Ferbian Milas Siswanto1* , Indah Mira Tiaraputri Wijaya2, Maria Dara Novi Handayani1
, Indah Mira Tiaraputri Wijaya2, Maria Dara Novi Handayani1 , Rita Dewi1
, Rita Dewi1 , Ana Lucia Ekowati3
, Ana Lucia Ekowati3 , Jojor Lamsihar Manalu4
, Jojor Lamsihar Manalu4 , and Novelya Novelya5
, and Novelya Novelya5
1Department of Chemistry and Biochemistry, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia.
2Master program on Anti-Aging Medicine, Faculty of Medicine, Udayana University, Bali, Indonesia.
3Department of Medical Biology, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia.
4Department of Physiology, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia.
5Undergraduate Medical Program, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia.
Corresponding Author E-mail: ferbian.siswanto@atmajaya.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2965
Abstract
Aging is a complex and inexorable phenomenon, entailing several physiological changes. Impaired memory skills are associated with cell death in the hippocampus as a result of the age-related buildup of free radicals. D-galactose can artificially accelerate brain aging, causing memory impairment in mice due to neuroinflammation and oxidative stress. Numerous phytoconstituents found in Angelica keiskei possess anti-oxidant, anti-inflammatory, and memory-enhancing properties. In this study, we sought to determine the effects of an ethanol extract from the leaves of A. keiskei (EELAK) on spatial memory in mice with impaired memory functions due to D-gal. Here, we showed that administering 300 mg/kg BW/day of D-gal orally for a duration of 28 days significantly decreased spatial memory as quantified by the Morris Water Maze, and this was linked to a marked increase in hippocampal acetylcholinesterase (AChE), inflammation, and oxidative stress. The spatial memory of D-gal-induced mice was markedly enhanced by 20 mg/kg BW/day of EELAK, demonstrating its potent memory-boosting properties. The mice treated with EELAK also showed a notable decrease in hippocampal neuroinflammation (p65 NF-kB, NO, and TNF-α protein) and an increase in antioxidant activity (elevated SOD activity and reduced MDA levels), suggesting its potent neuroprotective activity. In conclusion, our results establish for the first time that by reducing oxidative stress and neuroinflammation, EELAK enhances spatial memory. Hence, for the prevention and treatment of age-related neurodegenerative illnesses like Alzheimer's disease, EELAK may be a useful therapeutic approach.
Keywords
Angelica keiskei Extract; Brain Aging; D-Galactose; Mice; Spatial Memory
Download this article as:| Copy the following to cite this article: Siswanto F. M, Wijaya I, M. T, Handayani M. D. N, Dewi R, Ekowati A. L. Manalu J. L, Novelya N. Extract of Angelica keiskei Leaves Attenuates Spatial Memory Impairment on the D-galactose Model of Brain Aging in Mice. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Siswanto F. M, Wijaya I, M. T, Handayani M. D. N, Dewi R, Ekowati A. L. Manalu J. L, Novelya N. Extract of Angelica keiskei Leaves Attenuates Spatial Memory Impairment on the D-galactose Model of Brain Aging in Mice. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3TRZBBq |
Introduction
Aging is a natural process that will be experienced by all living things, including humans. Impaired memory is one of the most frequent symptoms of aging, which can be caused by oxidative stress.1 Many studies have shown that the pathogenesis of degenerative diseases and aging processes involves the formation of reactive oxygen species (ROS).2 Age-related memory impairments stem from the build-up of oxidative damage and the concomitant decline in antioxidant defenses, which lowers acetylcholine levels.3 Iron overload in childhood that induces oxidative stress may lead to cognitive impairments.4 Sleep deprivation, via oxidative stress, also contributes to cognitive impairments.5 Excessive amounts of radicals and low concentrations of antioxidants in the brain can cause cell damage.6 The hippocampus and cerebellum are the most vulnerable parts of the brain due to their low antioxidant capacity.7 Study reported that oxidative stress in the brain or hypothalamus results in decreased memory.8 Other factors that can affect memory function are aging, impaired brain perfusion, infectious and systemic diseases, chemical intoxication, head injury, and mental health conditions (depression and/or anxiety).9
Antioxidants compounds that is capable of preventing the oxidation of other molecules, is thought to play a role in improving memory.10 Lately, there has been growing evidence in the potential of phytochemical compounds to improve memory, learning and cognitive abilities.11 A prospective cohort study among Healthy Aging in Neighborhoods of Diversity across the Life Span with a total sampel of 1947 participants showed that regular flavonoid supplementation has a positive effect on improving cognitive function.12 A recent meta-analysis also highlights a strong indications that consuming flavonoids on a regular basis has a positive effect on neurocognitive performance.13
One of the natural ingredients with high flavonoid content is Angelica keiskei, especially the leaf parts. There have been many studies on the benefits of Angelica keiskei as anti-obesity,14 anti-diabetes,15 anti-hyperpigmentation,16 antithrombotic,17 anti-inflammation,18 and anti-myophaty.19 However, its effect on spatial memory in mice with brain aging has yet to be investigated. Herein, we explored the effects of an ethanol extract from the leaves of A. keiskei (EELAK) on spatial memory in mice with impaired memory functions due to D-gal.
