Manuscript accepted on :21-05-2024
Published online on: 17-07-2024
Plagiarism Check: Yes
Reviewed by: Dr. Alaa Saadi Abbood and Dr. Ayan Chatterjee
Second Review by: Dr. Dito Anurogo
Final Approval by: Dr. Gul Ozcan
Agnis Pondineka Ria Aditama1 , Siti Asiyah2*
, Siti Asiyah2* , Tintin Hariyani2, Wahyu Nuraisya2
, Tintin Hariyani2, Wahyu Nuraisya2 , Linda Andri Mustofa2
, Linda Andri Mustofa2 and Anis Setyowati2
and Anis Setyowati2
1Health Polytechnic of Jember, Jember, Indonesia.
2Department of Midwifery, STIKES Karya Husada Kediri, Kediri, Indonesia.
Corresponding Author E-mail:aninkamila@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2985
Abstract
Osteoporosis is a disease characterized by bone loss, which can be treated by increasing the bone formation process. A marker of increased bone formation processes is the activity of the transcription factor Osx which stimulates the formation of Ocn. M. crenata leaves contains phytoestrogens which are thought to increase bone formation. The purpose of this research was to prove that the n-butanol fraction from the leaves of M. crenata can increase bone formation in hFOB 1.19 osteoblast cells by increasing the activity of the transcription factors Osx and Ocn. hFOB 1.19 cells that had reached confluence were given n-butanol fraction at a dose of 62.5, 125, and 250 µg/L, and Genistein 2.5 µg/ml as a positive control for phytoestrogens. Increased bone formation was identified through the expression of Osx and Ocn using the immunocytochemical method with CLSM. The findings indicate that the application of various dosages of the n-butanol fraction derived from the leaves of M. crenata has a positive impact on bone formation in hFOB 1.19 cells. The best dose was determined to be 250 µg/L, with a statistically significant difference of p<0.005. The n-butanol fraction obtained from M. crenata leaves was found to increase the expression of Osx and Ocn in hFOB 1.19 cells, indicating its potential to enhance bone production.
Keywords
hFOB 1.19; Marsilea crenata C Presl.; n-butanol fraction; Osteri; Osteocalcin
Download this article as:| Copy the following to cite this article: Aditama A. P. R, Asiyah S, Hariyani T, Nuraisya W, Mustofa L. A, Setyowati A. Enhancement of Osteoblastogenesis in the hFOB 1.19 Cell Line by the Induction of the n-Butanol Fraction Derived from Marsilea crenata C Presl. Leaves. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Aditama A. P. R, Asiyah S, Hariyani T, Nuraisya W, Mustofa L. A, Setyowati A. Enhancement of Osteoblastogenesis in the hFOB 1.19 Cell Line by the Induction of the n-Butanol Fraction Derived from Marsilea crenata C Presl. Leaves. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3LouPf0 |
Introduction
Osteoporosis is a bone disease in humans characterized by reduced bone strength and density, leading to a higher risk of fractures. Bone density often decreases with age, showing variations based on the gender and race of individuals. Key irreversible factors contributing to osteoporosis development are age, menopause, and female gender1. Estrogen deficiency in women over 40 leads to reduced bone mass, raising the likelihood of osteoporosis2,3.
The process of bone remodeling is characterized by its dynamic nature, wherein osteoclasts engage in the resorption of aged bone tissue and osteoblasts are responsible for the generation of new bone tissue. It is responsible for healing injured bones4,5. The formation stage involves the proliferation and differentiation of osteoblast precursors, followed by the mineralization of the new bone matrix4.
The estrogen-receptor (E-ER) complex in the nucleus or cell membran can be influenced by estrogen to affect the proliferation, differentiation, and maturation of osteoblast in different species6,7. One transcription factor that is important for osteoblast development is called Osterix (Osx). By upregulating the expression of proteins such as osteocalcin (Ocn), osteopontin (OPN), bone sialoprotein (BSP), and collagen type I (COL-I), it promotes the production of new bone8,9. As an adult, Ocn is a major protein in bones. It helps bones mineralize by interacting with γ-carboxyglutamic acid (Gla) residues and increasing the absorption of hydroxyapatite10.
