Maytham Razaq Shleghm* , Ahmed F. Abed Mansoor
, Ahmed F. Abed Mansoor and Tahseen Ch Naeemah
and Tahseen Ch Naeemah
College of Pharmacy, National University of Science and Technology, Dhi Qar, Iraq
Corresponding Author E-mail: maytham.r.abdlhasan@nust.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/3008
Abstract
Background: This study aimed to evaluate the antibacterial effects of Punica granatum extract on such pathogenic bacteria as Staphylococcus aureus and Escherichia coli. Materials and methods: The samples from 130 patients with skin infections in Baghdad, Iraq, aged between 15 and 60 over years were collected for this study. The study collected. Each isolate was positively identified using morphological, cultural, and biochemical assays as detailed in the reference. The P. granatum peels were air-dried and powdered. Then 25g were extracted using 500 mL of water and ethanol on Soxhlet equipment for 72 hours. The extracts were then cooled, filtered, and concentrated at 40oC to get the crude extract; it was kept at four degrees centigrade in dark vials until use. The extracts were tested for the presence of alkaloids, tannins, flavonoids, glycosides, as well as steroidal terpenes. The efficacy of antimicrobial effects was calculated using well-diffusion techniques on Muller Hinton Agar (MHA). The plates were injected with a standardized suspension of the test isolates against McFarland tube 0.5. Five wells, each measuring five millimeters in diameter, were evenly spaced out using a sterile standard core borer. The well bottoms were sealed with sterile molten nutritional agar to prevent the extract from leaking out from beneath the agar. The aqueous and ethanolic crude extracts dissolved in DMSO served as positive controls, while sterile water and 10% DMSO served as negative controls. Each extract was diluted to a final concentration of 50, 100, or 200mg/ml, and 25 ml was added to the appropriate well on the infected plate. The plates were then incubated for 24 hours at 37 degrees Celsius. A millimeter-calibrated ruler was used to measure the size of the resultant inhibitory zones. The zone of inhibition of the test microorganisms at that dose was calculated as the mean of three measurements. Results: Clinical isolates of E. coli and S. aureus were inhibited by pomegranate extracts at a concentration of 200mg/ml compared to other concentrations, and this extract concentration showed a non-significant difference with chloramphenicol (P<0.01). The study revealed that pomegranate peel extract significantly reduced E. coli levels in feces and increased survival rates in rats. On the first day, E. coli concentrations were much higher in the control group (G2) compared to the treatment group (G3). By day 6, all rats in the control group had died, while all rats in the treatment group survived. Pomegranate peel extract shows notable antibacterial properties, impacting bacterial membrane permeability and cell survival. The variation in extract composition affects its efficacy. Conclusion, Pomegranate peel extract significantly reduced E. coli levels and improved survival rates in rats. On day 6, all rats in the control group died, while all in the treatment group survived. The extract's antibacterial effects and impact on bacterial membranes highlight its potential as a therapeutic agent.
Keywords
Antibacterial; E. coli; Plant extract; Punica granatum; S. aureus
Download this article as:| Copy the following to cite this article: Shleghm M. R, Mansoor A. F. A, Naeemah T. C. Evaluation of the Antibacterial Effects of the Punica granatum Peels Extracts Against some Pathogenic Bacteria: An In vivo and In vitro Study. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Shleghm M. R, Mansoor A. F. A, Naeemah T. C. Evaluation of the Antibacterial Effects of the Punica granatum Peels Extracts Against some Pathogenic Bacteria: An In vivo and In vitro Study. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3ZxF74z |
Introduction
Traditional herbal treatments have been used for centuries. Many people in third-world nations rely heavily on herbal treatments as their first line of defense against illness.1,2 As bacteria develop resistance to chemical manufactured pharmaceuticals, scientists are looking for safer alternatives, such as herbal medicines and the active components they contain.3,6
Pomegranate, or Punica granatum Linn, is a deciduous shrub or small Asian tree (growing to a height of 5–8 meters) of the family Punicaceae. It has been farmed for centuries throughout the Asia-Mediterranean area8, Europe, and Africa, although its natural habitat is Iran and northern India. Cancer, heart disease, diabetes mellitus, dental disease, bacterial infection, antibiotic resistance, sun-damaged skin, diarrhoea, bloody diarrhoea, and haemorrhoids are all treated using the bioactive chemicals found in Punica granatum. Some forms of sore throat may also be alleviated by using P. granatum as a mouth rinse. Flowers, trunk skin, fruits, roots, seeds, and even the plant itself have therapeutic use.7,9
Flowers of the Punica granatum are red or reddish in colour, with a diameter of 3.5 to 7 cm, and may be campanulate or cylindrical in shape. You may find both fertilised and unfertilized flowers. The unfertilized flower has tiny, sterile petals that are short in style and short in stamens, and the stigma is located low on the flower, below the anthers. In Iranian alternative medicine, the unfertilized blooms are called “Golnar”.10
Pomegranate tea may be used to heal sore throats and oral irritation, while the skin and roots of the trunk are effective against parasite infections and diarrhoea, including bloody diarrhoea. In ancient Greek medicine, pomegranate was used as a hemostatic agent and to treat diabetic mellitus.11,12
P. granatum’s antibacterial activities have only lately been recognized.13,15 P. granatum has potent antibacterial activities against Gram-positive and Gram-negative nonoral microorganisms when extracted in ethanol, water, methanol, and acetone.16,17 However, only a few research14,17 have examined the effectiveness of this plant’s antibacterial activities against oral bacteria.
