Rahmi Annisa , Yasmin Erly Agustin, Nabila Rahmadani
, Yasmin Erly Agustin, Nabila Rahmadani and Roihatul Mutiah
and Roihatul Mutiah
Department of Pharmacy, Faculty of Medicine and Health Science, Universitas Islam Negeri Maulana Malik Ibrahim, Malang, Indonesia
Corresponding Author E-mail:roiha@farmasi.uin-malang.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2974
Abstract
The aim of this study was to determine the effect of different concentrations of yellow root extract (Arcangelisia Flava (L.) Merr.) and film-forming hydrogel (FFH) polymer on the physicochemical characteristics and stability of the preparation. The method for making FFH is carried out using varying concentrations (%) of 0,5 extract, 1, and 1,5, as well as variations in concentration (%) of PVP and PVA (2,8:15,2; 2,8:17,2; 2,8:19,2). F1 – F9 were evaluated for physicochemical characteristics, including organoleptic, pH, spreadability, adhesive power, drying time, viscosity, stickiness, and mechanical properties. Next, freeze-thaw stability and data analysis were carried out using the LSD test at a confidence level of 95%. This research indicates that the FFH formulation of yellow root extract has good characteristics in the F1 formula with an extract concentration of 0.5% and a concentration (%) of PVP and PVP polymer (2.8:15.2), which meets all physicochemical characteristic tests. The results of data analysis using SPSS showed that the extract concentration and polymer concentration had a significant effect (0.05<0.05) on the characteristics and stability of the Yellow Root Extract Film Forming Hydrogel (FFH) preparation.
Keywords
Burns; Film Forming Hydrogel; Polymers; Wound; Yellow Root Extract
Download this article as:| Copy the following to cite this article: Annisa R, Agustin Y. E, Rahmadani N, Mutiah R. Formulation and Characteristics of Film Forming Hydrogel (FFH) of Yellow Root Extract (Arcangelisia Flava (L.) Merr.) As Wound Healing for Burns. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Annisa R, Agustin Y. E, Rahmadani N, Mutiah R. Formulation and Characteristics of Film Forming Hydrogel (FFH) of Yellow Root Extract (Arcangelisia Flava (L.) Merr.) As Wound Healing for Burns. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3Wa7Hph |
Introduction
Burn injuries remain a global health threat. The rate of injury is 92 %, and burn injury is one of them 1. In a previous study, the prevalence of burns reached 0.6 percent 2. Burns are the tissue response of the skin to temperature-induced trauma. Several things, such as heat, steam, chemicals, and electricity, can cause burns. Most burn injuries were caused by high temperatures or heat, accounting for 95 percent of the total incidence. The types of heat-related injuries were categorized into three categories, including blisters (50 percent), burns from direct contact with fire (24 percent), and burns (26 percent) 3.
Indonesia is rich in natural resources, including a wealth of plants with medicinal properties. The culture of using natural ingredients as medicine has been implemented by society for a long time, and the latest trend is towards a more natural lifestyle, so people are again using various natural ingredients for treatment 4. Much research has been carried out on beneficial plants in medicine. The use of medicinal plants is a tradition that has been passed down from generation to generation. The use of natural ingredients as treatment continues to experience rapid growth in the field of medicine and modern medicine to date. One plant proven to have medicinal benefits is yellow root (Arcangelisia flava (L.) Merr.) 5.
Yellow root (Arcangelisia flava (L.) Merr.) is a native Indonesian plant that the community has widely used as a medicine. Compounds in yellow root plants, including alkaloids, flavonoids, and saponins, can accelerate burn healing 4. Yellow root plant stem extract with a concentration of 0,025 percent can accelerate the wound healing process through anti-inflammatory mechanisms 5. In research conducted by 6, It was mentioned that the yellow root plant stem extract at a concentration of 1 gram in 100 grams of topical gel preparation gave optimal bacterial inhibition results. In addition, the research conducted by 4 mentioned that the yellow root’s alkaloid, flavonoid, and saponin content has activity in epithelial cell proliferation.