The use of estrogen in women with estrogen deficiency, such as long-term hormone replacement therapy (HRT), carries a carcinogenic risk to female reproductive organs and other side effects11. Therefore, research on estrogen alternatives prefers natural ingredients, especially phytoestrogen compounds, which provide bone protective effects by inhibiting bone resorption and increasing bone formation due to menopause12,13. Plant chemicals called phytoestrogens can act like estrogen by making osteoblast cells work harder by attaching to the estrogen receptor (ER) with low side effects14.
The aquatic plant Marsilea crenata C Presl. which is commonly consumed as a staple meal in Surabaya, East Java, Indonesia, has been identified as a source of phytoestrogens 15,16,17. Numerous investigations conducted on M. crenata have revealed the presence of phytoestrogens in various forms, including triterpenoids, steroids, and isoflavones, within the ethanol extract, n-hexane, ethyl acetate, and the n-butanol fraction of M. crenata leaves. Additionally, it has the potential to stimulate the activation of ER-β and enhance the elevation of alkaline phosphatase (ALP) levels in the MC3T3-E1 preosteoblast cell line, hence promoting cellular proliferation and differentiation 18,19. In vivo using male and female mice shows that M. crenata induces the proliferation of trabecular bone osteoblast cells 20,21.
The purpose of this research is to prove that the n-butanol fraction (BF) of M. crenata leaves can enchance bone formation in human fetal osteoblast (hFOB 1.19) by measuring the Osx and Ocn expressions using a confocal laser scanning microscop (CLSM) instrument. These cells are used to study how human osteoblasts differentiate, how osteoblasts work, and how cytokines affect osteoblast function22,23.
Material and methods
Materials
UPT Materia Medika, Batu, East Java, identified M. crenata leaves that were gathered from the Benowo region of Surabaya, East Java, Indonesia (code: 1a17b-18a-1)24. The hFOB 1.19 cell line (CRL-11372) was obtained from American Type Cell Culture (Virginia, USA). Quercetin, dimethyl sulfoxide (DMSO), paraformaldehyde (PFA), bovine serum albumin (BSA), and phosphate-buffered saline (PBS) were supplied by Sigma-Aldrich (Missouri, USA). Primary antibodies against mice osteocalcin and anti-rabbit osterix were acquired from Abcam (Cambridge, Britain). The Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin, fetal bovine serum (FBS), and G418 were provided by Aretha Laboratory (Bandung, Indonesia). Anti-mouse secondary antibody Rhodamine, anti-rabbit secondary antibody fluorescein isothiocyanate (FITC), paraformaldehyde and Tween-80 were provided by the Central Laboratory of Life Science at Brawijaya University (Malang, Indonesia).
Method
Extraction, Fractionation, and Preparation of BF
A technique of extraction was performed using 1.5 kg of M. crenata leaf powder and a solvent consisting of 96% ethanol, with the assistance of ultrasonics wave. This procedure yielded a total of 70 g of extract17. Liquid-liquid extraction was done in a 1:1 ratio, the extract was combined with 600 mL of water and subsequently separated using n-Hexane. Ethyl acetate and n-butanol were employed to separate the aqueous phase. The isolation and drying of the n-butanol phase were conducted using a Heidolph Hei-VAP ML/GG3 rotary evaporator.
A stock solution with a concentration of 5000 ppm was prepared by combining a 50 mg BF with 0.5% Tween-80 in 0.5% DMSO (w/v). The solution is formulated with concentrations of 62.5, 125, and 250 µg/L.
Cell Culture
The hFOB 1.19 cells were cultured in a culture flask with a complete media including DMEM, FBS 10%, G418, and penicillin-streptomycin 1%. The flask was then placed in an incubator with a CO2 5% and maintained at a temperature of 37°C for a period of 6 days. Cell proliferation was monitored at 24-hour intervals, and the media was routinely replenished until it reached a concentration of 80–90%. This was followed by the transfer of the cells to a microplate.