Nevertheless, the aforementioned research is quite small and mostly lacks a suitable microbiological technique. Frequently, their procedures lack clarity or provide insufficient explanation. One research on the effect of P. granatum flower (petal) water extract on oral microbial pathogens was found after searching the literature, and it mostly dealt with S. sanguinis.(18) On the other hand, Menezes et al.19 found that P. granatum hydroalcoholic extract was very efficient against biofilm-forming microbes in patients’ mouths.
This study conducted for evaluation of antibacterial effects of Punica granatum extract against some pathogenic bacteria.
Materials and Methods
Staphylococcus aureus and Escherichia coli samples were collected from 130 patients with skin infections in Baghdad, Iraq, between the ages of 15 and 60 over the course of a year. Each and every isolate was positively identified by means of the morphological, cultural, and biochemical assays detailed in reference.14
After air drying and powdering the P. granatum peels, 25g were extracted using 500mL of water and ethanol on Soxhlet equipment for 72 hours. The contents of the flasks were cooled, then filtered, concentrated at 40 degrees Celsius to get the crude extract, and then kept at four degrees centigrade in dark vials until use.16
Following the procedures outlined in (17) and (18), we tested for the presence of flavonoids, alkaloids, glycosides, tannins, as well as steroidal terpenes.
The efficacy was calculated using well diffusion techniques using Muller Hinton Agar (MHA). The plates were injected with a suspension of the test isolates that had been standardised against McFarland tube 0.5. After drying the plates for 30 minutes at 37oC in the incubator, a sterile standard core borer was used to evenly space out five wells, each measuring five millimetres in diameter. To stop the extract from leaking out from beneath the agar, the well bottoms were sealed with sterile molten nutritional agar. The aqueous and ethanolic crude extracts dissolved in DMSO served as positive controls, while sterile water and 10% DMSO served as negative controls. Each extract was diluted to a final concentration of 50, 100, or 200mg/ml, and 25 ml was added to the appropriate well on the infected plate.
The plates were then incubated for 24 hours at 37 degrees Celsius. A millimeter-calibrated ruler was used to assess the size of the resultant inhibitory zones. The zone of inhibition of the test microorganisms at that dose was calculated as the mean of three measuremens. 19,20
For in vivo study, experiment made use of thirty male rats ranging in age from 6 to 8 weeks, weighing 180-220gm. All animals were provided with water and commercial solid food ad libitum. These were divided into 3 groups (each group contain 10 animals):
G1 control negative
G2 control positive for E. coli 1×108 CFU/ml
G3 oral infection by E. coli 1×108 CFU/ml then treated by orally administered 5 mg (using gavage needle) of extract (21).
Numbers of bacteria per gramme of faeces were assessed by collecting faecal samples 0, 1, 2, 3, 4, 5, and 6 days after the bacterial suspensions were provided. Duplicate MacConkey agar plates (Difco) were incubated overnight at 37°C after receiving 100 μl aliquots of faecal suspensions that had been serially diluted in PBS. using the counting of typical colonies on plates containing 30–300 colonies.
Statistical analysis was done by using SPSS version 23.