Materials And Methods
Animals
Thirty experimentally naive mice, strain BALB/c, male, 3-month-old, weighing 20–30 g was purchased from Faculty of Medicine, Udayana University, Indonesia. Throughout the experiment, the animals were housed in a standard experimental animal housing with a consistent temperature of 22–24 °C, a 12-hour light–dark cycle, 45–65% humidity, and unrestricted access to a standard food and water (ad libitum). Prior to the experiment, the animals were given a week to get used to the circumstances of the animal facility. After that, they were randomly assigned to three groups, with ten animals each group. The vehicle was administered by orogastric gavage to the healthy control group (HC group). The D-galactose-induced brain aging group (BA group) received 300 mg/kg BW/day of D-gal (Merck, Darmstadt, Germany) orally for a duration of 28 days to mimic aging and were orally treated with the same volume of vehicle.20,21 The EELAK-treated group (EELAK group) received 300 mg/kg BW D-gal in physiological saline and 20 mg/kg BW/day of EELAK dissolved in distilled water orally for a duration of 28 days.22
Preparation EELAK
The Angelica keiskei leaves were prepared using earlier techniques.22 In brief, dried A. keiskei leaves were ground into a coarse powder and then passed through a 40-mesh filter (425 µm). Five liters of 70% ethanol were mixed with the powdered A. keiskei leaves, and the mixture was kept for 48 hours. The mixture was then filtered through Whatman No. 1 filter paper, and the extracts were collected and the solvent was removed using a rotary evaporator. Then, the ethanol extract from A. keiskei leaves (EELAK) was kept at -20 °C.
Morris Water Maze
We tested the mice’s spatial memory using the established Morris Water Maze.23,24 The maze was made up of a recording system and a circular stainless-steel tank that measured 100 cm in diemeter and 60 cm in height. The tank held water with a temperature of 20±1°C and around 30 cm deep. The tank was split into four quadrants geographically. One hidden circular black escape platform measuring 10 cm in diameter and secured one centimeter below the water’s surface was located in one of these quadrants. For four days in a row, oriented navigation trials were conducted twice a day for sixty seconds each, with a thirty-minute break in between. On the fifth day, the platform was removed. The ratio of time spent in the target quadrant to escape latency before reaching the platform were recorded as Probe test results, and the number of target crossings over the previous location of the target platform were recorded as the number of platform crossings.
Analysis of Acetylcholinesterase (AChE) Activity
The activity of acetylcholinesterase (AChE) was assessed from the hippocampus homogenates after the spatial memory assasement. Mice were euthanized by cervical dislocation under anasthesia with 40 mg/kg sodium pentobarbital (I.P.); the intact brains were harvested and and placed into ice-cold PBS (pH 7.4). The cerebellum was removed using a surgical blade, and then the hippocampus was collected from the brain. The hippocampus was either stored frozen at -80 °C or used immediately. To examine the AChE activity, the hippocampus was homogenized in Dulbecco’s Modified Eagle Medium (Wako), centrifuged at 10,000 rpm at 4°C for 20 minutes, and the obtained supernatant was subjected to examination using a colorimetric enzyme-linked immunosorbent (ELISA) test (Cat. No. ab138871, Abcam, Cambridge, UK).
Examination of Neuroinflammatory Markers
The levels of p65 NF-kB (Cat. No. ab176648, Abcam), NO (ab285318, Abcam), and TNF-alpha (ab208348, Abcam) were quantified using commercially available mouse ELISA kits in strict accordance with the manufacturer’s instruction.
SOD Activity Assay
Superoxide dismutase (SOD) activity was measured using a modified version of an earlier methodology.25 Ice-cold ethanol (0.15 ml) was added after 0.1 ml of hippocampus tissue supernatant and 0.25 ml of ice-cold chloroform were added. For ten minutes, the mixture was centrifuged at 4 °C and 3000 rpm. Then, the supernatant was mixed with distilled water (2 ml), EDTA (1.25 ml), and carbonate buffer (3.75 ml). To start the reaction, 1 mL epinephrine (Wako, Osaka, Japan) was added. The absorbance was observed at 480 nm. The SOD activity was then normalized to the total protein in the sample quantified by Lowry’s method and shown as U/mg protein.
Measurement of Malondialdehyde Level
We used the standard calorimetric methods for thiobarbituric acid reactive substances (TBARS) to quantified the level of hippocampus malondialdehyde (MDA).26 In brief, one gram of hippocampal tissue was homogenized in Tris-HCl buffer (pH 7.5) and centrifuged for ten minutes at 1000 g. The supernatant (0.1 mL) was mixed with two milliliters of the MDA working solution (thiobarbituric acid 0.37%, 0.25 N HCl, and 15% TCA). The mixture was allowed to cool to room temperature and centrifuged for 10 minutes at 4 °C and 1500 rpm after being incubated for 15 minutes at 100 °C. After that, 96-well plates were filled with the clear supernatant, and the absorbance at 535 nm was determined. Lowry’s method was used to quantify the protein content, and the hippocampal MDA levels were adjusted.