One treatment for burns can be done using conventional semisolid topical preparations such as ointments, gels, and creams. However, these preparations have limitations when applied to the wounded skin 6. These limitations include the contact time between the preparation and the skin, which cannot last long, is easily erased due to friction, and frequent use, which affects compliance with the use of the preparation. Technological developments in the health sector encourage continued improvement of drug delivery systems to overcome the limitations of previous preparations. Film Forming Hydrogel (FFH) is a topical preparation applied directly to the skin surface, leaving a thin layer immediately after use. The development of Film Forming Hydrogel (FFH) aims to overcome the problems found in previous preparations with the ability to absorb good wound exudate. In addition, the formed film has properties that can follow the skin’s elasticity, providing comfort when used.10
The concentration of polymer used affects the characteristics of the film formed. The polymer concentration should be manageable because it can cause the film layer formed to be stiff and rigid, making it brittle and easily broken10. Meanwhile, the film formed will have good resistance if the polymer concentration increases. Film Film-forming hydrogel (FFH) from ethanol extract of yellow root plant stems is made in 9 formulas, namely, F1, F2, F3, F4, F5, F6, F7, F8, and F9. These formulas have the same plasticizer and solvent concentrations but different concentrations of active ingredients and polymers to see the effect on the physicochemical characteristics of the preparations made and their stability. The active ingredient used in the FFH preparation is yellow root extract (Arcangelisia flava (L.) Merr.) with a concentration of % (0,5; 1; 1,5). Meanwhile, the polymers used in the formula are PVP and PVA with a concentration ratio of % (2,8: 15,2; 2,8: 17,2; 2,8: 19,2). Therefore, this study aims to obtain a formula with good physical and chemical characteristics of FFH preparations using variations in the concentration of PVP-PVA polymers and extract concentration.
Material and methods
Extraction of yellow root plant
The extraction of yellow root plant stems was conducted using Ultrasound-Assisted Extraction (SONICA) using 70% ethanol solvent (PT. Bratachem). The extraction was performed with a solvent ratio 1:10 for 10 minutes at 50oC. The filtrate that has been obtained is then filtered and concentrated using a rotary evaporator (IKA RV 10 Basic) at 400C and in the oven at 500C 8. In this extraction, a yield of 4.8 percent was obtained. The requirement for the yield of yellow root extract based on the Indonesian Herbal Pharmacopoeia is >3.5 percent. 7
Yield Calculation
The formula calculates calculation of the yield of yellow root extract:
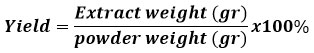
Preparation of Film Forming Hydrogel (FFH)
FFH formula consists of yellow root dry extract as the active ingredient, PVA-PVP as the polymer (PT. Bratachem), Propylene glycol as a plasticizer (PT. Bratachem), nipagin and nipasol as preservatives (PT. Bratachem), and distilled water as a solvent (PT. Bratachem). The preparation of FFH (Table 1) started with the preparation of hydrogel base (PVA-PVP), and the other components were mixed gradually. Stirring was done using a homogenizer (IKA) at 3000 rpm for 10 minutes 9.
Table 1: Formula Film Forming Hydrogel (FFH) yellow root extract (A.flava)
|
Name |
Function |
Concentration % (b/b) |
|||||||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
F10 |
F11 |
F12 |
||
|
Ekstrak |
Active compound |
0,5 |
0,5 |
0,5 |
1 |
1 |
1 |
1,5 |
1,5 |
1,5 |
– |
– |
– |
|
PVP |
Polymer |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
2,8 |
|
PVA |
Polymer |
15,2 |
17,2 |
19,2 |
15,2 |
17,2 |
19,2 |
15,2 |
17,2 |
19,2 |
15,2 |
17,2 |
19,2 |
|
PG |
Plasticizer |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
Nipagin |
preservative |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
0,18 |
|
Nipasol |
preservative |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
|
Aquades |
Solvent |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
ad 100 |
Characteristic Evaluation of Film Forming Hydrogel (FFH)
Organoleptic
Organoleptic testing is done by visually examining the preparation’s shape, color, and smell.
pH
The pH test was conducted using a pH meter (Mettler Toledo) and repeated three times. The pH values that meet the criteria are 4-6 10.
Adhesiveness
The adhesion test was carried out by placing 0.5 g of preparation on a glass slide, then covered with another glass slide and giving a load of 200 g for 5 minutes. Determination of adhesion is done by calculating the time required until both glass objects are released. The requirement for the adhesion test is >4 seconds 11.
Spreadability
The spreadability test was carried out by placing 0.5 g of preparation on a round glass with a diameter, then covering it with another glass on top and leaving it for 1 minute. After one minute, weights of 50, 100, 150 g were given. Diameter measurements were taken after being given weights for 1 minute. The requirements for the spreadability test are 5-7 cm 12.