Osx Measurement
After reaching confluence, hFOB 1.19 cells were treated for 48 hours with BF at doses of 62.5, 125, and 250 µg/L and genistein 2.5 µg/ml as a positive control. After that, cells were fixed with 4% PFA and cleaned with PBS. Subsequently, Osx primary antibodies, BSA, and Triton X-100 were added, and the mixture was incubated for a full day at 4°C. Subsequently, Osx expression was examined by immunocytochemistry using an Olympus Fluoview Ver.4.2a CLSM at 488 nm, and anti-rabbit Osx (Secondary Antibody-FITC) was added.
Ocn Measurement
The hFOB 1.19 cells that had reached confluence were given BF at a dose of 62.5, 125, and 250 µg/L, and Genistein 2.5 µg/ml as a positive control, then incubated for 48 hours. Next, cells were washed with PBS and fixed with 4% PFA. After that, Triton Followed by the addition of anti-mouse Ocn (secondary antibody-Rhodamine) and analysis of Ocn expression using immunocytochemical techniques using the Olympus Fluoview Ver.4.2a CLSM instrument at a wavelength of 543 nm.
Data analysis
After obtaining data using CLSM, the next step is to carry out quantification to obtain numerical data, which will then be carried out using one-way ANOVA and post-hoc LSD analysis using the statistical software Statistical Product and Service Solutions (SPSS) with a significant difference of p<0.05.
Results and Discussion
To get 70 g of extract, 1.6 kg of dry powdered M. crenata leaves were extracted using 96% ethanol. The extract was then suspended in 1:1 aqua destillates. The suspension is then fractionated with the n-butanol solvent liquid extraction method using a separating funnel. From this process, the weight of the BF was 5.97 grams.
Improvement of the bone formation process in vitro using the BF from M. crenata leaves has been studied in hFOB 1.19 cells. The immunocytochemistry method using CLSM was carried out to measure the expression of Osx and Ocn. Measurements were carried out at a wavelength of 488 nm for Osx and 543 nm for Ocn. Osx expression was shown by green fluorescence intensity, while Ocn expression was shown by red fluorescence intensity in hFOB 1.19 cells (Figures 1 and Figure 3).
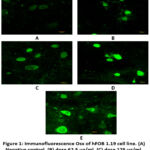 |
Figure 1: Immunofluorescence Osx of hFOB 1.19 cell line. (A) Negative control, (B) dose 62.5 µg/ml, (C) dose 125 µg/ml, (D) dose 250 µg/ml, (E) genistein 2.5 µg/ml. |
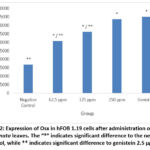 |
Figure 2: Expression of Osx in hFOB 1.19 cells after administration of BF of M. crenata leaves. The “*” indicates significant difference to the negative control, while ** indicates significant difference to genistein 2.5 µg/ml. |
Figure 1 shows Osx immunofluorescence in hFOB 1.19 cells in all groups. The strongest intensity was shown in the genistein positive control group, followed by the treatment group according to concentration level, while the weakest intensity was shown in the negative control group. Figure 2 shows the 250 µg/ml dose group gave the best results by increasing Osx expression significantly compared to the negative control (p = 0.000) and not significantly compared to 2.5 µg/ml genistein (p = 0.129). The increase in Osx expression at the dose of 62.5 µg/ml was significantly different from the negative control (p = 0.000) and from genistein 2.5 µg/ml (p = 0.000), while the 125 µg/ml dose group was also significantly different from the control negative (p = 0.000) and from genistein 2.5 µg/ml (p = 0.000). This shows that genistein and the treatment group were able to increase Osx expression in hFOB 1.19 cells.
