cross-Sectional Study. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/47EsYwT |
Introduction
Sleep quality is essential for overall health. Research shows that a substantial portion of the general population, between 20% and 65%, experiences poor sleep quality, with roughly one-third of adults affected by various sleep disorders. (1,2) Among medical college students, 20-40% are reported to be sleep-deprived, highlighting the prevalence of this issue in academia. (3,4) Recent research indicates that inadequate sleep quality can negatively impact health through biological pathways, including pro-inflammatory responses. (5) Higher levels of systemic inflammation markers, including fibrinogen, Interleukin 6 (IL-6), and C-reactive protein (CRP), have been associated with suboptimal sleep quality, where elevated levels correlating with worse sleep outcomes. (6)
Recently, there has been an increasing trend in utilizing hemogram-based inflammatory indices to evaluate systemic inflammation. These indices, including the SII, PLR, and NLR, are derived from routine and cost-effective complete blood count tests. They are reported to offer greater specificity compared to traditional markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). (7,8) Additionally, these indices have demonstrated potential in predicting the outcomes of various inflammation-related health conditions. (9) This study explores whether these parameters can provide insights into the relationship between prevalent sleep deprivation and underlying sub-chronic inflammation.
Aim
This study aims to investigate the association between sleep inconsistency and systemic inflammation using Hemogram Based Inflammatory Indices
Methodology
This institution-based cross-sectional study was approved by the ethics committee and involved 90 undergraduate medical students aged 18 to 25 years, including 48 males and 42 females, who participated after providing informed consent. Individuals using sedatives, narcotics, or any central nervous system suppressant for acute or chronic medical conditions were excluded. The questionnaire was designed to screen for subjects who met the exclusion criteria. Demographic details (age, gender, weight, height, and medical history) were collected, and Body Mass Index (BMI) and waist-to-hip ratio (WHR), was calculated.
The study used the Pittsburgh Sleep Quality Index (PSQI), comprising of 19 items and 7 components to evaluate sleep quality. (10) After obtaining informed consent, the participants were instructed to track their sleep patterns and other specified parameters outlined in the questionnaire during weekdays. The participants then completed a questionnaire on weekends.
Whole blood samples were collected from all the participants. Participants were divided into 2 categories based on their PSQI scores: Group 1 (PSQI ≤ 5, signifying good sleep quality) and Group 2 (PSQI > 5, signifying poor sleep quality).
The samples were processed in an automated cell counter to assess the complete blood count including the red blood cell count, hemoglobin content, and differential cell count. Further, the hemogram-based inflammatory indices had been derived accordingly.
SII: calculated as peripheral platelet count × neutrophil count/lymphocyte count. SII = P * N/L
PLR: Ratio of peripheral platelet count to lymphocyte blood counts, PLR = P/L
NLR: Ratio of neutrophils to lymphocytes, NLR= N/L
Statistical Analysis
The data analysis was conducted utilizing SPSS Version 16.0 (Chicago, Illinois, USA), with descriptive findings presented in percentage format. Group comparisons were performed using an unpaired t-test, while correlations were evaluated through Pearson’s correlation analysis. A P-value of less than 0.05 was considered as statistically significant.
Results
The study included 90 participants, with a gender distribution of 53% male and 47% female. The average PSQI score among the participants was 5.9±2.9, and 53% of the participants had a PSQI score greater than 5 (fig-1).
The participants were categorized into two groups based on their PSQI scores: Group 1 (PSQI ≤ 5) and Group 2 (PSQI > 5). Demographic characteristics, including mean age, BMI, WHR, Hb, and blood indices, were comparable between the two groups. (Table-1) The group with good sleep quality (PSQI score ≤ 5) had a higher proportion of males (57%). (Fig-2)
Analysis of inflammatory markers revealed that subjects with poor sleep quality (Table-2) had elevated levels of PLR (84±23 vs. 100±37; P<0.090), NLR (1.36 ± 0.46 vs. 1.57 ± 0.54: P<0.166), and SII (344±126 vs. 439±193; P<0.059).