Viscosity
The viscosity of the preparation was measured using a tool viscometer Brookfield spindle 64. The viscosity test requirements are 7100-83144 cPs 13.
Drying Time
The drying time test on the formed film was carried out by applying the preparation on a glass object that had previously been heated at 370C. The test parameters for drying time are <5 minutes 14.
Mechanical Properties
Assessment of tensile strength and elongation can describe the film formed. The description of the film also describes its resistance to abrasion and flexibility. Assessment based on the mechanical properties of human skin determined the value of tensile strength and elongation of the film formed is 5-32 Mpa and 30-115% 15.
Stickiness
The testing process is done by pressing the paper against the dry film with low pressure. The film formed should stick to something other than the paper 16.
Stability
Stability testing of FFH using freeze-thaw method. FFH was stored for 24 hours at 40C and 24 hours at 400C 17. This process is repeated for three cycles or six days 18.
Data Analysis
Statistical analysis was carried out using parametric statistical tests, multivariate analysis using SPSS version 23 software. In order to know whether there was a significant influence between the formulas, further analysis was carried out using the least significant difference (LSD) with a confidence level of 95% (p-value <0,05).19
Results and Discussion
This research is developing a delivery system, namely Film Forming Hydrogel (FFH), aimed at overcoming the problems found in conventional topical preparations such as gels, ointments, and creams because conventional preparations provide a fast contact period and are less stable in storage. The use of FFH in wound therapy management can absorb wound exudate well 20. In addition, the formed film has properties that can follow the skin’s elasticity, providing comfort when used 20.
Characteristic Evaluation of Film Forming Hydrogel (FFH)
Organoleptic
Based on the organoleptic evaluation results, F7, F8, and F9 with 1.5 percent extract concentration have a darker color and a more intense characteristic odor when compared to F1, F2, and F3 with 0.5 percent extract concentration and F4, F5, F6 with 1 percent extract concentration. FFH that meets the organoleptic requirements has a color like the active substance, a distinctive aroma of the yellow root, and a thick appearance.
The results of the dosage form observation show that the formula can be poured with varying viscosity. This is due to the increase in the viscosity of the preparation. By naked eye observation, the preparations with suitable viscosity are F1, F4, and F7 because they are not too thick and liquid.
Table 2: Organoleptic evaluation results before and after stability test of formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava)
|
Formula |
Observations |
Stability |
||
|
Before stability test |
After stability test |
|||
|
F1 |
Color |
Yellow to light brown |
Yellow to light brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Yellow root characteristic odor |
Yellow root characteristic odor |
||
|
F2 |
Color |
Yellow to light brown |
Yellow to light brown |
|
|
Shape |
thick gel-like |
thick gel-like |
||
|
Smell |
Yellow root characteristic odor |
Yellow root characteristic odor |
||
|
F3 |
Color |
Yellow to light brown |
Yellow to light brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Yellow root characteristic odor |
Yellow root characteristic odor |
||
|
F4 |
Color |
Dark brown |
Dark brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Sharp odor typical of yellow root |
Sharp odor typical of yellow root |
||
|
F5 |
Color |
Dark brown |
Dark brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Sharp odor typical of yellow root |
Sharp odor typical of yellow root |
||
|
F6 |
Color |
Dark brown |
Dark brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Sharp odor typical of yellow root |
Sharp odor typical of yellow root |
||
|
F7 |
Color |
Blackish brown |
Blackish brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Sharp odor typical of yellow root |
Sharp odor typical of yellow root |
||
|
F8 |
Color |
Blackish brown |
Blackish brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Sharp odor typical of yellow root |
Sharp odor typical of yellow root |
||
|
F9 |
Color |
Blackish brown |
Blackish brown |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Sharp odor typical of yellow root |
Sharp odor typical of yellow root |
||
|
F10 |
Color |
Yellowish white |
Yellowish white |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Typical odor of PVA polymer |
Typical odor of PVA polymer |
||
|
F11 |
Color |
Yellowish white |
Yellowish white |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Typical odor of PVA polymer |
Typical odor of PVA polymer |
||
|
F12 |
Color |
Yellowish white |
Yellowish white |
|
|
Shape |
Thick gel-like |
Thick gel-like |
||
|
Smell |
Typical odor of PVA polymer |
Typical odor of PVA polymer |
||
Organoleptic test observations on FFH preparation were done twice before and after stability. After the stability test, the observation showed no change in the preparation’s color, odor, and shape.
pH
The pH measurement was carried out using a pH meter. Topical preparations must have a pH by the normal pH of the skin, namely 4-6 10. The pH around the wounded skin tends to increase between 7-8. An alkaline pH will inhibit wound healing, so a slightly acidic pH is required.