Statistical Analysis
All data were shown as mean ± S.D. A one-way analysis of variace (ANOVA) was used to determine statistical significance, and Tukey’s post-hoc test was then performed. The findings were considered significant at p < 0.05.
Results
EELAK Enhances Spatial Memory and Reduces Hippocampal AChE Activity in D-gal-induced Mice
Reduced learning and memory abilities and cognitive impairment are the two most important clinical indicators of aging.27 We used the Morris Water Maze task to assess spatial memory in this study. A significant difference was seen between treatment groups in the ratio of time spent in the target quadrant (F2,29 = 76.887, p < 0.001) and the number of target platform crossings (F2,29 = 50.011, p < 0.001), according to the one-way ANOVA analysis of the data. Ratios of the time spent in the target quadrant and the number of target platform crossings were lower in mice treated with D-gal alone (BA group) than in the control group (p < 0.001 and p < 0.01, respectively). These findings lend credence to the established fact that D-gal intervention can significantly impair the capacity of spatial memory and is being used as a model for brain aging. In contrast, the ELAAK group saw fewer target platform crossings and a lower ratio of time spent in the target quadrant when compared to the BA group. Notably, the EELAK group showed improvements in both the number of target platform crossings and the ratio of time spent in the target quadrant, by 48% and 62%, respectively (p < 0.001). Next, as AChE is the specific serine hydrolase of Ach, its levels in the hippocampus were measured. Since AChE is essential for memory, a decrease in its activity is anticipated to cause an increase in ACh levels. A one-way ANOVA analysis revealed a significant variation in AChE activity between the groups (F2,29 = 62.554, p < 0.001). The group treated with D-gal showed an increase in AChE activity in the hippocampus (p < 0.001), and the increase in AchE activity was reduced (p < 0.01) by 20 mg/kg BW/day of EELAK (Figure 1). In conclusion, EELAK can significantly enhance mice’s spatial memory with brain aging, which may be connected to a depletion in AChE activity.
 |
Figure 1: EELAK affects AChE activity and spatial memory of mice treated with D-galactose. |
EELAK Decreases Hippocampal NF-κB and Inflammatory Markers in D-gal-stimulated Mice
Several studies have indicated that D-gal activates NF-kB pathway, which in turn causes hippocampal cytokine storms, such as TNF-α, and releases other inflammatory factors, including nitric oxide (NO).28 In the present study, ELISA was used to measure hippocampal NF-kB (canonical p65), NO, and TNF-α levels to establish if EELAK treatment influences inflammation in the hippocampus. ANOVA revealed significant differences between group effects for p65 NF-kB (F2,29 = 76.633, p < 0.001), NO (F2,29 = 72.509, p < 0.001), and TNF-α (F2,29 = 39.386, p < 0.001). Subsequent posthoc analyses revealed that D-gal treatment alone to the BA group significantly increased p65 NF-kB (p < 0.001), NO (p < 0.01), and TNF-α (p < 0.01) levels by 245%, 130%, and 66%, respectively, compared to healthy control group (HC group), which is consistent with several previous reports.29,30 In the EELAK-treated group, the levels of p65 NF-kB (p < 0.001), NO (p < 0.01), and TNF-α (p < 0.05), in the hippocampus were notably downregulated compared to D-gal only-treated group (BA group) (Figure 2). Together, these data demonstrated that EELAK mediates the inhibition of hippocampal inflammation in the D-gal-treated mice.
 |
Figure 2: Effects of EELAK on hippocampal levels of inflammatory markers on D-galactose-induced brain aging in mice. |
D-gal Leads to Reduce SOD Activity and Increase MDA Level in Hippocampus, and EELAK Attenuates these Changes
To determine the antioxidant-related protective ability of EELAK on D-gal-induced aging brain, we quantified the activity of antioxidant enzyme in mouse hippocampus samples. ANOVA revealed significant between group effects for SOD activity (F2,29= 18.027, p < 0.001) and MDA levels (F2,29= 169.820, p < 0.001). As indicated in Figure 3A, our result demonstrated that D-gal significantly affected the SOD activity and MDA content in the hippocampus of mice (p < 0.01 and p < 0.001, respectively). However, co-treatment with EELAK significantly reversed the reduced SOD activity (p < 0.05) and elevated MDA levels (p < 0.001) caused by D-gal. These results indicate that EELAK enhances the antioxidant capacity of the hippocampus in D-gal-induced mice, which may be related to its memory-enhancing mechanism.
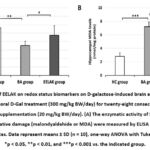 |
Figure 3: Effects of EELAK on redox status biomarkers on D-galactose-induced brain aging in mice. |
Discussion
Aging is a progressive and irreversible natural process that can be delayed. The primary cause of incapacity and dependence among the elderly is dementia and/or cognitive impairment, which is why dementia is designated as a global public health priority.31 It has been recognized that humans are susceptible to brain aging problems, and the number of persons with dementia is expected to double every 20 years.32 Synthetic drugs normally have a lot of toxicities and side effects.33 Angelica keiskei, a member of the Umbelliferae family, demonstrated an extensive range of pharmacological actions and safety, which led to its widespread use as a functional food or medicinal herb.34 Based on the currently available information, Angelica keiskei is believed to be able to alleviate physiological decline associated with aging.35 The most prevalent bioactive ingredients in Angelica keiskei are coumarins, chalcones, and flavonoids.36 In this article, we found for the first time that ethanol extract from the leaves of A. keiskei (EELAK) minimized D-gal-induced oxidative stress, neuro-inflammation, and memory impairment in a mouse model of aging.