The PSQI score was comparable between male and female participants (Table-3), but an elevated NLR (1.38 ± 0.5 v/s. 1.62 ± 0.5: P < 0.120), PLR (89.4 ± 33 v/s 98.3 ±28; P < 0.352), and SII (338 ± 136 v/s 471±186; P<0.014) was observed in female subjects (Table-4), and the increase in SII was statistically significant. Additionally, correlation analysis revealed that the PSQI score had a significant positive correlation with NLR, PLR, and SII, particularly among female subjects (r=0.322; p<0.049) (Table 5).
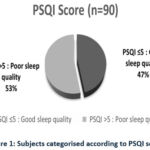 |
Figure 1: Subjects categorised according to PSQI score |
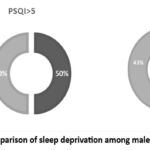 |
Figure 2: comparison of sleep deprivation among males and females |
Table 1: Comparison of study parameters among subjects in group-1 and 2
|
|
Group 1 PSQI ≤ 5 |
Group 2 PSQI > 5 |
P value |
|
PSQI |
3.4 ± 1.23 |
8.12 ± 2.04 |
|
|
Gender (M/F) |
24/18 |
24/24 |
|
|
Age |
21 ± 1 |
21 ± 1 |
|
|
BMI |
24 ± 3 |
24 ± 4 |
0.437 |
|
WHR |
0.83 ± 0.05 |
0.85 ± 0.07 |
0.448 |
|
HB (g/dL) |
14.75 ± 1.77 |
14.01 ± 1.69 |
0.171 |
|
TLC (/µL) |
7.93 ± 1.96 X10³ |
8.09 ± 1.98 X10³ |
0.795 |
|
MCV (fL) |
92.85 ± 4.1 |
90.23 ± 6.78 |
0.823 |
|
MCH (pg) |
30.66 ± 2.35 |
29.39 ± 2.60 |
0.097 |
|
MCHC (g/dL) |
32.97 ± 1.24 |
32.53 ± 0.88 |
0.201 |
|
RDW (%) |
13.78 ± 1.48 |
14.1 ± 1.13 |
0.440 |
|
PLT (/µL) |
273 ± 74 X10³ |
267 ± 65 X10³ |
0.794 |
|
PDW (fL) |
18.62 ± 23.34 |
16.90 ± 21.37 |
0.800 |
|
MPV (fL) |
11.04 ± 1.15 |
10.76 ± 1.14 |
0.430 |
|
NEU (/µL) |
4.26 ± 1.51 X10³ |
4.65 ± 1.83 X10³ |
0.447 |
|
LYM (/µL) |
2.86 ± 0.81 X10³ |
3.06 ± 0.71 X10³ |
0.394 |
Table 2: Comparison of inflammatory indices (NLR, PLR, SII) among subjects in group-1 and 2
|
|
Group 1 PSQI ≤ 5 |
Group 2 PSQI > 5 |
P value |
|
PSQI |
3.4 ± 1.23 |
8.12 ± 2.04 |
|
|
NLR |
1.36 ± 0.46 |
1.57 ± 0.54 |
P < 0.166 |
|
PLR |
84 ± 24 |
100 ± 37 |
P < 0.090 |
|
SII |
344 ± 126 |
439 ±193 |
P < 0.059 |
Table 3: Comparison of study parameters between male and female subjects
|
|
MALE |
FEMALE |
P value |
|
Number |
48 |
42 |
|
|
Age |
21 ± 1 |
21 ± 0.7 |
0.560 |
|
PSQI |
5.96 ± 3.25 |
6.00 ± 2.51 |
0.964 |
|
BMI |
23 ±4 |
22 ± 4 |
0.607 |
|
WHR |
0.87 ±0.04 |
0.82 ±0.07 |
0.041 |
|
HB (gm/dl) |
15.59 ± 0.99 |
12.72 ±1.03 |
0.000 |
|
TLC (/µL) |
7.64 ± 2.03 X10³ |
8.52 ± 1.76 X10³ |
0.130 |
|
MCV (fL) |
92.55 ±4.22 |
89.94 ±7.26 |
0.173 |
|
MCH (pg) |
30.61 ±2.02 |
29.12 ±2.94 |
0.068 |
|
MCHC (g/dL) |
33.04 ±0.96 |
32.34 ±1.09 |
0.034 |
|
RDW (%) |
13.56 ±1.06 |
14.47 ±1.42 |
0.026 |
|
PLT (/µL) |
249.64 ±59.38 X10³ |
297.58 ±72.36 X10³ |
0.025 |
|
PDW (fL) |
17.07 ±20.97 |
18.50 ±23.93 |
0.837 |
|
MPV (fL) |
10.76 ±1.06 |
11.06 ±1.24 |
0.395 |
|
NEU (/µL) |
4.14 ±1.78 X10³ |
4.92 ±1.48 X10³ |
0.121 |
|
LYM (/µL) |
2.92 ±0.92 X10³ |
3.01 ±0.50 X10³ |
0.697 |
Table 4: Comparison of inflammatory indices (NLR, PLR, SII) among male and female subjects
|
|
MALE |
FEMALE |
P value |
|
NLR |
1.38 ± 0.50 |
1.62 ± 0.50 |
P < 0.120 |
|
PLR |
89.44 ± 33.81 |
98.32 ±28.65 |
P < 0.352 |
|
SII |
338.84 ± 136.22 |
471.74 ± 186.90 |
P < 0.014 |
