Mariia Shanaida1* , Olha Korablova2
, Olha Korablova2 , Dzhamal Rakhmetov2
, Dzhamal Rakhmetov2 , Bohdanna Sydor1
, Bohdanna Sydor1 , Volodymyr Shanaida3
, Volodymyr Shanaida3 , Nataliia Hudz4,5
, Nataliia Hudz4,5 and Ján Brindza6
and Ján Brindza6
1Department of Pharmacognosy and Medical Botany, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
2M.M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine, Kyiv, Ukraine
3Design of Machine Tools, Instruments and Machines Department, Ternopil Ivan Puluj National Technical University, Ternopil, Ukraine;
4 Department of Pharmacy and Ecological Chemistry, University of Opole, Opole, Poland
5Department of Drug Technology and Biopharmacy, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
6Faculty of Agrobiology and Food Resources, Slovak University of Agriculture in Nitra, Nitra, Slovakia
Corresponding Author E-mail:shanayda@tdmu.edu.ua
DOI : https://dx.doi.org/10.13005/bpj/2956
Abstract
This study aimed to analyze the compositions of flavonoids and phenolic acids in the aerial parts of two Artemisia L. species (Artemisia ludoviciana Nutt. and Artemisia campestris L.) when grown in Ukraine. High-performance liquid chromatography analysis (HPLC) detected the presence of 11 flavonoids and 10 phenolic acids in the plant materials under study. Among the phenolic acids, chlorogenic acid was the most abundant in the raw material of both species (14.503 mg/g in Artemisia ludoviciana and 4.504 mg/g in Artemisia campestris). The main flavonoids in the Artemisia ludoviciana herb decreased in the following order: flavanone-7-O-glycoside (naringin) (21.924 mg/g) > fisetin (13.068 mg/g) > kaempferol-3-b-glucoside (5.119 mg/g) > rutin (1.295 mg/g). In comparison, in Artemisia campestris raw material the order was: flavanone-7-O-glycoside (7.525 mg/g) > fisetin (2.933 mg/g) > rutin (1.355 mg/g). Since the predominant polyphenols have demonstrated valuable therapeutic potential, the data obtained could be considered for further exploration of their biological activities.
Keywords
Aerial part; Artemisia species; Flavonoids; High-performance Liquid Chromatography; Phenolic acids
Download this article as:| Copy the following to cite this article: Shanaida M, Korablova O, Rakhmetov D, Sydor B, Shanaida V, Hudz N, Brindza J. Chromatographic Profiles of Polyphenols in the Herbs of Artemisia campestris L. and Artemisia ludoviciana Nutt. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Shanaida M, Korablova O, Rakhmetov D, Sydor B, Shanaida V, Hudz N, Brindza J. Chromatographic Profiles of Polyphenols in the Herbs of Artemisia campestris L. and Artemisia ludoviciana Nutt. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/3MEG57H |
Introduction
Medicinal plants are rich sources of valuable natural compounds with promising therapeutic potential, which play a crucial role in traditional and evidence-based medicine1. The gradual development of more advanced instrumental extraction techniques and analytical methods has allowed for the comprehensive identification of numerous bioactive plant metabolites2. There is no doubt that polyphenols, synthesized in plants, are a diverse and essential group of bioactive components3-5.
The genus Wormwood (Artemisia L., family Asteraceae Dumort.) includes many species that have long been of interest to phytotherapists and researchers as valuable sources of healing substances. They are considered to be among the most well-known medicinal plants in the world6. These plants have significant potential in the treatment of numerous diseases, and more recently, in the treatment of malaria7. It’s important to note that Artemisia absinthium L. was previously included in the European Pharmacopoeia (6.4)8. However, it was later removed from pharmacopoeial monographs due to the neurotoxic effects of thujone, one of the main compounds in its essential oil.
The aerial parts of several Artemisia plants have been used in traditional medicine in many countries due to their antimicrobial, antiparasitic, nematocidal, insecticidal, hypoglycemic, secretolytic, etc. effects. These effects are attributed to the diverse chemical compounds found in the essential oils and polyphenols of Artemisia species9. Researchers in the pharmaceutical industry have shown significant interest in the varied chemical compositions of Artemisia representatives, leading to numerous studies on their potential medical uses6,7,9,10.
It is evident from the analysis of available scientific sources that there has been relatively limited study of the phytochemical profiles and pharmacological properties of certain Artemisia species. For instance, as of July 01, 2024, a search on the PubMed international database of scientific publications revealed only 38 articles for “Artemisia ludoviciana“, 69 articles for “Artemisia campestris“, and 6159 articles for “Artemisia vulgaris“. This indicates the significant differences in the levels of scientific interest in studying the bioactive components of various Artemisia representatives. Therefore, there is a considerable key point in further exploring the phytochemicals of Artemisia ludoviciana Nutt. and Artemisia campestris L.