In the present study, we induced aging in mice using D-galactose, which has been widely used in various studies for aging modeling in experimental animals and is considered the most effective to mimics physiological aging, particularly to artificially create brain senescence.28 D-gal causes oxidative stress on various tissues, including brain, by increasing the production of ROS and Advance Glycation Endproduct (AGEs) due to galactose oxidase activityand nonenzymatic glycosylation reaction, respectively, which also occur in the natural aging process.37 In addition, several studies indicated that D-gal results in neuroinflammation and significant cognitive deterioration.38,39 The effect of D-gal on cognitive function is mediated by decreased brain-derived neurotrophic factor (BDNF) expression, neuronal apoptosis, and dysfunction of synaptic proteins in the brain.40 The current work effectively established and used an aging rat model driven by D-gal to investigate the memory-enhancing properties and potential mechanism of EELAK. Mice administered with D-gal in the present study for 28 days displayed significant deficits in spatial memory, the activated NF-kB pathway (increased NO and TNF-α), and reduced antioxidant capacity (impaired SOD activity and elevated MDA levels). Additionally, a study showed that D-gal-injected mice exhibit the aging phenotype such as a dull appearance, a slight bow, and sluggish behavior.41
The transcription factor family NF-κB is crucial for controlling the inflammatory response.42 Two components of NF-κB called p50 and p65 are cytoplasmically sequestered alongside their inhibitor protein, IκBα, in a physiological state.43 Once TLR4 and MyD88 are activated by D-gal, they trigger the phosphorylation, ubiquitination, and destruction of IκBα.44 After translocating to the nucleus, free NF-κB dimers increase the expression of genes that code for various proinflammatory proteins or enzymes. These include interleukin-6, interleukin-1β, and TNF-α, as well as COX2 and NO.45 To bolster this, we detected changes in NF-κB, NO, and TNF-α in this study. Next, the biomarkers of oxidative stress, an end product of lipid peroxidation (MDA), and the activity of antioxidative defense enzymes (SOD) were altered by D-gal. MDA is a significant biomarker of membrane lipid peroxidation under oxidative stress and is widely used as a gauge of the severity of aging. Important natural enzymes in the antioxidative system that efficiently lower free radicals, such as SOD, have also been used as markers for oxidative damage in various tissues and to predict the severity of aging-related phenotypes.26,46
Since oxidative stress influences the development of various neurodegenerative disorders, including Alzheimer’s disease, the use of radical scavengers or antioxidant agents is a major strategy for managing the progression of neurodegenerative disorders.47 Antioxidant compounds, whether synthetic or natural, have previously been shown experimentally as effective treatment choices against aging in experimental animal models, including oxidative stress-related brain aging induced by D-gal.39,48 In the present study, we examined the protective role of EELAK on D-gal-induced brain aging in mice. Our findings demonstrated the potent anti-inflammatory, antioxidant, and memory-enhancing activities of EELAK. EELAK effectively reversed the detrimental effects of D-gal on spatial memory and biochemical parameters of the hippocampus. This finding aligned with earlier research, indicating that administering EELAK to mice could shield them from D-gal-induced inflammation and oxidative stress through its anti-inflammatory and antioxidant properties.
The EELAK used in the present study contains a high amount of flavonoid, reaching a concentration of 11523.66 mg QE-Eq/g. Its antioxidant capacity is 28294 mg/L GAEAC (data not shown). A study showed that flavonoids can improve acetylcholine (ACh) activity by inhibiting acetylcholinesterase (AChE), a specific serine hydrolase for ACh responsible for the termination of neuronal transmission and signaling between cholinergic synapses.49 The role of ACh on the memory process is related to the muscarinic acetylcholine receptor (mAChR) called M1. mAChR activities facilitate the process of synaptic plasticity in learning and memory.50 Flavonoids act as potent AChE inhibitors that inhibit the hydrolysis of ACh.49,51 At the molecular level, flavonoid interacts with AChE molecules through hydrophobic interaction, halogen bonding, and aromatic stacking interactions.49 In addition to their AChE inhibitor activity, flavonoids activate signaling pathways required for controlling synaptic plasticity, promote vascularization, stimulate the growth of new nerve cells, and improve the recovery of damaged neurons in the central nervous system.52,53 These biological functions of flavonoids are essential for the neuronal healing process following D-gal treatment.54 AChE inhibitors approved by the FDA for the treatment of AD, including donepezil, rivastigmine, and galantamine, are among the frequently prescribed drugs.55 Two of these three medications are secondary metabolites found in plants, which is why we examined the EELAK in this study. In this study, EELAK showed a significant effect, reducing AChE enzymatic activity by 25%.