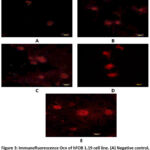 |
Figure 3: Immunofluorescence Ocn of hFOB 1.19 cell line. (A) Negative control, (B) dose 62.5 µg/ml, (C) dose 125 µg/ml, (D) dose 250 µg/ml, (E) genistein 2.5 µg/ml. |
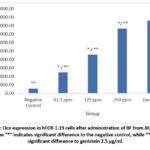 |
Figure 4: Ocn expression in hFOB 1.19 cells after administration of BF from M. crenata leaves. |
Figure 3 shows Ocn immunofluorescence in hFOB 1.19 cells in all groups. The strongest intensity was shown in the genistein positive control group, followed by the treatment group according to concentration level, while the weakest intensity was shown in the negative control group. Figure 4 shows the 250 µg/ml dose group gave the best results, with a significant increase in Ocn expression compared to the negative control (p = 0.000) and also significant compared to genistein 2.5 µg/ml (p = 0.000). The increase in Ocn expression at the dose of 62.5 µg/ml was also significantly different from the negative control (p = 0.000) and from genistein 2.5 µg/ml (p = 0.000), while the 125 µg/ml dose group also showed a significant difference compared to the negative control (p = 0.000) and genistein 2.5 µg/ml (p = 0.000). Meanwhile, the 250 ppm treatment group was only significantly different from the negative group, which shows that the ability to increase Osx was similar to the positive control group.
The BF from M. crenata leaves has been identified as a potential phytoestrogen that can function as an alternative to estrogen via the ER-β pathway17. Phytoestrogens are known to be able to stimulate ER-β activation, which plays a role in regulating transcriptional gene expression and triggering osteoblast cell proliferation and differentiation8,9. In the context of bone formation, osteoblast cell differentiation becomes a key factor, which is influenced by the activation of transcription factors such as Osx and runt-related transcription factor 2 (Runx-2), as well as the production of important proteins such as ALP, Ocn, type I collagen, and BSP, along with the mineralization process and increased bone density8,9,10.
Because Osx plays a crucial role as a specific transcription factor for osteoblast cells, it regulates the process of osteoblast cell maturation and bone formation, including the expression of key proteins like Ocn25. On the other hand, Ocn was chosen as a marker factor because, as the main non-collagen protein produced by mature osteoblast cells, it is often used in the characterization of human bone cells. Ocn has a crucial role in bone formation and mineralization processes10,26.
Genistein 2.5 µg/ml was chosen as a positive control because of its ability as a phytoestrogen, which can substitute the role of estrogen in preserving bone balance, and also because of its ability to increase the bone formation process in both in vivo and in vitro experiments27. Genistein is known to have high affinity for ER-β28, inhibit nuclear factor-kappa B (NF-κB) activation29, and increase Ocn expression14,30. Activity of the BF from M. crenata leaves. Similar to genistein, which was used as a positive control in this study, it is possible that it has activity similar to genistein. Dose 250 ppm of the of the BF from M. crenata leaves. This is the most optimal dose for increasing Osx and Ocn because the amount and intensity measured are similar to those of genistein.
Research data shows that the BF extracted from the leaves of M. crenata is able to increase the expression of Osx and Ocn. These findings indicate the presence of phytoestrogen compounds in the BF, which have a role in increasing the expression of Osx and Ocn, both of which are important indicators in the bone formation process.
Conclusion
The BF of M. crenata leaves at doses of 62.5, 125, and 250 µg/ml has been shown to enchance the expression of Osx and Ocn in hFOB 1.19 cells, with the optimal dose at 250 ppm. These results indicate that the BF from M. crenata has the potential to improve the bone formation process by inducing osteoblastogenesis. However, further preclinical and clinical research needs to be carried out to confirm the osteoblastogenesis effect of BF.
Acknowledgment
None
Conflict of Interest
The authors declare that there are no possible conflicts of interest with respect to the research, authors, and/or publication of this article.
Funding Source
No funding agency in the governmental, commercial, private, or not-for-profit sectors provided a particular grant for this research.
References
- Delkash P., Farsad F. Osteoporosis: A Review of the Factors Affecting Osteoporosis and its Management. Systematic Reviews in Pharmacy. 2020; 11(8).