Recent studies have revealed compelling data regarding the chemical composition and medicinal properties of Artemisia ludoviciana andArtemisia campestris, plants with a history of traditional use in North American and Eurasian indigenous medicine, respectively10-12. Despite this, there has been limited scientific research on the biomedical potential of these plants. Their essential oils were much better studied than polyphenols or other bioactive compounds 6,10,12-15. Besides it, Artemisia campestris is a polymorphic species consisting of many subspecies, varieties, and chemotypes that differ in the chemical composition of biocompounds11.
It should be noted thatArtemisia ludoviciana andArtemisia campestris have been successfully introduced to botanical gardens and research institutions, including in Ukraine. Considering this, we advocate for comprehensive phytochemical research attention for these two species.
Materials and Methods
Plant raw material
The herbs of Artemisia campestris and Artemisia ludoviciana were harvested in 2022 during the flowering period from the plots in M.M. Gryshko National Botanical Garden (Kyiv, Ukraine) which is located in the Forest-Steppe zone (30°33′44″ east longitude and 50°24′45″ north latitudes). The above-ground parts of plants were dried at 30-35°C.
The phytochemical research of flavonoids and phenolic acids in the studied raw materials was carried out by the method of high-performance liquid chromatography (HPLC). Before the HPLC analysis, the grinded raw material was extracted with 80% methanol using an ultrasonic bath (in sealed glass vials with Teflon caps for 2 hours at 70∘С). The obtained extracts were centrifuged and then filtered through membrane filters (pores 0.22 μm).
Chromatographic Analysis
The HPLC analis was performed on an Agilent Technologies 1200 liquid chromatograph with a Zorbax SB-C18 column (3.5 μm, 150 x 4.6 mm).
During the study of flavonoids, acetonitrile (A) and a 0.1% solution of formic acid in water (B) were used as the mobile phase. The elution was performed in the gradient mode: 0 min – A (5%) : B (95%); 20 min – A (30%) : B (70%); 30 min – A (60%) : B (40%); 50 min – A (100%) : B (0%); 60 min – A (100 %) : B (0 %). The flow rate was 0.25 mL/min. The injection volume was 4 μL. The detection was carried out using a diode-matrix detector with signal registration at a wavelength of 280 and 365 nm. The research was carried out using standard solutions of flavonoids (rutin, quercetin, kaempferol, naringenin, naringin, neohesperidin, quercetin-3-b-glycoside, kaempferol-3-b-glucoside, apigenin, luteolin, baicalein, rhamnetin, fisetin and silibenin).
To analyse the phenolic acids, methanol (A) and a 0.1% solution of formic acid in water (B) were chosen as the mobile phase. The elution was carried out in the gradient mode: 0 min – A (10%): B (90%); 40 min – A (75%) : B (25%); 45 min – A (100%) : B (0%); 55 min – A (100 %) : B (0 %). The flow rate through the column was 0.6 mL/min. The injection volume was 3 μL. The detection was carried out using a diode-matrix detector with signal registration at 275 and 330 nm. The identification and quantification were performed using standard solutions of phenolic acids (gallic, hydroxyphenylacetic, benzoic, quinic, syringic, sinapic, rosmarinic, caffeic, chlorogenic, p-coumaric, trans-cinnamic, and trans-ferulic).
The content of identified phenolic compounds (X) (mg/g) was calculated according to the formula:
X = C×V/m,
Where: C – is the concentration of the compound determined chromatographically, mg/mL;
V – the volume of the extract, mL;
m – the weight of the extracted raw material, g.
Results and Discussion
The conducted HPLC study revealed the composition and contents of phenolic acids and flavonoids in the raw materials of Artemisia campestris and Artemisia ludoviciana. In the analysis, 10 phenolic acids were identified (Table 1, Fig. 1). Hydroxycinnamic chlorogenic acid was the most abundant in the herbs of both species, with its content being 3.2 times higher in Artemisia ludoviciana compared toArtemisia campestris. Rosmarinic acid was the second most prevalent phenolic acid in the aerial parts of the studied plants. Other phenolic acids were found in much smaller quantities. It is important to note that caffeic and quinic acids were identified only in the Artemisia campestris herb while trans-ferulic acid was found only in the Artemisia ludoviciana.