Results and Discussion
Clinical isolates of E. coli and S. aureus were inhibited by pomegranate extracts at concentration of 200mg/ml as compared with other concentration and this extract concentration was showed non-significant difference with chloramphenicol (P<0.01), as shown in Table 1.
Table 1: Comparison between different concentrations of extract with chloramphenicol
|
Bacteria |
50 mg/ml |
100 mg/ml |
200 mg/ml |
Chloramphenicol 0.03 mg |
|
S. aureus |
3.4±0.4 |
4.7±0.9 |
12.5±1.2 |
14.6±2.3 |
|
E. coli |
4.2±0.8 |
5.3±0.1 |
13.7±2.4 |
15.2±1.7 |
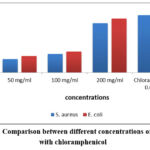 |
Figure 1: Comparison between different concentrations of extract with chloramphenicol
|
Table 2 shows that there were significant effects of extract treatment on mortality and the quantity of live E. coli bacteria retrieved from faeces. On the first day after infection, ten rats from the G2 and G3 groups were observed to excrete viable E. coli bacteria in their faeces. The G2 group’s faeces contained bacteria at a concentration of 103-6 CFU g−1, while the G3 group’s faeces contained bacteria at a concentration of 102-3 CFU g−1. Also, at 6 days after injection, no mice in group G3 had perished, whereas all five rats in group G2 had died.
Table 2: Effects of treatment with pomegranate extract on fecal shedding of E. coli (CFU g−1) by rats.
|
|
Day 0 |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
|
G2-1 |
0 |
3 × 103 |
2 × 103 |
1 × 104 |
2 × 104 |
1 × 104 |
Death |
|
G2-2 |
0 |
2 × 104 |
5 × 104 |
7 × 105 |
Death |
Death |
Death |
|
G2-3 |
0 |
3 × 104 |
1 × 105 |
2 × 105 |
Death |
Death |
Death |
|
G2-4 |
0 |
7 × 104 |
3 × 104 |
2 × 104 |
1 × 106 |
Death |
Death |
|
G2-5 |
0 |
4 × 105 |
2 × 105 |
1 × 105 |
3 × 106 |
Death |
Death |
|
G3-1 |
0 |
1 × 103 |
1 × 103 |
1 × 103 |
6 × 103 |
2 × 103 |
0 |
|
G3-2 |
0 |
2 × 103 |
1 × 103 |
2 × 103 |
3 × 103 |
2 × 103 |
2 × 103 |
|
G3-3 |
0 |
2 × 102 |
2 × 103 |
8 × 103 |
3 × 103 |
3 × 102 |
2 × 102 |
|
G3-4 |
0 |
1 × 103 |
3 × 102 |
2 × 102 |
1 × 102 |
3 × 102 |
2 × 102 |
|
G3-5 |
0 |
4 × 102 |
8 × 102 |
1 × 103 |
2 × 102 |
3 × 102 |
1 × 102 |
Pomegranate peel extract was shown to have the highest antibacterial activity 21 among the alcoholic extracts of pomegranate seed, fruit, peel, and juice tested against several bacterium strains, including S. aureus. Methanolic pomegranate peel extract, as revealed by 18, is more effective against gram-positive bacteria than against gram-negative bacteria. Because of this, methanolic extracts of both sour and sweet pomegranate peels are very effective against S. aureus 18. Due to its effective antibacterial activity against S. aureus 22, pomegranate peel extract was recommended by 22 for use in popular chicken meat items in order to extend their shelf life.
The content of plant extracts varies, which explains why bacteria’s reactions to them vary. To test the antibacterial effects of pomegranate peels against P. aeruginosa and S. aureus (23), (16) extracted them at room temperature using several polar solvents.
The minimum inhibitory concentrations (MICs) for active pomegranate extracts against the investigated bacteria, including S. aureus and P. aeruginosa, were reported by Duman et al. to vary from 40 to >90 g/ mL (24). Pomegranate extracts suppress or postpone S. aureus development at doses ranging from 0.01 to 1% v/v 25.
Antimicrobial properties of pomegranate water, ethanolic, and butanolic extracts against Escherichia coli, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus have been described in (26,27). Antimicrobial activity (inhibition zone) against S. aureus and P. aeruginosa was shown to be significantly greater in pomegranate peel fractions than in the control group28.