Table 5: Correlation of inflammatory indices (NLR, PLR, SII) with PSQI in female subjects
|
|
r value |
P value |
|
NLR |
0.283 |
0.085 |
|
PLR |
0.192 |
0.249 |
|
SII |
0.322 |
0.049 |
Discussion
Sleep quality among medical students has been studied worldwide because of its negative health outcomes and consequences on their academic routines and personal lives. A metanalysis of 57 studies involving 25,735 medical students worldwide revealed a high prevalence of poor sleep quality, with 52.7% of participants experiencing sleep disturbance with a mean PSQI score of 6.1. (11) Our findings align with these studies, showing a high rate of sleep inconsistency among our participants, with a mean PSQI score of 5.9 ± 2.9 and 53% categorized as poor sleepers.
Previous studies have explored the association between sleep quality, and body mass index have reported an association between short sleep duration and obesity. (12,13) Contrary to these findings, our analysis did not reveal a significant association between BMI and sleep quality. This outcome could largely be attributed to the fact that the mean BMI of our study population was 24 which falls within the normal weight range according to the World Health Organization’s BMI classifications.
Gender differences in sleep quality and patterns, as well as sleep disorders, are well-documented. Studies have consistently revealed that women experience a higher prevalence of various sleep disturbances compared to men. (14) Conversely, men tend to be better sleepers, exhibiting superior sleep quality, longer sleep duration, and higher sleep efficiency. Our findings support this, as we observed that the group with good sleep quality (PSQI score ≤ 5) predominantly consisted of males. This is consistent with previous research showing that female college students tend to have poorer sleep quality, more awakenings, and longer sleep latency. (15,16)
Recent studies have explored the relationship between sleep quality and the immune system. Some studies have reported that sleep disturbances can affect the immune system by weakening its defenses, rendering the body more susceptible to various disorders. (17) Conversely, other studies have observed that sleep patterns can affect the functionality of the immune system, highlighting a reciprocal and intricate interaction between these two critical aspects of health (18,19).
A meta-analysis investigating the relationship between NLR and obstructive sleep apnea (OSA) found that NLR levels were significantly higher in OSA patients compared to controls. This indicates that NLR may serve as a reliable marker for systemic inflammation and a predictor of disease severity in OSA patients. (20) In addition, the role of the synaptic adhesion molecule Neuroligin-1 (NLG1) in sleep-wake regulation has been demonstrated, suggesting a potential mechanism through which sleep can influence the NLR. (21) Even though we observed an elevation in NLR among students with poor sleep quality (PSQI score >5), these results were not statistically significant and when compared based on gender, it was higher in females.