Table 1: The amounts of phenolic acids in the studied Artemisia herbs evaluated by the HPLC method
|
Phenolic acid |
Retention time, min |
Content, mg/g |
|
|
Artemisia campestris |
Artemisia ludoviciana |
||
|
Gallic acid |
5.9 |
0.081 |
0.117 |
|
Hydroxyphenylacetic acid |
9.4 |
0.109 |
0.136 |
|
Chlorogenic acid |
11.3 |
4.504 |
14.503 |
|
Caffeic acid |
12.2 |
0.216 |
Not detected |
|
Syringic acid |
14.5 |
0.089 |
0.223 |
|
Benzoic acid |
15.8 |
0.044 |
0.079 |
|
trans-Ferulic acid |
17.6 |
Not detected |
0.324 |
|
Sinapic acid |
19.3 |
0.051 |
0.041 |
|
Rosmarinic acid |
20.4 |
1.774 |
3.326 |
|
Quinic acid |
23.1 |
0.117 |
Not detected |
 |
Figure 1: HPLC chromatograms of phenolic acids detected in the Artemisia campestris (a) and Artemisia ludoviciana (b) herbs: |
Chlorogenic acid (Fig. 2) as the major phenolic acid of both studied herbs has various pharmacological activities such as antioxidant, anticancer, hepatoprotective, immunomodulating, antimicrobial, antidiabetic, etc.16. It finds applications in multiple industries, including healthcare, food, and chemicals17. As it was discovered recently, chlorogenic acid was responsible for the observed antioxidant activity of the water-alcohol extract of Artemisia campestris18.
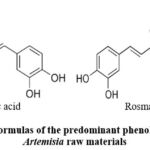 |
Figure 2: Structural formulas of the predominant phenolic acids in the studied Artemisia raw materials |
Using the HPLC method, Ochkur19 identified chlorogenic acid in ethanolic extracts of 5 species of wormwood harvested in Ukraine: Artemisia vulgaris L., Artemisia abrotanum L., Artemisia austriaca Jacq., Artemisia dracunculus L. and Artemisia absinthium. The liquid chromatography-mass spectrometry assessment of methanol extracts of five Artemisia representatives collected from Romanian flora (Artemisia vulgaris, Artemisia absinthium, Artemisia austriaca, Artemisia pontica L. and Artemisia annua L.) led to the identification of 26 flavonoids (mainly flavone derivatives) and 15 phenolic acids20. It should be noted that chlorogenic acid was the common predominant compound of all studied Artemisia speciesfrom Romania20. Chlorogenic acid was also regarded as a predominant component with antioxidant activity among several caffeoylquinic acids detected in theArtemisia absinthium and Artemisia ludoviciana grown in Lithuania9. In confirmation of the above data, Ickovski and co-authors21 found that chlorogenic acid was the most abundant phenolic acid in the methanolic extracts of Artemisia vulgaris and Artemisia absinthium aerial parts collected in Serbia.
The main biological activities of rosmarinic acid include anti-inflammatory, antioxidant, antidiabetic, antiviral, antitumor, neuroprotective, and hepatoprotective effects22.The accumulation of rosmarinic acid is very specific for some taxons of the Lamiaceae as well as Asteraceae families23-24. Interesting scientific data has been revealed regarding the rosmarinic acid, identified in Artemisia annua extracts. It showed a synergistic interaction with artemisinin when acting on a malarial plasmodium strain25.
Among 11 identified flavonoids detected in the herbs of Artemisia species by us, it was determined the prevailing flavanone-7-O-glycoside (naringin) (Table 2, Fig. 3). Its content was 2.9 times higher in Artemisia ludoviciana compared toArtemisia campestris. Regarding flavonoid fisetin, its amount was 4.6 times higher in the Artemisia ludoviciana herb.
Table 2: The amounts of flavonoids in the studied Artemisia herbs evaluated by the HPLC method
|
Flavonoid |
Retention time, min |
Content, mg/g |
|
|
Artemisia campestris |
Artemisia ludoviciana |
||
|
Rutin |
22.6 |
1.355 |
1.295 |
|
Quercetin-3-b-glycoside |
23.6 |
0.109 |
0.080 |
|
Kaempferol-3-b-glucoside |
25.2 |
0.181 |
5.119 |
|
Flavanone-7-O-glycoside |
26.1 |
7.525 |
21.924 |
|
Fisetin |
27.7 |
2.933 |
13.068 |
|
Quercetin |
33.3 |
0.296 |
Not detected |
|
Luteolin |
36.9 |
0.404 |
0.101 |
|
Naringenin |
38.1 |
Not detected |
0.304 |
|
Apigenin |
38.7 |
Not detected |
0.739 |
|
Baicalein |
39.4 |
0.266 |
Not detected |
|
Kaempferol |
47.3 |
0.718 |
0.148 |
As can be seen from Table 2, the Artemisia ludoviciana herbaccumulates apigenin and naringenin, while Artemisia campestris does notcontain these flavonoids at all. Regarding quercetin and baicalein on the contrary, in Artemisia campestris herb, some amounts of these flavonoids. The studied species differ quite significantly in terms of kaempferol-3-b-glucoside content, since in Artemisia ludoviciana herb it was detected 28.3 times more than in Artemisia campestris raw material. As for the content of rutin, it was found at the same level in the herbs of both species.