Recently observed that pomegranate fruit extracts exhibit potent antibacterial activity in vitro against a wide range of bacteria, including E. coli, S. aureus, Enterobacter spp., Bacillus spp., and Micrococcus spp. According to research by 29, pomegranate extracts in chloroform, ethanol, and water were very effective against E. coli O157:H7.
Variation in extract composition is a significant contributor to MIC variation 30. Location, harvest time, plant age, growth stage, drying procedure, and extraction method all have a role in determining the extract’s chemical make-up 31,32,33,34.
Many authors revealed an antibacterial effect of pomegranate on E. coli in vivo, the effects of P. granatum peel extract was the primary focus of this investigation. These actions alter plasma membrane permeability, increase cell protein concentrations, and ultimately cause cell death 35. While research on P. granatum derivatives’ antibacterial activity has been well-documented 36,37 , the impact on in vivo circumstances is less well understood.
 |
Figure 2: Gross appearance of S. aureus
|
 |
Figure 3: Gross appearance of E. coli
|
Conclusion
In conclusion, pomegranate peel extracts exhibit significant antibacterial activity against E. coli and S. aureus, particularly at a concentration of 200 mg/mL, where they perform comparably to chloramphenicol. The in vivo study further demonstrated that rats receiving the extract showed reduced bacterial load in feces and better survival rates compared to the untreated control group. These findings underscore the potential of pomegranate peel extracts as an effective antibacterial agent and suggest their utility in controlling bacterial infections and enhancing survival in clinical or experimental settings.
Acknowledgement
We acknowledge the assistance we received from the members of the National University of Science and Technology ( NUST), College of Pharmacy
Conflict of Interest
The authors do not have any conflict of interest
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
References
- Arun N, Singh DP. Punica granatum: a review on pharmacological and therapeutic properties. Int J Pharm Sci Res. 2012 1;3(5):1240.
- Debnath A, Das A. Isolation of bioactive compounds from low-cost agricultural resources and its utilization in daily life. Access Microbiology. 2024 1;6(6):000660-v4.
- Abdallah EM, Alhatlani BY, de Paula Menezes R, Martins CH. Back to Nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants. 2023 28;12(17):3077.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology. 1966 1;45(4_ts):493-6.
- Anesini C, Perez C. Screening of plants used in Argentine folk medicine for antimicrobial activity. Journal of ethnopharmacology. 1993 1;39(2):119-28.
- Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. Chapman and Hall; 1998.
- Ashraf MV, Pant S, Khan MH, Shah AA, Siddiqui S, Jeridi M, Alhamdi HW, Ahmad S. Phytochemicals as antimicrobials: prospecting Himalayan medicinal plants as source of alternate medicine to combat antimicrobial resistance. Pharmaceuticals. 2023 15;16(6):881.
- Mansoor AF, Raghif AR, Al-Sudani IM, Aldabagh MA. Therapeutic Effects of Rivastigmine in induced Cytokine Storm in Mice: Dose Standardization. Journal of Carcinogenesis. 2022 1;21(2).
- Junaid SA, Olabode AO, Onwuliri FC, Okwori AE, Agina SE. The antimicrobial properties of Ocimum gratissimumextracts on some selected bacterial gastrointestinal isolates. African journal of Biotechnology. 2006;5(22).
- Counts JM, Astles JR, Tenover FC, Hindler J. Systems approach to improving antimicrobial susceptibility testing in clinical laboratories in the United States. Journal of clinical microbiology. 2007;45(7):2230-4.
- Scaglione E, Sateriale D, Mantova G, Di Rosario M, Continisio L, Vitiello M, Pagliarulo C, Colicchio R, Pagliuca C, Salvatore P. Antimicrobial efficacy of Punica granatum Lythraceae peel extract against pathogens belonging to the ESKAPE group. Frontiers in Microbiology. 2024 22;15:1383027.
- Ge S, Duo L, Wang J, Yang J, Li Z, Tu Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. Journal of ethnopharmacology. 2021 10;271:113877.
- Khan MK, Malik A. Antibiotic resistance and detection of β-lactamase in bacterial strains of Staphylococci and Escherichia coli isolated from foodstuffs. World Journal of Microbiology and Biotechnology. 2001;17:863-8.
