Shrinidhi Nathany1 , Siddharth Sagar2
, Siddharth Sagar2 , Padmapriya Jaiprakash3
, Padmapriya Jaiprakash3  and Mridula Madiyal4
and Mridula Madiyal4
1Molecular diagnostics, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India.
2Microbiologist, Dr. Khanna’s Pathcare Pvt Ltd, New Delhi, India.
3Department of Pathology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Sciences, Karnataka, Manipal, India.
4Department of Microbiology, KS Hegde Academy of Medical Sciences (KSHEMA), NITTE (Deemed to be University) Mangalore, India.
Corresponding Author E-mail: padmapriya.j@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2964
Abstract
Introduction and objectives: Herpes Simplex Virus (HSV) is a common viral pathogen, known to cause symptomatic disease in the immunocompromised hosts; however, it may occur in healthy individuals as well. Among the varied manifestations of the disease, involving skin, central nervous system and gastrointestinal systems, HSV esophagitis is a less studied entity from the Indian perspective. The aim of the study is to compare clinical, histologic and serologic data of HSV esophagitis patients. Materials and methods: 27 cases, proved to have HSV esophagitis on endoscopic biopsy were included, and the pertinent clinical and serologic characteristics were studied. Results: We found a male preponderance (p<0.01), which has been a well-established risk factor. Other risk factors are retroviral coinfection, Type 2 Diabetes Mellitus, immunosuppression, chemoradiation etc. among others. Eleven patients had retroviral coinfection, with CD4 counts of > 200/μL in all. Anti HSV 1 IgM antibody was most commonly detected on serology. HSV esophagitis affects immunocompromised patients more often than immunocompetent ones. Odynophagia and dysphagia are the most common symptoms. Histological evaluation for the characteristic inclusions helps in early diagnosis. The novelty of this study rests on the clinicopathological and serologic correlation, for a better understanding of the disease process, to prompt future large scale studies on the same. Conclusion: Herpes simplex esophagitis is most seen in immunocompromised patients. Apart from retroviral illness, other risk factors include diabetes mellitus, patients undergoing chemotherapy and other malignancy. CD4 counts in our series was >200/μL, in contrast to the cutoff of <200/μL described in literature.
Keywords
Herpes Simplex Virus; Histopathology; Serology; Esophagitis
Download this article as:| Copy the following to cite this article: Nathany S, Sagar S, Jaiprakash P, Madiyal M. Clinicopathological Study of Herpes Simplex Esophagitis in a Tertiary Centre in India. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Nathany S, Sagar S, Jaiprakash P, Madiyal M. Clinicopathological Study of Herpes Simplex Esophagitis in a Tertiary Centre in India. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4e0CJHV |
Introduction
The most common causes of infectious esophagitis are Candida (88%), herpes simplex virus (10%) and cytomegalovirus (2%). Herpes simplex virus (HSV) is the most common cause of viral esophagitis. Histopathological evaluation forms an unusual armamentarium in the evaluation of viral infections. The many cytopathic effects of viruses are sometimes the earliest manifestation of an ongoing disease process. One such is Herpes Simplex Virus (HSV). The word “herpes” (from the Greek, “to creep”) has been around in medical literature since time immemorial.1 The Roman physician Herodotus in 100 AD described cold sores (herpes febrilis) in his anecdotes.1 In 1976, John Astruc, a French physician first described genital herpes.1 Among the immunocompetent individuals, HSV esophagitis typically occurs as a primary infection with a self-limited course.2 There is evidence that impaired mucosal barrier may augment oesophageal HSV infection.2 The part of the gastrointestinal tract that is commonly affected is the oesophagus, with an incidence of 0.5% to 2% based on an autopsy series.3-6 The correlation between the clinical manifestations, endoscopic and histopathological findings, and the viral antibodies has not been widely described in literature. Therefore, in the present study we aimed to investigate the clinical manifestations and associated comorbidities of patients diagnosed to have herpes simplex esophagitis based on findings of endoscopy, serology, and histopathology.