In line with our finding, many studies have reported the anti-inflammatory and antioxidant activities of EELAK. A study reported that A. keiskei leaves extract (10 μg/mL) ameliorates the elevated synthesis of nitric oxide (NO) and the elevation of inflammatory cytokines such as TNF-α and IL-1B in lipopolysaccharide-stimulated macrophage cell line.56 Another study found that the production of NO synthase (iNOS) in macrophage-induced toll-like receptors (TLR) is inhibited by the isobavachalcone of A. keiskei.57 Regarding the antioxidant capacity, a study showed that a methanol extract of A. keiskei leaves exhibit an IC50 value of 129.40 ± 7.36 ppm, indicating moderate antioxidant activity.58 In the present study, we found that the ethanol extract had an IC50 value of 80.16 ppm. Additionally, previous study demonstrated an IC50 value of 7.73 ppm.59 Together, the current study and others suggest that ethanol is a better solvent to prepare A. keiskei leaves extract. The major and specific flavonoid in A. keiskei leaf is chalcones that have been documented for their diverse pharmacological actions, such as their antioxidative functions.34 It is believed that the α,β-double bond and free hydroxyl groups present in chalcones contribute to their antioxidant activity.60 Chalcones display bith direct and indirect antioxidant action, reducing the generation of ROS, RNS, and superoxide to prevent oxidative stress.61
Several plant-based compounds have also been reported to attenuate spatial memory deficits. Persimmon (Diospyros kaki) leaves extract with a flavonoid content of 957.75 mg/100 g was proven to inhibit cognitive impairment in D-galactose-induced mice.20 Rosemary extract containing 176.5 mg/100 g flavonoid has been shown to increase spatial memory in middle-aged mice.62 Furthermore, administration of green tea extract with a flavonoid content of 255.96 mg/100 g increases spatial memory in ischemic mice by modulating oxidative and inflammatory stress responses.63When compared with other plants, the flavonoid content of EELAK is relatively higher. Therefore, it is expected that EELAK would be more effective in preventing brain aging.
Natural foods and nutrients are abundant in the world and have shown consistent beneficial effects for human health.64 Because they contain bioactive ingredients, plant extracts are becoming more and more significant additions to medicine these days, and their interventions are still seen as potential solutions to prevent and/or treat several diseases. 65 In this context, based on the present study, we could suggest that EELAK is a potential candidate to be used as a sole or supportive therapy for age-related neurodegenerative diseases in clinical settings.
Conclusion
Our study indicates that Angelica keiskei leaves extract ameliorated D-gal-induced impaired spatial memory in BALB/c mice. Considering that A. keiskei leaves extract reverse the D-Galactose-induced increases in hippocampal AChE activity, inflammation, and oxidative stress, the present finding is the first evidence that A. keiskei enhances the cholinergic system through its anti-inflammatory and antioxidant activities, thereby improving cognitive function. In conclusion, these results suggest A. keiskei as a promising natural product for the prevention of memory disorders and age-related neurodegenerative disorders such as Alzheimer’s disease. Since we artificially accelerated brain aging using oral D-galactose, further studies using naturally old mice are needed to prove the memory-enhancing effects of A. keiskei leaves extract.
Acknowledgement
We thank the Animal Laboratory Unit, Faculty of Medicine, Udayana University, Indonesia for providing facilities to perform animal care and experimental treatment.
Conflict of Interest
The authors declare that there are no competing interests.
Funding Sources
This study was supported by Grant-in-Aid from Atma Jaya Catholic University of Indonesia (Hibah Dosen Pemula tahun 2024 to F.M.S. and Hibah Desentralisasi tahun 2024 to M.D.N.H), and a Postdoctoral Grant from WCU UNDIP Batch IV-2023 (to F.M.S.).
Data Availability Statement
This statement does not apply to this article.
Ethical Approval
The experimental protocols used in this study comply with international guidelines for humane animal treatment and were backed by the Ethics Committee on the Use of Animals of Udayana University, Indonesia.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Author Contributions
Conception and design of study: FMS, IMTW; Acquisition of data: FMS, IMTW, MDNH, RD; Analysis and/or interpretation of data: FMS; Drafting the manuscript: FMS, NN; Revising the manuscript critically for important intellectual content: MDHN, ALE, JLM. All authors have read and approved the version of the manuscript to be published.