- Samreen, Ahmad I, Siddiqui SA, Naseer A, Nazir A. Efflux Pump Inhibition-Based Screening and Anti-Infective Evaluation of Punica granatum Against Bacterial Pathogens. Current Microbiology. 2024;81(1):51.
- Abed Mansoor AF, Abu Raghif AR. Attenuated effects of rivastigmine in induced cytokine storm in mice. Journal of Emergency Medicine, Trauma & Acute Care. 2022;2022(3):12.
- Erdogrul ÖT. Antibacterial activities of some plant extracts used in folk medicine. Pharmaceutical Biology. 2002 1;40(4):269-73.
- Nuamsetti T, Dechayuenyong P, Tantipaibulvut S. Antibacterial activity of pomegranate fruit peels and arils. Science Asia. 2012 1;38(3):319-22.
- Cowan MM. Plant products as antimicrobial agents. Clinical microbiology reviews. 1999 1;12(4):564-82.
- Voravuthikunchai SP, Sririrak T, Limsuwan S, Supawita T, Iida T, Honda T. Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohemorrhagic Escherichia coli O157: H7. Journal of health science. 2005;51(5):590-6.
- Mathabe MC, Nikolova RV, Lall N, Nyazema NZ. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. Journal of ethnopharmacology. 2006 21;105(1-2):286-93.
- Choi JG, Kang OH, Lee YS, Chae HS, Oh YC, Brice OO, Kim MS, Sohn DH, Kim HS, Park H, Shin DW. In vitro and in vivo antibacterial activity of Punica granatum peel ethanol extract against Salmonella. Evidence‐Based Complementary and Alternative Medicine. 2011;2011(1):690518.
- Kanatt SR, Chander R, Sharma A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. International journal of food science & technology. 2010;45(2):216-22.
- Negi PS, Jayaprakasha GK, Jena BS. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food chemistry. 2003 1;80(3):393-7.
- Duman AD, Ozgen M, Dayisoylu KS, Erbil N, Durgac C. Antimicrobial activity of six pomegranate (Punica granatum L.) varieties and their relation to some of their pomological and phytonutrient characteristics. Molecules. 2009 13;14(5):1808-17.
- Braga LC, Shupp JW, Cummings C, Jett M, Takahashi JA, Carmo LS, Chartone-Souza E, Nascimento AM. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. Journal of ethnopharmacology. 2005 4;96(1-2):335-9.
- Al-Zoreky NS. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International journal of food microbiology. 2009 15;134(3):244-8.
- Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of ethnopharmacology. 2001 1;74(2):113-23.
- Opara LU, Al-Ani MR, Al-Shuaibi YS. Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food and Bioprocess Technology. 2009;2:315-21.
- Piyawan VS, Treechada S, Surasak L, Thanomjit S, Tetsuya I, Takeshi H. Inhibitory Effects of Active Compounds from Punica granatum Pericarp on Verocytotoxin Production by Enterohemorrhagic Escherichia coli O157: H7. JOURNAL OF HEALTH SCIENCE. 2005;51(5):590-6.
- Fateh MV, Ahmed S, Ali M, Bandyopadhyay S. A review on the medicinal importance of pomegranate. J Pharm sci. 2013;3(4):23-5.
- Prashanth D, Asha MK, Amit A. Antibacterial activity of Punica granatum. Fitoterapia. 2001 1;72(2):171-3.
- Fonmboh DJ, Abah ER, Fokunang TE, Herve B, Teke GN, Rose NM, Borgia NN, Fokunang LB, Andrew BN, Kaba N, Bathelemy N. An overview of methods of extraction, isolation and characterization of natural medicinal plant products in improved traditional medicine research. Asian J Res Med Pharm Sci. 2020;9(2):31-57.
- Bhandari PR. Pomegranate (Punica granatum L). Ancient seeds for modern cure? Review of potential therapeutic applications. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2012 1;2(3):171-84.
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991 1;30(12):3875-83.
- Kantachumpoo A, Chirapart A. Components and antimicrobial activity of polysaccharides extracted from Thai brown seaweeds. Kasetsart Journal (Natural Science). 2010 30;44(2):220-33.
- Dahham SS, Ali MN, Tabassum H, Khan M. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.). Am. Eurasian J. Agric. Environ. Sci. 2010;9(3):273-81.
- Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. Journal of ethnopharmacology. 2012 28;143(2):397-405.