Materials and Methods
Study design and population – laboratory based, retrospective study, done on rural population in Coastal Karnataka, South India,
Methods: From a total of 6,782 esophageal biopsies received in the department of Pathology, between November 2011- January 2017, 5683 were endoscopically found to have an ulcerated esophageal mucosa. Of these 5683 cases, filters were given in the laboratory information system to isolate cases with a clinical suspicion of HSV infection, which gave a total of 173 cases, owing to the endoscopic findings and in correlation with the underlying comorbidities. Inclusion criteria was biopsy proven cases of herpes simplex esophagitis. Exclusion criteria: Biopsy negative for HSV changes.
Ethical consideration: This study was conducted according to the WMA Declaration of Helsinki, after approval of scientific review committee. The data collected was coded to maintain the confidentiality of the patients. The demographic and clinical details like alcohol consumption, smoking habits, underlying comorbidity, immunocompromised state, retroviral disease status, were collected from patient records from the archives. Direct and indirect identifiers were avoided by coding the sample population case wise (e.g. case 1, 2, etcetera). The indications for an upper gastrointestinal (GI) endoscopy including dysphagia and/or odynophagia, heartburn, epigastric pain, nausea/vomiting, GI bleeding and endoscopic findings like friable mucosa, erosions/ ulcers, polypoid/ nodular pattern and site of involvement were noted. Biopsy should be performed from the margin / edge of the ulcer rather than from the base. Serological parameters like anti-HSV 1 IgM and anti-HSV 2 IgM were also recorded. The biopsy slides were reviewed for the cytopathic effects and the characteristic inclusions of HSV. The slides were reviewed independently by two pathologists. Special stains including periodic acid Schiff (PAS) and/ or Gomori’s methenamine silver (GMS) stains were performed to rule out concomitant fungal infection.
Data analyses: The data on categorical variables is shown as in (% of cases) and the data on normally distributed continuous variables is presented as Mean and Standard deviation (SD). For non-normally distributed continuous variables, median (min – max) was used. The inter-group statistical comparison of distribution of categorical variables is tested using Chi-Square test or Fisher’s exact probability test.
Results
27 of the 173 clinically suspected patients were diagnosed as HSV esophagitis based on histological and/or serological investigation. The median age of the study population was 50 years (range 34-71 years) while the male to female ratio was 2.3:1. The most common clinical manifestations amongst the diagnosed cases, were epigastric pain (21/27 patients, 77.8%), odynophagia/dysphagia (16/27 patients, 59.2%), heartburn (15/27 patients, 55.5%) and nausea/vomiting (11/27 patients, 40.7%). Cigarette smoking and alcohol consumption were present among (11/27 patients, 40.7%) and (12/27 patients, 44.4%), respectively (table1). Of the 27 diagnosed patients, 8 (29.6%) had Type 2 DM, 5 (18.5%) had COPD, 2 (7.4%) had end stage renal disease, 1 (3.7%) had cirrhosis of liver, 5 (18.5%) had a diagnosed malignancy and were under chemotherapy and/or irradiation or both for the same. 17 (62.9%) were under steroid therapy while none had undergone an organ transplantation. HIV co-infection was present among 11 cases. Upper GI endoscopy reports revealed erosions and ulcers with surrounding erythema of the mucosa in 19/27 patients (70.3%), friable mucosa in 5/27 patients (18.5) and polypoid/nodular pattern in 3/27 patients (11.1%). 18.5% of patients (5/27) had lesions on the upper third of the oesophagus, 59.2% (16/27) in the middle third, while 22.2% (6/27) on the distal third. None of our patients had HSV lesions involving the entire oesophagus. The CD4 counts were available in 11 cases with HIV coinfection with all patients having a count > 200/μL. Anti-HSV 1 IgM antibodies were positive among 20/27 patients, negative among 2/27 patients and not done among 5/27 patients. Anti-HSV 2 IgM antibodies were positive among 15/27 patients, negative among 7/27 patients and not done among 5/27 patients. There were 48.1% (13/27) patients who had antibodies present against both HSV 1 and HSV 2 (Table 2). With respect to the HIV coinfected population, all cases (n=11) showed anti-HSV 1 IgM positivity (11/11) and 6/11 also showed anti-HSV 2 IgM positivity. However, there was no significant relation with the CD4 counts in these patients. HSV usually affects the epithelial cells of the oesophagus, hence histologic examination reveals large intranuclear inclusion bodies, commonly referred to as Cowdry A bodies, (Figure 1 A and B) and/or multinucleated giant cells (Figure 2) with ground glass nuclei and marginated chromatin in the epithelial cells (9). The surrounding mucosa may show necro-inflammatory slough, mixed inflammatory infiltrate and reactive atypia with few koilocytes (Figure 3).