References
- Craik FIM. Memory, aging and the brain: Old findings and current issues. Aging Brain. 2023;4:100096. doi:10.1016/j.nbas.2023.100096
CrossRef - Leyane TS, Jere SW, Houreld NN. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int J Mol Sci. 2022;23(13):7273. doi:10.3390/ijms23137273
CrossRef - Singh P, Barman B, Thakur MK. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front Aging Neurosci. 2022;14. doi:10.3389/fnagi.2022.944697
CrossRef - Gao G, You L, Zhang J, Chang YZ, Yu P. Brain Iron Metabolism, Redox Balance and Neurological Diseases. Antioxidants. 2023;12(6):1289. doi:10.3390/antiox12061289
CrossRef - Atrooz F, Salim S. Sleep deprivation, oxidative stress and inflammation. In: ; 2020:309-336. doi:10.1016/bs.apcsb.2019.03.001
CrossRef - Lee KH, Cha M, Lee BH. Neuroprotective Effect of Antioxidants in the Brain. Int J Mol Sci. 2020;21(19):7152. doi:10.3390/ijms21197152
CrossRef - Davidson TL, Stevenson RJ. Vulnerability of the Hippocampus to Insults: Links to Blood–Brain Barrier Dysfunction. Int J Mol Sci. 2024;25(4):1991. doi:10.3390/ijms25041991
CrossRef - Li J, Cao D, Yu S, et al. Functional specialization and interaction in the amygdala-hippocampus circuit during working memory processing. Nat Commun. 2023;14(1):2921. doi:10.1038/s41467-023-38571-w
CrossRef - Hill NL, Bratlee‐Whitaker E, Wion RK, Madrigal C, Bhargava S, Mogle J. Factors that influence the emotional impact of memory problems in older adults: A qualitative descriptive study. Int J Older People Nurs. 2022;17(3). doi:10.1111/opn.12439
CrossRef - Fukui K, You F, Kato Y, et al. A Blended Vitamin Supplement Improves Spatial Cognitive and Short-Term Memory in Aged Mice. Int J Mol Sci. 2024;25(5):2804. doi:10.3390/ijms25052804
CrossRef - Kennedy DO. Phytochemicals for Improving Aspects of Cognitive Function and Psychological State Potentially Relevant to Sports Performance. Sport Med. 2019;49(S1):39-58. doi:10.1007/s40279-018-1007-0
CrossRef - Fanelli Kuczmarski M, Crawford SB, Sebastian RS, et al. Association between Flavonoid Intake and Cognitive Executive Function among African American and White Adults in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) Study. Nutrients. 2024;16(9):1360. doi:10.3390/nu16091360
CrossRef - Cheng N, Bell L, Lamport DJ, Williams CM. Dietary Flavonoids and Human Cognition: A Meta‐Analysis. Mol Nutr Food Res. 2022;66(21). doi:10.1002/mnfr.202100976
CrossRef - Fu K, Gao X, Hua P, et al. Anti-obesity effect of Angelica keiskei Jiaosu prepared by yeast fermentation on high-fat diet-fed mice. Front Nutr. 2023;9. doi:10.3389/fnut.2022.1079784
CrossRef - Ohta M, Fujinami A, Oishi K, Kobayashi N, Ohnishi K, Ohkura N. Ashitaba ( Angelica Keiskei) Exudate Prevents Increases in Plasminogen Activator Inhibitor-1 Induced by Obesity in Tsumura Suzuki Obese Diabetic Mice. J Diet Suppl. 2019;16(3):331-344. doi:10.1080/19390211.2018.1458366
CrossRef - Lee JH, Mei HC, Kuo IC, Lee TH, Chen YH, Lee CK. Characterizing Tyrosinase Modulators from the Roots of Angelica keiskei Using Tyrosinase Inhibition Assay and UPLC-MS/MS as the Combinatorial Novel Approach. Molecules. 2019;24(18):3297. doi:10.3390/molecules24183297
CrossRef - Ohkura N, Taniguchi M, Oishi K, Inoue K, Ohta M. Angelica keiskei (Ashitaba) has potential as an antithrombotic health food. Food Res. 2022;6(2):18-24. doi:10.26656/fr.2017.6(2).121
CrossRef - Rochma E. The Analgesic and Anti-inflammatory Activity of Fraction Ashitaba (Angelica keiskei (Miq.) Koidz.) Leaves On Male White Rat Wistar Strain and Its Safety on The Gastric. J Farm Indones. 2022;19(1):14-29. doi:10.31001/jfi.v19i1.827
CrossRef - Kweon M, Lee H, Park C, Choi YH, Ryu JH. A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy. Nutrients. 2019;11(10):2419. doi:10.3390/nu11102419
CrossRef - Ma Y, Ma B, Shang Y, et al. Flavonoid-rich ethanol extract from the leaves of Diospyros kaki attenuates cognitive deficits, amyloid-beta production, oxidative stress, and neuroinflammation in APP/PS1 transgenic mice. Brain Res. 2018;1678:85-93. doi:10.1016/j.brainres.2017.10.001
CrossRef - Sadigh-Eteghad S, Majdi A, McCann SK, Mahmoudi J, Vafaee MS, Macleod MR. D-galactose-induced brain ageing model: A systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. Ariga H, ed. PLoS One. 2017;12(8):e0184122. doi:10.1371/journal.pone.0184122
CrossRef - Siswanto FM. Angelica keiskei leaves extract attenuates psychosocial stress in overcrowding-subjected rats. Published online 2024:1-23.