 |
Figure 3: HPLC chromatograms of flavonoids detected in the Artemisia campestris (a) and Artemisia ludoviciana (b) herbs: |
Naringin (Fig. 4), the common predominant flavonoid compound in the herbs of both studied species, is one of the main polyphenols in grapefruits, giving them a bitter taste. Naringin is known for its anti-inflammatory, antioxidant, and antitumor properties26. Fisetin possesses an antioxidant effect and positively influences the brain cortex cells, improving cognitive functions27. Rutin possesses antioxidant, anti-inflammatory and capillary-strengthening properties28.
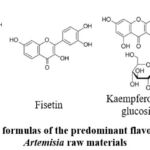 |
Figure 4: Structural formulas of the predominant flavonoids in the studied |
Recently, it was revealed that the Artemisia campestris extract with high total phenolic content, demonstrated antioxidant activity as well as an anti-hyperglycemic effect via the inhibiting of α-amylase and α-glucosidase29.
As it is known, significant differences in the chemical composition of raw material collected from the same species can exist due to geographical distribution, varying climatic conditions during the year of harvest, or the presence of genetic variations. Thus, Anaya-Eugenio and co-authors30 identified an O-methylated flavone eupatilin in the herb Artemisia ludoviciana collected in Mexico. Eupatilin and sesquiterpene lactone estafiatin were isolated from the Artemisia ludoviciana subsp. mexicanaaerial part demonstrated the anti-Helicobacter pylori activity31. Bioactive flavons eupatilin and dimethoxycentaureidin were revealed in the Artemisia campestris leaves collected in Tunis14. Eupatilin and 3-O-methylquercetin prevailed in the 80% aqueous methanol extracts of Artemisia campestris and Artemisia herba-alba collected in Algeria32.
There is no doubt that the choice of solvent and extraction method substantially impacts the extraction level of polyphenols from plant raw materials. Thus, it was found33 that methanolic extract from the leaves of Tunisian Artemisia campestris subjected to maceration under shaking conditions exhibited a higher content of polyphenols and antioxidant effects compared to the hexane extract. The methanolic extracts of aerial part of some endemic Artemisia representatives from Central Asia were the most promising source of polyphenolic compounds34. Similarly to our findings, 70% aqueous methanol extract from Artemisia argyi stems prepared using ultrasonic waves was considered optimal for obtaining high content of flavonoids35.
Conclusions
The HPLC method was utilized to analyze the chromatographic profiles and quantitative content of flavonoids and phenolic acids in the Artemisia ludoviciana and Artemisia campestris herbs cultivated in Ukraine. The study revealed the presence of 10 phenolic acids, with the dominant compounds being hydroxycinnamic chlorogenic acid (14.503 mg/g in Artemisia ludoviciana and 4.504 mg/g in Artemisia campestris). Additionally, 11 flavonoid compounds were discovered, with the highest content observed for flavanone-7-O-glycoside (naringin) in both species. The predominant flavonoids in the herb of Artemisia ludoviciana decreased in the order of flavanone-7-O-glycoside (21.924 mg/g) > fisetin (13.068 mg/g) > kaempferol-3-b-glucoside (5.119 mg/g) > rutin (1.295 mg/g), while in the raw material of Artemisia campestris the following decline was observed: flavanone-7-O-glycoside (7.525 mg/g) > fisetin (2.933 mg/g) > rutin (1.355 mg/g). The major polyphenolic compounds revealed possess significant therapeutic potential, and the data obtained could be considered for further research into their biological activities.
Acknowledgment
The article was prepared with the active participation of researchers in the international network AGROBIONET, as a part of the international program “Agricultural biodiversity to improve nutrition, health and quality of life” within the project MVTS-SR/UA-6/14 «The use of lesser-known and little-used plant species to improve nutrition, health and quality of life». Experimental work was conducted in the laboratories of the Institute of Plant and Environmental Sciences at the Faculty of Agrobiology and Food Resources, Slovak Agricultural University in Nitra.