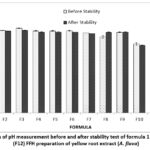 |
Figure 1: Graph of pH measurement before and after stability test of formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava). |
The pH test results show that all formulas meet the pH range according to the standard, namely 4-6 20. It can be seen in the graph that as the extract increases, the pH of the preparation will decrease. This is because the pH of the extract is in the acidic pH range, namely 5.08. In the pH testing graph after stability, the results show a decrease in pH. A decrease in pH value during storage can occur due to the influence of CO2 reacting with the water phase, so the pH tends to become more acidic 21. However, the decrease is still within the range of pH testing parameters. Based on the results of LSD statistical analysis, there is a significant effect between the concentration of extracts and polymers on the pH of FFH preparations in pH measurement (p-value <0,05). In addition, the concentration of extract and polymer also had a significant effect on the stability of the preparation (p-value <0,05).
Adhesiveness
Testing the adhesive power of the preparation is done to know the ability of the hydrogel preparation to adhere to the skin’s surface when used. A good hydrogel preparation will produce adhesion of more than 4 seconds 11. The concentration of PVA influences the adhesive power used where the higher the concentration of PVA, the longer the adhesive power will be 12.
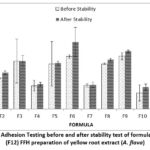 |
Figure 2: Graph of Adhesion Testing before and after stability test of formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava). |
The adhesion test results showed that all formulas met the pH range according to the standard, namely >4 seconds 11. The graph shows that the polymer concentration affects the adhesion of the preparation, where the greater the concentration of the PVP polymer ratio: PVA polymer ratio, the more the adhesion of the preparation increases. It can be seen in F3 at the concentration ratio of PVP: PVA (2.8: 19.2) percent has a higher graph when compared to F1 at a concentration ratio of PVP: PVA (2.8: 15.2) percent. After the freeze-thaw stability test, the adhesion of the preparation increased. This is because the viscosity of the preparation increases after the stability test is carried out. The results of the LSD statistical analysis show that the extract concentration has a non-significant effect (p-value> 0,05) at 1 percent of the extract concentration against F7, F8, and F9. 1.5 percent extract concentration against F4, F5, and F6. While the polymer concentration gives significantly different test results (p-value < 0,05) against all formulas. As for stability, the concentration of extract and polymer had a significant effect (p-value <0,05).
Spreadability
The spreadability test was conducted to determine the ability of FFH preparations to spread when applied to the skin. It is expected to spread quickly when applied so that the effect is evenly distributed 22. Hydrogel preparations that provide good spreadability are expected to have better wound healing effectiveness 23. Spread ability is related to the viscosity of the preparation, where the more significant the viscosity value of preparation, the more difficult it will be applied so that the ability of the preparation to spread quickly on the skin is also lower. The greater the spreadability of the hydrogel preparation, the faster it will penetrate the skin and show its effectiveness 22. Spread ability testing parameters are 5-7 cm 12. Spread ability that is too high (> 7cm) or too low (< 5cm) will make it difficult when the preparation is applied to the skin 12.
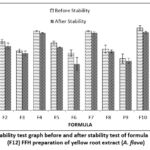 |
Figure 3: Spread ability test graph before and after stability test of formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava) |
The results of the spreadability test of formulas that meet the spreadability test standards of 5-7 cm 12 F1, F4, and F7 with a polymer concentration ratio of PVP: PVA (2.8: 15.2) percent. Polymer concentration affects the ability of the preparation to spread. The higher the polymer concentration used, the preparation’s spreadability will also decrease. After the stability test was carried out, the results obtained were that the spreadability of the preparation decreased. This shows that storage temperature affects the viscosity of the preparation. The thicker the preparation, the more the spreadability of the preparation will decrease. Based on the results of LSD, statistical analysis shows a significant effect of differences in extract concentration and polymer concentration on the results of the preparation spreadability test (p-value <0,05). The stability test showed that the concentration of 1 percent extract had no significant effect (p value>0.05) on F10, F11, and F12. At the same time, the polymer concentration has a significant effect (p-value <0.05).