CrossRef - Othman MZ, Hassan Z, Che Has AT. Morris water maze: a versatile and pertinent tool for assessing spatial learning and memory. Exp Anim. 2022;71(3):21-0120. doi:10.1538/expanim.21-0120
CrossRef - Lu Z, Yang T, Wang L, et al. Comparison of different protocols of Morris water maze in cognitive impairment with heart failure. Brain Behav. 2020;10(2). doi:10.1002/brb3.1519
CrossRef - Senthilkumar M, Amaresan N, Sankaranarayanan A. Estimation of Superoxide Dismutase (SOD). In: ; 2021:117-118. doi:10.1007/978-1-0716-1080-0_29
CrossRef - Pradhany RC, Siswanto FM, Sukoco H, Suarsana IN, Suartini IGAA. L-carnitine Prevents Hepatic Steatosis in Deep-Frying Oil-Treated Rat. Biomed Pharmacol J. 2022;15(3):1751-1758. doi:10.13005/bpj/2514
CrossRef - Brito DVC, Esteves F, Rajado AT, et al. Assessing cognitive decline in the aging brain: lessons from rodent and human studies. npj Aging. 2023;9(1):23. doi:10.1038/s41514-023-00120-6
CrossRef - Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol. 2018;101:13-36. doi:10.1016/j.exger.2017.10.029
CrossRef - Ma Y, Ma B, Shang Y, et al. Flavonoid-Rich Ethanol Extract from the Leaves of Diospyros kaki Attenuates D-Galactose-Induced Oxidative Stress and Neuroinflammation-Mediated Brain Aging in Mice. Oxid Med Cell Longev. 2018;2018:1-12. doi:10.1155/2018/8938207
CrossRef - Zhang B, Lian W, Zhao J, Wang Z, Liu A, Du G. DL0410 Alleviates Memory Impairment in D-Galactose-Induced Aging Rats by Suppressing Neuroinflammation via the TLR4/MyD88/NF-κB Pathway. Mendoza-Núñez VM, ed. Oxid Med Cell Longev. 2021;2021:1-31. doi:10.1155/2021/6521146
CrossRef - Farrow M, Fair H, Klekociuk SZ, Vickers JC. Educating the masses to address a global public health priority: The Preventing Dementia Massive Open Online Course (MOOC). Lavorgna L, ed. PLoS One. 2022;17(5):e0267205. doi:10.1371/journal.pone.0267205
CrossRef - Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Heal. 2022;7(2):e105-e125. doi:10.1016/S2468-2667(21)00249-8
CrossRef - Elkordy AA, Haj-Ahmad RR, Awaad AS, Zaki RM. An overview on natural product drug formulations from conventional medicines to nanomedicines: Past, present and future. J Drug Deliv Sci Technol. 2021;63:102459. doi:10.1016/j.jddst.2021.102459
CrossRef - Wahyuni I, Aulifa DL, Rosdianto AM, Levita J. The pharmacology activities of Angelica keiskei Koidzumi and its efficacy and safety in humans. Heliyon. 2024;10(2):e24119. doi:10.1016/j.heliyon.2024.e24119
CrossRef - Liu H, Wei G, Wang T, et al. Angelica keiskei water extract Mitigates Age-Associated Physiological Decline in Mice. Redox Rep. 2024;29(1). doi:10.1080/13510002.2024.2305036
CrossRef - Jasim HA, Nahar L, Jasim MA, Moore SA, Ritchie KJ, Sarker SD. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules. 2021;11(8):1203. doi:10.3390/biom11081203
CrossRef - Pantiya P, Thonusin C, Ongnok B, et al. Chronic D-galactose administration induces natural aging characteristics, in rat’s brain and heart. Toxicology. 2023;492:153553. doi:10.1016/j.tox.2023.153553
CrossRef - Ahmad S, Khan A, Ali W, et al. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.612078
CrossRef - Ma Y, Wang X, Li X, et al. COP-22 Alleviates d-Galactose–Induced Brain Aging by Attenuating Oxidative Stress, Inflammation, and Apoptosis in Mice. Mol Neurobiol. Published online February 12, 2024. doi:10.1007/s12035-024-03976-1
CrossRef - Lalert L, Maneesri le-Grand S, Techarang T, Huntula S, Punsawad C. Neuroprotective effect of low-dose paracetamol treatment against cognitive dysfunction in d-galactose-induced aging mice. Heliyon. 2022;8(10):e11108. doi:10.1016/j.heliyon.2022.e11108
CrossRef - Li H, Zheng L, Chen C, Liu X, Zhang W. Brain Senescence Caused by Elevated Levels of Reactive Metabolite Methylglyoxal on D-Galactose-Induced Aging Mice. Front Neurosci. 2019;13. doi:10.3389/fnins.2019.01004
CrossRef - Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. doi:10.1038/sigtrans.2017.23
CrossRef - Siswanto FM, Okukawa K, Tamura A, Oguro A, Imaoka S. Hydrogen peroxide activates APE1/Ref-1 via NF-κB and Parkin: a role in liver cancer resistance to oxidative stress. Free Radic Res. 2023;57(3):223-238. doi:10.1080/10715762.2023.2229509
CrossRef - Wang H, Wei X, Wei X, et al. 4-hydroxybenzo[d]oxazol-2(3H)-one ameliorates LPS/D-GalN-induced acute liver injury by inhibiting TLR4/NF-κB and MAPK signaling pathways in mice. Int Immunopharmacol. 2020;83:106445. doi:10.1016/j.intimp.2020.106445