Table 1: Clinical Characteristics and Underlying Risk Factors with respect to the type of ulcer on Endoscopy findings in patients with Herpes Simplex Infection.
|
|
Type 1 (n=5) |
Type 2 (n=19) |
Type 3 (n=3) |
Total (N=27) |
P |
|
Age > 60 years |
1 |
6 |
0 |
7 |
0.48 |
|
Male sex |
4 |
15 |
0 |
19 |
0.01* |
|
Personal Habits |
|||||
|
Smoking |
2 |
9 |
0 |
11 |
0.30 |
|
Alcohol |
4 |
8 |
0 |
12 |
0.08 |
|
Underlying Disease Condition |
|||||
|
DM |
2 |
6 |
0 |
8 |
0.45 |
|
COPD |
2 |
2 |
1 |
5 |
0.25 |
|
ESRD |
0 |
2 |
0 |
2 |
0.63 |
|
Cirrhosis |
0 |
1 |
0 |
1 |
0.80 |
|
Malignancy |
1 |
4 |
0 |
5 |
0.68 |
|
HIV infection |
1 |
9 |
1 |
11 |
0.52 |
|
Status of Infection |
|||||
|
Sepsis |
0 |
5 |
0 |
5 |
0.27 |
|
Other Factors |
|||||
|
Steroids |
5 |
11 |
1 |
17 |
0.11 |
|
WBC |
7140 ± 5326 |
10110 ± 4759 |
9166 ± 1800 |
9455 ± 4652 |
0.46 |
|
DM – Diabetes Mellitus, COPD – Chronic Obstructive Pulmonary Disease, ESRD – End Stage Renal Disease, HIV – Human Immunodeficiency Virus |
|||||
Table 2: Correlation of CD4 counts with the type of anti-HSV IgM antibody in patients with HIV coinfection (n=11).
|
Anti-HSV 1 IgM |
Anti-HSV 2 IgM |
CD4 cell count (/μl) |
|
Positive |
Negative |
477.82 |
|
Positive |
Positive |
432.67 |
|
Positive |
Negative |
272 |
|
Positive |
Positive |
384.65 |
|
Positive |
Positive |
354.45 |
|
Positive |
Positive |
276.84 |
|
Positive |
Negative |
456.66 |
|
Positive |
Positive |
475.66 |
|
Positive |
Positive |
478.99 |
|
Positive |
Negative |
479.08 |
|
Positive |
Negative |
485.56 |
Discussion
The main aim is to study the clinical features, including comorbidities and endoscopic findings, diagnosed to have herpes simplex esophagitis based on histopathology along with correlation of serological findings.
HSV esophagitis is known to occur in immunocompromised individuals including those affected with AIDS; however there have been reports of its occurrence in the immunocompetent as well.1 The hallmark of HSV esophagitis endoscopically are multiple, small, superficial ulcers most commonly along the distal third of the oesophagus7 whereas in our study the most frequent site was the middle third. Viral culture of the biopsy sample is the most sensitive diagnostic modality for HSV-18-10, however in our study we could not employ the same.