- Compston J. E., McClung M. R., Leslie W. D. Osteoporosis. The Lancet. 2019; 393(10169): 364–376. doi:10.1016/s0140-6736(18)32112-3.
CrossRef - Noh J. Y., Yang Y., Jung H. Molecular Mechanisms and Emerging Therapeutics for Osteoporosis. International Journal of Molecular Sciences. 2020; 21(20): 7623. doi:10.3390/ijms21207623.
CrossRef - Raggatt L. J., Patridge N. C. Cellular and Molecular Mechanisms of Bone Remodelling. The Journal of Biological Chemistry. 2010; 285(33).
CrossRef - Šromová V, Sobola D, Kaspar P. A Brief Review of Bone Cell Function and Importance. Cells, 2023; 12(21): 2576.
CrossRef - Liu G., Lu Y., Mai Z., Liu R., Peng Z., Chen L., Ai H. Suppressing MicroRNA-30b by Estrogen Promotes Osteogenesis in Bone Marrow Mesenchymal Stem Cells. Stem Cells International. 2019: 1–13.
CrossRef - Fuentes N., Silveyra P. Estrogen Receptor Signaling Mechanisms. Advances in Protein Chemistry and Structural Biology. 2019; 116: 135-170
CrossRef - Tu Q., Valverde P., Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun. 2006; 341: 1257–65. [PubMed: 16466699].
CrossRef - Zhang C., Tang W., Li Y., Yang F., Dowd D. R., MacDonald P. N. Osteoblast-Specific Transcription Factor Osterix Increases Vitamin D Receptor Gene Expression in Osteoblasts. PLoS ONE. 2011; 6(10): e26504.
CrossRef - Rathore B, Singh M, Kumar V, Misra A. Osteocalcin: an emerging biomarker for bone turnover. International Journal of Research in Medicinal Sciences. 2016; 4(9): 3670-3674. Doi: http://dx.doi.org/10.18203/2320-6012.ijrms20162899
CrossRef - Bretler D. M., Hansen P. R., Lindhardsen J., Ahlehoff O., Andersson C., Jensen T. B., … & Gislason G. H. Hormone replacement therapy and risk of new-onset atrial fibrillation after myocardial infarction-a nationwide cohort study. PLoS One. 2012; 7(12): e51580.
CrossRef - Yang T. S., Wang S. Y., Yang Y. C., Su C. H., Lee F. K., Chen S. C., Tseng C. Y., Jou H. J., Huang J. P., Huang K. E. Effects of standardized phytoestrogen on Taiwanese menopausal women. Taiwanese Journal of Obstetrics & Gynecology. 2012; 51: 229-235.
CrossRef - Jayusman P. A., Nasruddin N. S., Baharin B., Ibrahim N. I., Ahmad H. H., Shuid A. N. Overview on postmenopausal osteoporosis and periodontitis: The therapeutic potential of phytoestrogens against alveolar bone loss. Frontiers in pharmacology. 2023; 14: 1120457.
CrossRef - Sirotkin A. V., Harrath A. H. Phytoestrogens and their effects. European Journal of Pharmacology. 2014; 741: 230–236. doi:10.1016/j.ejphar.2014.07.057.
CrossRef - Yacoeb A. M., Nurjanah., Arifin M., Sulistiono W., Kristiono S. S. Deskripsi Histologis dan Perubahan Komposisi Daun dan Tangkai Semanggi (Marsilea crenata Presl., Marsileaceae) akibat perebusan. Jurnal Pengolahan Hasil Perikanan Indonesia. 2010; (2): 81-95.
- Laswati H. Green clover potentiates delaying the increment of imbalance bone remodeling process in postmenopausal women. Folia Medica Indonesiana. 2011; 47(2): pp.112-117
- Ma’arif B., Aditama A. P., Muslikh F. A., Sari D. P., Purbosari I., Laswati H., Agil M. Inhibitory Effect of Free-ERβ Expression by n-Butanol Fraction of Semanggi (Marsilea crenata Presl.) Leaves on hFOB 1.19 Cells. Jurnal Sains dan Kesehatan. 2021; 3(4): 475-481.