Viscosity
The viscosity test was conducted to determine whether or not the FFH preparation was easy to apply. It shows the ability of the preparation to flow. In addition, viscosity can be used as a stability parameter and affect the preparation’s spreadability and stickiness 23.
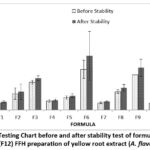 |
Figure 4: Viscosity Testing Chart before and after stability test of formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava) |
Based on the table above, the results of viscosity testing on FFH preparation of yellow root extract produce different results. The higher the polymer concentration, namely PVA, used, the greater the viscosity of the preparation. Formulas that meet the viscosity test standards 7100-83144 cPs 13 are F1 and F7. This is because increasing the concentration of PVA can increase the number of polymer fibers; besides that, PVA has properties that bind water so that much liquid is retained and pulled by PVA 24. After the stability test, the viscosity increased. This change may be due to the effect of the polymer on temperature changes. The last cycle test in the formula is at a cold temperature of 40C. When preparation is stored at cold temperatures, the polymer chains will shorten and join each other to increase viscosity 25. Based on the results of LSD statistical analysis, it was found that 1 percent extract concentration did not have a significant effect (p value>0.05) on formulas F10, F11, and F12. Polymer concentration has a significant effect (p-value <0.05). Meanwhile, the stability results showed that the concentration of 1 percent extract had no significant effect (p value>0.05) on formulas F10, F11, and F12. At the same time, the polymer concentration has a significant effect (p-value <0.05).
Drying Time
The drying time test was conducted to see the ability of FFH preparation to form a thin film on the skin surface shortly after the preparation was applied. A good FFH preparation will enable the formation of a thin film within 5 minutes 14. The faster the FFH preparation dries, the faster the active ingredients are released and penetrated. Based on the research journal conducted by the study10, it is stated that after the preparation is applied to the skin, the solvent will evaporate, resulting in a thin film residue. When the solvent evaporates, the drug will be released and penetrated.
 |
Figure 5: Drying time testing chart before and after stability test of formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava). |
The drying time test was done by applying the preparation to a glass object previously heated at 370C and then counting until the preparation dried. Heating the glass object at 370C aims to provide a temperature comparable to the average human body temperature in the 36.20C – 37.50C so that the film can dry. All FFH formulas of yellow root extract met the test standard of drying time <5 minutes 14 except in the control formula 1 (F10). The concentration of polymer used affects the drying time of the preparation; when used with an increasing concentration, the drying time will be faster 26.
After the stability test, the preparation’s drying time decreases; the preparation’s viscosity influences this. The thicker the preparation, the faster the drying time will be because the solvent in the preparation is less, and the evaporation of the solvent is faster. Based on the results of LSD, statistical analysis shows that the difference in extract concentration and polymer concentration has a significant effect (p-value <0.05). In the LSD statistical test on stability, the results showed that the concentration of extract and polymer had a significant effect (p-value <0.05).
Stickiness
The stickiness test is carried out to determine whether the film formed has sticky properties. This test is done by attaching a piece of paper pressed on the box with the film. The film is said to be good if not sticky on the paper’s surface 16.
Tabel 3: Stickiness Testing Results before and after stability test formula 1 (F1)-formula 12 (F12) FFH preparation of yellow root extract (A. flava)
|
Formula |
Before Stability Test |
After Stability Test |
|
F1 |
No film sticking |
No film sticking |
|
F2 |
No film sticking |
No film sticking |
|
F3 |
No film sticking |
No film sticking |
|
F4 |
No film sticking |
No film sticking |
|
F5 |
No film sticking |
No film sticking |
|
F6 |
No film sticking |
No film sticking |
|
F7 |
No film sticking |
No film sticking |
|
F8 |
No film sticking |
No film sticking |
|
F9 |
No film sticking |
No film sticking |
|
F10 |
No film sticking |
No film sticking |
|
F11 |
No film sticking |
No film sticking |
|
F12 |
No film sticking |
No film sticking |
The results of sickness testing showed that all formulas fulfilled the stickiness test; no film was attached to the paper’s surface. This shows that when the preparation is applied to the skin surface, it does not cause discomfort because it is easy to stick to clothes or fabrics. In the stickiness test, it was found that there were no changes in all formulas from the stickiness test results after the freeze-thaw stability test was carried out.