HSV esophagitis, per se, shows a slight male predilection (4, 10-14) and affects individuals in the 5th to 6th decades of their lives owing to increasing risk factors. In our study group also, the median age was found to be 50 years with a male preponderance (M:F – 2.3:1) which was in concordance with two studies (M:F – 3:1, mean age 63.1 years and M:F – 4.7:1, mean age 38 years). 4,10 However, this may represent just the tip of the iceberg as most infections may remain subclinical or latent. The most common risk factors include male sex (P < 0.01), age > 60 years, personal habits like cigarette smoking and alcohol consumption, underlying comorbidities like Type 2 DM, COPD, ESRD, cirrhosis of liver, malignancy, and HIV coinfection and other predisposing factors like steroid intake and chemoradiation, as compared to another study which reported chemoradiation as a risk factor (P < 0.05) 7 as also associated co-infections like fungal, bacterial etc.11
The most common clinical manifestations in our study were odynophagia/dysphagia (59.2%) which is concordant with several contemporary reports of 61% 2, 71%13 and 76%.12 GI bleeding has been reported to occur in HSV esophagitis; however, none of our patients had frank GI bleeding in contrast to a study11 which reported GI bleeding in 30% of their study group (n=23). This may be attributed to the underlying comorbidities, the severity of infection and sociodemographic diversities.
The three types of HSV esophagitis, observed endoscopically4, include: Type 1 – small, punched-out lesions with raised margins having a yellowish exudate, Type 2 – small, punched-out lesions without raised margins or exudate, Type 3 – multiple, confluent ulcers with a map-like appearance; all the three types were recorded in our series.
The CD4 counts in those with HIV coinfection (n=11), based on literature is usually <200 for HSV infection to set in. In our study, all the patients had a CD4 count of over 200, which may be attributable to racial and geographic differences with different immunological constitution, and to the small sample size.
All the patients in our study were subjected to an upper GI endoscopic biopsy which on routine haematoxylin and eosin-stained microscopic examination revealed ulcerated oesophageal mucosae with necro-inflammatory slough. The squamous epithelial cells showed perinuclear clearing (koilocytosis) along with intranuclear inclusions (Cowdry A) (Figure 1 and 2) suggesting a viral aetiology, possibly HSV. This was in concordance with other studies. 4,7,9,13 Ancillary immunohistochemistry specific for HSV could not be done due to unavailability of the antibody. The diagnosis was rendered based on morphology with clinical suspicion and serological evidence. The serology for anti-HSV 1 and 2 IgM were done for all the cases in the study group; however, to the best of our knowledge no other study has compared or studied the relation of positive antibody serology with histological evidence.
Antiviral therapy, including acyclovir, famciclovir, and Val acyclovir, is used to treat HSV esophagitis in immunocompromised hosts.15, 16 However, the benefit of antiviral therapy for HSV esophagitis is controversial in immunocompetent patients because of reported rare complications.12,13,17 In our retrospective study, 22/27 patients (81.5%) received antiviral therapy, and their symptoms, especially dysphagia and/or odynophagia showed an obvious improvement within 3 to 5 days as documented in the medical charts.
The present retrospective study has some limitations. First, laboratory data about immune status such as CD4 count were not checked on all HSV esophagitis patients, and only in those with HIV coinfection. Second, typically endoscopic typing includes type 1, type 2, and type 3 may not be mutually exclusive with overlapping or combined lesions in the same patient, which did not yield statistically significant results. Third, the small sample size of biopsy proven cases restricted comparison with clinically suspected cases. Fourth, immunohistochemistry could not be specifically carried out on the biopsies due to unavailability. Fifth, an adequate follow up was not available to carry out survival and mortality related studies. Although the mortality of the disease per se, is low, but when compounded by the underlying disease status, it may increase.
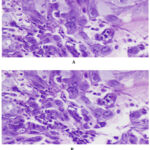 |
Figure 1: A and B. Intranuclear inclusions in the squamous epithelial cells with ground glass appearance (H and E, x400, x200). |
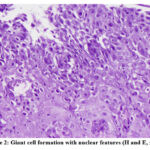 |
Figure 2: Giant cell formation with nuclear features (H and E, x200) |
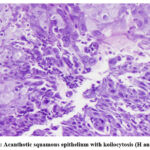 |
Figure 3: Acanthotic squamous epithelium with koilocytosis (H and E, x100) |
Conclusion
In conclusion, HSV esophagitis primarily affects males and immunocompromised hosts. An early laboratory work-up of the at-risk population and those with typical clinical manifestations can help improve the detection rates. Early and correct diagnosis based on histopathological evaluation of characteristic inclusions by the pathologist is useful for appropriate treatment.