The PLR is associated with sleep quality and is significantly elevated in patients with OSA, these increases correlate with OSA severity. (22) Additionally, PLR serves as an independent marker of cardiovascular disease in individuals with sleep apnea. (23) Factors such as poor sleep quality, fatigue, and vital exhaustion contribute to higher platelet counts, potentially leading to increased PLR. (24) In our study, we also observed an increase in PLR among students with a score above 5, and when compared based on gender, it was higher in females.
As suggested by previous research, the SII combines neutrophils, platelets, and lymphocytes to represent the systemic immune response and inflammation within the body. (25) In a recent cohort study, it was shown that the SII strongly correlates with the severity of OSA and exhibits superior performance compared to both NLR and PLR. (26) Despite these findings, research on the connection between sleep quality, sleep duration, and SII is still relatively unexplored and complex. (27) We noted a statistically significant increase in SII in female participants with sleep disturbance. In addition, a significant positive correlation was identified between the SII and PSQI scores in female subjects compared to NLR and PLR. These findings are in line with earlier studies that reported women are more susceptible to the effects of poor sleep on systemic inflammation than men, and sleep related disorders had a strong correlation with SII than PLR and NLR. (5)
However, this study’s limitations include a potentially limited sample size and restricted generalizability to larger populations, as it exclusively involved medical college students, a single ethnic group. Potential confounding variables were not controlled including socioeconomic status, psychiatric history, physical activity levels, diet, medication use, or other lifestyle factors that can influence both sleep quality and inflammation. Furthermore, the adoption of a cross-sectional study design limits the inference of causal relationships between sleep quality, inflammatory markers, and health outcomes.
Future research should explore advanced technologies such as wearable devices and AI-based tools for more accurate and real-time monitoring of sleep patterns and inflammatory markers to better understand the molecular mechanism involved. The gender-specific variation in inflammation underscores the importance of considering hormonal, lifestyle, and genetic factors in future research.
These findings support the development of targeted educational programs on the importance of sleep, especially in environments like medical schools, where students face high stress and irregular schedules. The simplicity of calculating SII, PLR, and NLR from routine blood counts makes these indices valuable for use in clinical practice.
Conclusion
Our findings emphasize the link between irregular sleep patterns and increased systemic inflammation. This highlights the need to identify and address sleep disturbances among medical students, ultimately enhancing the effectiveness of medical education programs.
Acknowledgment
I would like to sincerely thank everyone who supported me in my studies: my guide, my co-authors, and all the participants
Conflict of Interest
There is no conflict of interest to be declared.
Funding Sources
Our study is funded by Manipal Academy of Higher Education Seed Grand 2023. The grant number is 205501002/315/2022
References
- Schlarb A, Friedrich A, Claben M. Sleep problems in university students: an intervention. Neuropsychiatr Dis Treat. 2017;13:1989-2001.
CrossRef - Azad MC, Fraser K, Rumana N, Ahmad A, Shahana N, Hanly PJ. Sleep disturbances among medical students: a global perspective. J Clin Sleep Med. 2015;11(1):69-74.
CrossRef - Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. 2016;5(4):780-784.
CrossRef - Mills PJ, von Känel R, Norman D, Natarajan L, Ziegler MG, Dimsdale JE. Inflammation and sleep in healthy individuals. Sleep. 2007;30(6):729-735.
CrossRef - Dzierzewski JM, Donovan EK, Kay DB, Sannes TS, Bradbrook KE. Sleep inconsistency and markers of inflammation. Front Neurol. 2020;11:1042.
CrossRef - Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702-715.
CrossRef - Fest J, Ruiter R, Ikram MA, van Eijck CHJ, Stricker BH, Ikram MK. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566.