Mechanical Properties
Plasticizers play an essential role in providing flexibility to the film and increasing the tensile strength of the film formed. The choice of plasticizer must be tailored to the type of polymer used and have a low level of permeability to the skin. Common plasticizers include glycerin, propylene glycol, polyethylene glycol, and sorbitol 10.
Table 4: Evaluation results of mechanical properties before and after stability test of formula 1 (F1) FFH preparation of yellow root extract (A. flava)
|
Formula |
Elongation (Mpa) |
Tensile Strength (%) |
|
R1 |
2,9 |
30,00 |
|
R2 |
3,5 |
43,33 |
|
R3 |
4,2 |
60,00 |
|
Rerata±SD |
3,53±0,650 |
44,44±15,030 |
Assessment of tensile strength and flexibility can describe the film formed. In addition to the description of the film formed, it also describes the film’s resistance to abrasion and its flexibility. Based on the graph of the tensile strength and flexibility test results in Formula 1, the results are 3.53 N and 44.44 percent.
The FFH research has been widely developed using the active ingredients 27, pepper extract, and cloves 27. It is essential to pay attention to the use of polymer concentration in the FFH system because it can affect the physical and chemical properties of the FFH preparation. They used concentration (%) of PVP and PVA polymer with a ratio of 2.8:17.2 to produce FFH preparations with optimal characteristics. In this study, variations in concentration (%) of PVP and PVA were carried out within limits30. The lowest and highest are at a ratio of 2.8:15.2, 2.8:17.2, and 2.8:19.2. The use of polymers influences the characteristics of the Film film-forming hydrogel (FFH) preparation, including formation time, adhesion, pH value, viscosity, spreadability, stickiness 28.
Mechanical properties are affected by the type and amount of polymer. Concentrations that are too low cause discontinuous film formation, while concentrations that are too high produce stiff films, causing discomfort when applied. The improvement of mechanical properties is also influenced by adding plasticizers, which can increase the voids between polymer chains, thus increasing their mobility 15.
This research focuses on formulation so further research is needed regarding its effectiveness in healing burn wounds through pre-clinical trials using experimental animals. Besides that in-depth study of formulations related to additional materials, especially the types of polymers used which can influence the physical and chemical characteristics of Film Forming Hydrogel (FFH) preparations.
Conclusion
The best Film Forming Hydrogel (FFH) preparation was shown in F1 with an extract concentration of 0,5 % and a concentration of polymers (%) of PVP and PVA (2,8:15,2), which met the physicochemical characteristics test which included a semisolid form with a characteristic odor. Yellow root extract is yellow to light brown, pH 5,58 ± 0,026, spreadability 5,45 ± 0,01, adhesive power (seconds) 1,02 ± 0,25, drying time (seconds) 3,95 ± 0,67, stickiness indicates no film adheres to the fiber, viscosity (Cps) 7120 ± 9078.58, elongation (Mpa) 3,53 ± 0,65, tensile strength (%) 44,44 ± 15,03. In line with existing theory, variations in the concentration (%) of PVP and PVA polymers (2,8:15,2; 2,8:17,2; 2,8:19,2 ) have a significant effect (p-value <0, 05) on the physical and chemical characteristics as well as the stability of the preparation which requires further development of this research on wound healing activities.
Acknowledgments
The authors also wish to thank the Pharmacy Department of State Islamic University, Maulana Malik Ibrahim of Malang, for providing the facilities to conduct this study.
Conflict of Interest
The authors declared no conflict of interest.
Funding Sources
This research was funded by the Faculty of Medical and Health Science, Universitas Islam Negeri Maulana Malik Ibrahim Malang superior faculty research collaboration between lecturers and students based on dean decision number 0910/FKIK/05/2023.
References
- Wisesa J, Revilla G, Yenita Y. Pengaruh Pemberian Human Bone Marrow Mesenchymal Stem Cell Terhadap Gambaran Mikroskopis Jaringan Adneksa Pada Kulit Tikus Luka Bakar Diabetes Melitus. Jurnal Ilmu Kesehatan Indonesia 2022;3(2):131–137; doi: 10.25077/jikesi.v3i2.822.