CrossRef - Ma’arif B., Agil M., Laswati H. Phytochemical assessment on n-hexane extract and fractions of Marsilea crenata Presl. leaves through GC-MS. Trad. Med. J. 2016; 21(2): 77-85.
- Ma’arif B., Agil M., Laswati H. Alkaline phosphatase activity of Marsilea crenata Presl. Extract and Fractions as Marker of MC3T3-E1 Osteoblast Cell Differentiation. Journal of Applied Pharmaceutical Science. 2018; 8(3): 55-59.
- Agil M., Ma’arif B., & Aemi N. Y. Antiosteoporotic activity of n-hexane fraction from Marsilea crenata Presl. leaves in increasing trabecular vertebrae bone density of female mice. Jurnal Tumbuhan Obat Indonesia. 2018; 11(2): 1-7.
CrossRef - Aditama A. P., Muslikh F. A., Shirvi I. N., Islamiyah F. R., Putra K. H., Inayatilah F. R., … & Rahayu A. Effect of induction of proliferation of trabecular bone osteoblast cells in male mice by 96% ethanol extract of clover leaves (Marsilea crenata Presl.). Jurnal Sains dan Kesehatan. 2021; 3(4): 429-435.
CrossRef - Yen M., Chien C. C., Chiu I., Huang H. I., Chen Y. C., Hu H. I., Yen B. L. Multilineage differentiation and characterization of the human fetal osteoblastic 1.19 cell line: a possible in vitro model of human mesenchymal progenitors. Stem Cells. 2007; 25(1): 125-131.
CrossRef - Wang S., Fu Y., Zhao X. H. The Cooperative Effect of Genistein and Protein Hydrolysates on the Proliferation and Survival of Osteoblastic Cells (hFOB 1.19). Molecules. 2016; 21(11): 1489. doi:10.3390/molecules21111489
CrossRef - Aditama A. P., Muslikh F. A., Shirvi I. N., Islamiyah F. R., Putra K. H., Inayatilah F. R., … & Rahayu A. Induction Effect of Proliferation of Osteoblas Cells Trabecular Bone Male Mice by 96% Ethanol Extract of Semanggi Leaves (Marsilea crenata Presl.). Jurnal Sains dan Kesehatan. 2021; 3(4): 429-435.
CrossRef - Tang W., Yang F., de Crombrugghe B., Jiao H., Xiao G., Zhang C. Transcriptional regulation of vascular endothelial factor (VEGF) by osteoblast-specific transcription factor Osterix (Osx) in osteoblasts. J Biol Chem. 2012; 2871671-1678.
CrossRef - Aonuma H., Miyakoshi N., Hongo M., Kasukawa Y., Shimada Y. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku Journal of Experimental Medicine. 2009; 218: 201–205.
CrossRef - Wang N., Wang L., Yang J., Wang Z., Cheng L. Quercetin promotes osteogenic differentiation and antioxidant responses of mouse bone mesenchymal stem cells through activation of the AMPK/SIRT1 signaling pathway. Phytotherapy Research. 2021; 35(5): 2639-2650.
CrossRef - Bitto A., Granese R., Triolo O., Villari D., Maisano D., Giordano D., Altavilla D., Marini H., Bianca-Adamo E., Nicotina P. A., D’Anna R., Squadrito F. Genistein aglycone: A new therapeutic approach to reduce endometrial hyperplasia. Phytomedicine. 2010; 17(11): 844–850. doi:10.1016/j.phymed.2010.03.024
CrossRef - Ming L. G., Chen K. M., Xian C. J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. Journal of Cellular Physiology. 2012; 228(3): 513–521. doi:10.1002/jcp.24158
CrossRef - Aditama A. P. R., Ma’arif B., Muslikh F. A. Effect of Osterix and Osteocalcin Enhancement By Quercetin (3,3’,4’,5,7-Pentahydroxyflavone) on Osteoblast hFOB 1.19 Cell line. International Journal of Applied Pharmaceutics. 2022; 14(1): 32-35.