CrossRef - Harrison M. Abnormal laboratory results: erythrocyte sedimentation rate and C-reactive protein. Aust Prescr. 2015;38(3):93.
CrossRef - Islam MM, Satici MO, Eroglu SE, Helvaci M, Altintop L, Basoglu D, Varol S, Akkoyun H, Cinar O. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: an extensive literature review. Turk J Emerg Med. 2024;24(1):8-19.
CrossRef - Pittsburgh Sleep Quality Index (PSQI) Calculator [Internet]. QxMD. Available from: https://qxmd.com/calculate/calculator_603/pittsburgh-sleep-quality-index-psqi. Accessed August 28, 2024.
- Rao WW, Li W, Qi H, Hong L, Cao X, Wen Y, Cheng WJ, Zhang QQ, Cheung T, Hall BJ, Ungvari GS, Xiang YT. Sleep quality in medical students: a comprehensive meta-analysis of observational studies. Sleep Breath. 2020; 24:1151-1165.
CrossRef - Prasad M, Pavithra US, Babu D, Priya SP. Correlation of sleep quality with body mass index and blood pressure among the healthcare students in Karnataka: a cross-sectional study. J Clin Diagn Res. 2023;17(2).
CrossRef - Alafif N, Alruwaili NW. Sleep duration, body mass index, and dietary behaviour among KSU students. Nutrients. 2023;15(3):510.
CrossRef - Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383-389.
CrossRef - Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, Fang SC. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943-1952.
CrossRef - Fatima Y, Doi SA, Najman JM, Al Mamun A. Exploring gender difference in sleep quality of young adults: findings from a large population study. Clin Med Res. 2016;14(3-4):138-144.
CrossRef - Gamaldo CE, Shaikh AK, McArthur JC. The sleep-immunity relationship. Neurol Clin. 2012;30(4):1313-1343.
CrossRef - Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457-467.
CrossRef - Barriga-Ibars C, Rodriguez-Moratinos AB, Esteban S, Rial RV. Interrelations between sleep and the immune status. Rev Neurol. 2005;40(9):548-556.
CrossRef - Rha MS, Kim CH, Yoon JH, Cho HJ. Association between the neutrophil-to-lymphocyte ratio and obstructive sleep apnea: a meta-analysis. Sci Rep. 2020;10(1):10862.
CrossRef - El Helou J, Bélanger-Nelson E, Freyburger M, Dorsaz S, Curie T, Faraguna U, Franken P. Neuroligin-1 links neuronal activity to sleep-wake regulation. Proc Natl Acad Sci. 2013;110(24):9974-9979.
CrossRef - Song YJ, Kwon JH, Kim JY, Kim BY, Cho KI. The platelet-to-lymphocyte ratio reflects the severity of obstructive sleep apnea syndrome and concurrent hypertension. Clin Hypertens. 2016;22:1-8.
CrossRef - Gabryelska A, Łukasik ZM, Makowska JS, Białasiewicz P. Obstructive sleep apnea: from intermittent hypoxia to cardiovascular complications via blood platelets. Front Neurol. 2018;9:635.
CrossRef - Krummenacher R, Lukas PS, Demarmels Biasiutti F, Begré S, Znoj H, von Känel R. Independent association of sleep quality, fatigue, and vital exhaustion with platelet count in patients with a previous venous thromboembolic event. Platelets. 2009;20(8):566-574.
CrossRef - Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212-6222.
CrossRef - Topuz MF, Ture N, Akdag G, Arik O, Gulhan PY. The importance of systemic immune-inflammation index in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2022;279(10):5033-5038.
CrossRef - Kadier K, Dilixiati D, Ainiwaer A, Abulikemu A, Niyazi A, Xia T, Aximujiang K, Haxim A, Abudureheman S, Zhao W, Zhou J, Aili A. Analysis of the relationship between sleep-related disorder and systemic immune-inflammation index in the US population. BMC Psychiatry. 2023;23(1):773.
CrossRef