CrossRef - Putu N, Anantarini D, Rosita ME, et al. Efektivitas Ekstrak Tanaman Obat Dalam Sediaan Gel Terhadap Penyembuhan Luka Bakar PENDAHULUAN Salah satu masalah kesehatan dunia yang menyebabkan sekitar 180 . 000 kematian setiap tahunnya adalah luka bakar . Berdasarkan data Riskesdas 2018 prevelensi lu. Jurnal Ilmu Kesehatan (JIKA) 2022;1(2):28–39.
- Wijaya GA, Adnyana IMS, Subawa IW. Gambaran Tingkat Pengetahuan Pedagang Gorengan Tentang Pencegahan Dan Penanganan Pertama Luka Bakar Di Denpasar Tahun 2017. Jurnal Medika Udayana 2019;8(9).
- Giri IM, Wardani K, Suena NM. Peran metabolit sekunder tumbuhan dalam pembentukan kolagen pada kulit tikus yang mengalami luka bakar. Jurnal Integrasi Obat Tradisional 2021;1(1):23–29.
- Tavita GE, Lestari D, Linda R, et al. Phytochemical Testing and In Vitro Anti-inflammatory Activity on Ethanol Extract of Akar Kuning (Arcangelisia flava L) Stems from West Kalimantan. Jurnal Biologi Tropis 2022;22(4):1334–1339; doi: 10.29303/jbt.v22i4.4431.
CrossRef - Mulyani E, Suratno S, Pratama MRF. Formulasi dan Evaluasi Gel Topikal Antibakteri Fraksi Aktif Akar Kuning (Arcangelisia flava Merr.). Jurnal Pharmascience 2020;7(1):116–124; doi: 10.20527/jps.v7i1.8081.
CrossRef - Susiloningrum D, Sari Dem. Optimasi Suhu Uae (Ultrasonik Asssisted Extraction) Terhadap Nilai Sun Protection Factor (Spf) Ekstrak Rimpang Bangle (Zingiber Purpureum Roxb) Sebagai Kandidat Bahan Aktif Tabir Surya. Cendekia Journal Of Pharmacy 2023;7(1):58–66; Doi: 10.31596/Cjp.V7i1.207.
CrossRef - Hasibuan Ne, Azka A, Basri, Et Al. Skrining Fitokimia Ekstrak Etanol Daun Avicennia Marina Dari Kawasan Bandar Bakau Dumai. Aurelia Journal 2022;4(2):137–142.
- Handayani W, Aryani N, Oktaviyanti N. Stabilitas Fisik Dan Ph Sediaan Gel Antiaging Ekstrak Buah Mengkudu (Morinda Citrifolia L.). CALYPTRA 2020;9(1):1–19.
- Crendhuty FD, Sriwidodo S, Wardhana YW. Sistem Penghantaran Obat Berbasis Biopolimer Kitosan sebagai Film Forming System. Majalah Farmasetika 2021;6(1):38–55; doi: 10.24198/mfarmasetika.v6i1.27457.
CrossRef - Forestryana D, Hayati A, Putri AN. Formulation and Evaluation of Natural Gel Containing Ethanolic Extract of Pandanus amaryllifolius R. Using Various Gelling Agents. Borneo Journal of Pharmacy 2022;5(4):345–356; doi: 10.33084/bjop.v5i4.1411.
CrossRef - Silvia BM, Dewi ML. Studi Literatur Pengaruh Jenis dan Konsentrasi Basis terhadap Karakteristik Masker Gel Peel Off. Jurnal Riset Farmasi 2022;2(1):31–40; doi: 10.29313/jrf.v2i1.702.
CrossRef - Mardhianto A. Uji Aktivitas Antioksidan Masker Gel (Peel-off) Ekstrak Kulit Delima (Punica granatum L.). Universitas 17 Agustus 1945 Jakarta 2019;2019:1–17.
- Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian Journal of Pharmaceutical Sciences 2017;12(6):487–497; Doi: 10.1016/J.Ajps.2017.07.004.
CrossRef - Susilowati R, Annisa R, Duhita Mr, Et Al. Pengembangan Sediaan Film Forming Hydrogel Ekstrak Habbatussauda Dan Hilbah Sebagai Penyembuh Luka Diabetik (Implementasi Hadist Dalam Industri Farmasi Halal). 2023.
- Reddy PS, Ramana Murthy K V. Formulation and evaluation of oral fast dissolving films of poorly soluble drug ezetimibe using transcutol Hp. Indian Journal of Pharmaceutical Education and Research 2018;52(3):398–407; doi: 10.5530/ijper.52.3.46.