CrossRef - Siswanto FM, Tamura A, Sakuma R, Imaoka S. Yeast β -glucan Increases Etoposide Sensitivity in Lung Cancer Cell Line A549 by Suppressing Nuclear Factor Erythroid 2-Related Factor 2 via the Noncanonical Nuclear Factor Kappa B Pathway. Mol Pharmacol. 2022;101(4):257-273. doi:10.1124/molpharm.121.000475
CrossRef - Maldonado E, Morales-Pison S, Urbina F, Solari A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants. 2023;12(3):651. doi:10.3390/antiox12030651
CrossRef - Kamaljeet, Singh S, Gupta GD, Aran KR. Emerging role of antioxidants in Alzheimer’s disease: Insight into physiological, pathological mechanisms and management. Pharm Sci Adv. 2024;2:100021. doi:10.1016/j.pscia.2023.100021
CrossRef - Mumtaz S, Ali S, Qureshi MZ, Muhammad A, Manan A, Akbar Mughal T. Antioxidant and anti-aging role of silk sericin in D-galactose induced mice model. Saudi J Biol Sci. 2023;30(12):103872. doi:10.1016/j.sjbs.2023.103872
CrossRef - Al-Joufi FA, Shah SWA, Shoaib M, et al. Flavonoid Derivatives as Potential Cholinesterase Inhibitors in Scopolamine-Induced Amnesic Mice: An In Vitro, In Vivo and Integrated Computational Approach. Brain Sci. 2022;12(6):731. doi:10.3390/brainsci12060731
CrossRef - Jardine KH, Wideman CE, MacGregor C, et al. Activation of cortical M1 muscarinic receptors and related intracellular signaling is necessary for reactivation-induced object memory updating. Sci Rep. 2020;10(1):9209. doi:10.1038/s41598-020-65836-x
CrossRef - Lima E, Rauter A, Medeiros J. Flavonoids as Promising Multitarget Agents in Alzheimer’s Disease Therapy. Appl Sci. 2023;13(8):4651. doi:10.3390/app13084651
CrossRef - Melrose J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants. 2023;12(3):663. doi:10.3390/antiox12030663
CrossRef - Cichon N, Saluk-Bijak J, Gorniak L, Przyslo L, Bijak M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants. 2020;9(11):1035. doi:10.3390/antiox9111035
CrossRef - Liang Z, Liang H, Guo Y, Yang D. Cyanidin 3-O-galactoside: A Natural Compound with Multiple Health Benefits. Int J Mol Sci. 2021;22(5):2261. doi:10.3390/ijms22052261
CrossRef - Sridhar GR. Acetylcholinesterase inhibitors (Galantamine, Rivastigmine, and Donepezil). In: NeuroPsychopharmacotherapy. Springer International Publishing; 2021:1-13. doi:10.1007/978-3-319-56015-1_418-1
CrossRef - Chang HR, Lee HJ, Ryu JH. Chalcones from Angelica keiskei Attenuate the Inflammatory Responses by Suppressing Nuclear Translocation of NF- κ B. J Med Food. 2014;17(12):1306-1313. doi:10.1089/jmf.2013.3037
CrossRef - Shin HJ, Shon DH, Youn HS. Isobavachalcone suppresses expression of inducible nitric oxide synthase induced by Toll-like receptor agonists. Int Immunopharmacol. 2013;15(1):38-41. doi:10.1016/ j.intimp.2012.11. 005
CrossRef - Muliasari H, Hanifa NI, Hajrin W, Andanalusia M, Hidayati AR. Determination of Antioxidants by DPPH Scavenging Activity of Ashitaba Herb (Angelica keiskei) Methanol Extract. J Biol Trop. 2023;23(4):482-490. doi:10.29303/jbt.v23i4.5686
CrossRef - Aulifa DL, Noefitri RY, Tristiyanti D, Budiman A. Formulation of serum gel containing angelica keiskei leaf extract as an antioxidant and tyrosinase enzyme inhibitor. Int J Appl Pharm. 2020;12(3):108-111. doi:10.22159/ijap.2020v12i3.37303
CrossRef - Stepanić V, Matijašić M, Horvat T, et al. Antioxidant Activities of Alkyl Substituted Pyrazine Derivatives of Chalcones—In Vitro and In Silico Study. Antioxidants. 2019;8(4):90. doi:10.3390/antiox8040090
CrossRef - Takac P, Kello M, Vilkova M, et al. Antiproliferative Effect of Acridine Chalcone Is Mediated by Induction of Oxidative Stress. Biomolecules. 2020;10(2):345. doi:10.3390/biom10020345
CrossRef - Rasoolijazi H, Mehdizadeh M, Soleimani M, Nikbakhte F, Eslami Farsani M, Ababzadeh S. The effect of rosemary extract on spatial memory, learning and antioxidant enzymes activities in the hippocampus of middle-aged rats. Med J Islam Repub Iran. 2015;29:187. http://www.ncbi.nlm.nih.gov/pubmed/26034740
- Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evidence-Based Complement Altern Med. 2012;2012:1-11. doi:10.1155/2012/163106
CrossRef - Proestos C. The Benefits of Plant Extracts for Human Health. Foods. 2020;9(11):1653. doi:10.3390/foods9111653
CrossRef - Ahmad Khan MS, Ahmad I. Herbal Medicine. In: New Look to Phytomedicine. Elsevier; 2019:3-13. doi:10.1016/B978-0-12-814619-4.00001-X
CrossRef