Acknowledgement
We would like to acknowledge the technical staff of departments of Microbiology and Pathology, Manipal Academy of Higher Education, Karnataka, Manipal, 576 104, India for the retrieval of data and conducting of the procedures.
Conflict of Interest
The author(s) declares no conflict of interest
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Corey JT. Herpes Simplex Virus. In R. D. John E. Bennett, Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Saunders. 8th ed. 2015; pp.1713-30.
CrossRef - Canalejo Castrillero E, Garcia Duran F, Cabello N, García Martínez J. Herpes esophagitis in healthy adults and adolescents: Report of 3 cases and review of the literature. Medicine (Baltimore). 2010;89(4):204–10.
CrossRef - Matsumoto J, Sumiyoshi A. Herpes simplex esophagitis—a study in autopsy series. Am J Clin Pathol. 1985;84:96-9.
CrossRef - Itoh T, Takahashi T, Kusaka K, Kawaura K, Nakagawa Y, Yamakawa JI, et al. Herpes simplex esophagitis from 1307 autopsy cases. J. Gastroenterol. Hepatol. 2003;18:1407-11.
CrossRef - Buss DH, Scharyj M. Herpesvirus infection of the esophagus and other visceral organs in adults: Incidence and clinical significance. Am J Med 1979;66:457-62.
CrossRef - Nash G, Ross JS. Herpetic esophagitis: a common cause of esophageal ulceration. Hum Pathol 1974;5:339-45.
CrossRef - Wang HW, Kuo C J, Lin WR, Hsu CM, Ho YP, Lin CJ, et al. Clinical Characteristics and Manifestation of Herpes Esophagitis: One Single-center Experience in Taiwan. Medicine (Baltimore) 2016;95: e3187.
CrossRef - Fatahzadeh M and Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol 2007; 57:737-63.
CrossRef - Lavery EA and Coyle WJ. Herpes simplex virus and the alimentary tract. Curr Gastroenterol Rep; 2008;10: 417-23.
CrossRef - Généreau T, Lortholary O, Bouchaud O, Lacassin F, Vinceneux P, Truchis PD, et al. Herpes simplex esophagitis in patients with AIDS: report of 34 cases. Clin Infect Dis 1996;22:926-931.
CrossRef - Jagtap SV, Jagtap SS, Nagar V, Varshney K. Invasive mucormycosis in post COVID-19 infection: Case report with review. IP Arch Cytol Histopathol Res 2021;6(2):135-139.
CrossRef - McBane RD and Gross JB. Herpes esophagitis: clinical syndrome, endoscopic appearance, and diagnosis in 23 patients. Gastrointest Endosc1991;37:600-3.
CrossRef - Ramanathan J, Rammouni M, Baran J, Khatib R. Herpes simplex virus esophagitis in the immunocompetent host: an overview. Am J Gastroenterol 2000;95:2171-76.
CrossRef - Kato S, Yamamoto R, Yoshimitsu S, Shimazaki K, Ogawa S, Itoh K, et al. Herpes simplex esophagitis in the immunocompetent host. Dis Esophagus 2005;18: 340-344.
CrossRef - Bando T, Matsushita M, Kitano M, Okazaki K. Herpes simplex esophagitis in the elderly. Diag Endosc. 2009;21(3):205-7.
CrossRef - Kurahara K, Aoyagi K, Nakamura S, Kuwano Y, Yamamoto C, Iida M, & Fujishima, M. (1998). Treatment of herpes simplex esophagitis in an immunocompetent patient with intravenous acyclovir: a case report and review of the literature. Am J Gastroenterol 1998;93:2239-40.
CrossRef - Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK. Treating opportunistic infections among HIV-infected adults and adolescents; recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. 2004.
CrossRef - Galbraith JC and Shafran SD. Herpes simplex esophagitis in the immunocompetent patient: report of four cases and review. Clin Infect Dis 1992;14:894-901.
CrossRef