CrossRef - Tungadi R, Sy. Pakaya M, D.as’ali PW. Formulasi dan Evaluasi Stabilitas Fisik Sediaan Krim Senyawa Astaxanthin. Indonesian Journal of Pharmaceutical Education 2023;3(1):117–124; doi: 10.37311/ijpe.v3i1.14612.
CrossRef - Rahmani SIP, Zulkarnian AK. Optimization of HPMC and Na-CMC as Gelling Agents on Physical Properties and Stability in Sunflower Seed Oil Gel Formulation. JFood PharmSci 2023;11(2):812–819.
CrossRef - Riswan, Dunan H. Desain Penelitian Dan Statistik Multivariate. Agustus 20. (Riswan, Dunan H. eds). CV. Anugrah Utama Raharja: Bandar Lampung; 2019.
- Arisanty, Anita. Uji Mutu Fisik Sediaan Krim Ekstrak Etanol Buah Belimbing Wuluh (Averrhoa Bilimbi L.) Dengan Variasi Konsentrasi Na. Lauril Sulfat. Media Farmasi 2018;14(1):110–115.
CrossRef - Phindo L. Formulasi Dan Evaluasi Fisik Masker Peel Off Yang Mengandung Ekstrak Etanol 96% Kulit Batang Nangka (Artocarpus Heterophyllus. Lamk) Asam Glikolat Dan Niasinamida. Universitas Islam Negeri Syarif Hidayatullah Jakarta; 2016.
- Rosari V, Fitriani N, Prasetya F. Optimasi Basis Gel dan Evaluasi Sediaan Gel Anti Jerawat Ekstrak Daun Sirih Hitam (Piper betle L. Var Nigra). Proceeding of Mulawarman Pharmaceuticals Conferences 2021;204–212.
- Thomas NA, Taupik M, Djuwarno EN, et al. Uji Penyembuhan Luka Bakar Gel Enzim Bromelin Menggunakan Carbopol 940 Secara In Vivo. Journal Syifa Sciences and Clinical Research 2023;5(2):232–244; doi: https://doi.org/10.37311/jsscr.v5i2.20364 Uji.
CrossRef - Arinjani S, Ariani LW. Fisik Sediaan Masker Gel Peel Off Ekstrak Daun Ungu (Graptophyllum pictum L. Griff). Media Farmasi Indonesia 2019;14(2):1525–1530.
- Mursyid AM. Evaluasi Stabilitas Fisik Dan Profil Difusi Sediaan Gel (Minyak Zaitun). Jurnal Fitofarmaka Indonesia 2017;4(1):205–211; doi: 10.33096/jffi.v4i1.229.
CrossRef - Eka Ermawati D, Prastyo Adi L. Pengaruh Konsentrasi Polivinil Alkohol terhadap Sifat Fisik dan Kimia Sediaan Peel-off Mask Ekstrak Etanol Kayu Secang (Caesalpinia sappan L.). Journal of Applied Agriculture, Health, and Technology 2023;02(01):43–53; doi: 10.20961/jaht.v2i1.638.
CrossRef - Ramadhani, F; Miratsi, L; Humaeroh, Z & Afriani, F. Sintesis dan Karakterisasi Hidrogel PVA/Alginat Mengandung Ekstrak Lada Sebagai Pembalut Luka Antibakteri. Journal of Physics. 2021;02(02):54-59; doi: 10.33369/nmj.v2i2.17752
CrossRef - Pertiwi, D; Ikhsanudin, A; Ningsih, A & Sugihartini, N. Formulasi dan Karakterisasi Sediaan Hydrogel Minyak Cengkeh (Syzygium aromaticum) Berbasis Kitosan. Media Farmasi. 2017;14(1):17-28;
CrossRef - Chamidah, N & Rohmawati, L. 2022. Pengaruh Konsentrasi Ekstrak Daun Sirih Hijau Dan Madu Terhadap Sifat Antibakteri Plester Luka Hidrogel Pva/Kitosan. Inovasi Fisika Indonesia. Volume 11, Nomor 1: 48–55; doi: 10.26740/ifi.v11n1.p48-55.
CrossRef - Saranya & Manoj. Formulation, Evaluation, and Optimization of Novel Silver Sulfadiazine Loaded Film Forming Hydrogel For Burns. Journal for drugs and medicines. 2016;8(2):1-10; doi: 10.15254/H.J.D.Med.8.2016.156
CrossRef