Conflict of interest
The authors declare no conflict of interest
Funding Source
The authors are thankful for financial support from “The National Scholarship Programme of the Slovak Republic” (SAIA). Mariia Shanaida expresses her gratitude to the SAIA Agency for the financial support of the research stay (ID N 47777), during which experiments were carried out.
References
- Tanvir R, Guo L, Wu H, Li L. Special issue: Manipulation/regulation of secondary metabolites in medicinal plants. Plant Physiol Biochem. 2024;211:108549.
CrossRef - Fitzgerald M, Heinrich M, Booker A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front Pharmacol. 2020;10:1480.
CrossRef - El Oirdi M. Harnessing the Power of Polyphenols: A New Frontier in Disease Prevention and Therapy. Pharmaceuticals. 2024; 17(6): 692.
CrossRef - Hudz N, Yezerska O, Shanaida M, Horčinová Sedláčková V, Wieczorek PP. Application of the Folin-Ciocalteu method to the evaluation of Salvia sclarea extracts. Pharmacia. 2019;66(4):209–215.
CrossRef - Marzullo L, Ochkur O, Orlandini S, Renai L, Gotti R, Koshovyi O, Furlanetto S, Del Bubba M. Quality by Design in optimizing the extraction of (poly)phenolic compounds from Vaccinium myrtillus berries. J Chromatogr A. 2022;1677:463329.
CrossRef - Ekiert H, Klimek-Szczykutowicz M, Rzepiela A, Klin P, Szopa A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules. 2022;27(19):6427.
CrossRef - Ali M, Abbasi BH, Ahmad N, Khan H, Ali GS. Strategies to enhance biologically active-secondary metabolites in cell cultures of Artemisia – current trends. Crit Rev Biotechnol. 2017;37(7):833–851.
CrossRef - European Pharmacopoeia: Supplement 6.4. Council of Europe. 2008.
- Kamarauskaite J, Baniene R, Raudone L, Vilkickyte G, Vainoriene R, Motiekaityte V, Trumbeckaite S. Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antioxidants (Basel). 2021;10(9):1405.
CrossRef - Sharifi-Rad J, Herrera-Bravo J, Semwal P, Painuli S, Badoni H, Ezzat SM, Farid MM, Merghany RM, Aborehab NM, Salem MA, Sen S, Acharya K, Lapava N, Martorell M, Tynybekov B, Calina D, Cho WC. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxid Med Cell Longev. 2022:5628601.
CrossRef - Dib I, El Alaoui-Faris FE. Artemisia campestris L.: review on taxonomical aspects, cytogeography, biological activities and bioactive compounds. Biomed Pharmacother. 2019;109:1884–1906.
CrossRef - Rivero-Cruz I, Anaya-Eugenio G, Pérez-Vásquez A, Martinez AL, Mata R. Analysis and Pharmacological Effects of Artemisia ludoviciana Aqueous Extract and Compounds. Natural Product Communications. 2017; 12(10):1531–1534.
CrossRef - Marghich M, Amrani O, Karim A, Harit T, Beyi L, Mekhfi H, Bnouham M, Aziz M. Myorelaxant and antispasmodic effects of the essential oil of Artemisia campestris L., and the molecular docking of its major constituents with the muscarinic receptor and the L-type voltage-gated Ca2+channel. J Ethnopharmacol. 2023;311:116456.
CrossRef - Metoui R, Bouajila J, Znati M, Cazaux S, Neffati M, Akrout A. Bioactive flavones isolated from Tunisian Artemisia campestris L. Leaves. Cell Mol Biol (Noisy-le-grand). 2017; 63(11): 86–91.
CrossRef - Korablova O, Levchuk I, Rakhmetov D, Palamar V, Sydor B, Shanaida M. Comparative analysis of essential oils from three species of the genus Artemisia cultivated in Ukraine. Botanica. 2023;29(2):41–49.
CrossRef - Woźniak D, Nawrot-Hadzik I, Kozłowska W, Ślusarczyk S, Matkowski A. Caffeoylquinic Acids. In: Xiao, J., Sarker, S.D., Asakawa, Y. (eds) Handbook of Dietary Phytochemicals. Springer, Singapore. 2021: 1065–1104.
CrossRef - Singh S, Varshney M. Exploring the Pharmacological Potential of Chlorogenic acid as an Anti-Cancer Agent and a Call for Advance Research. Comb Chem High Throughput Screen. 2024; June 20. Advance online publication.
CrossRef - Bakchiche B, Gherib A, Bronze MR, Ghareeb MA. Identification, Quantification, and Antioxidant Activity of Hydroalcoholic Extract of Artemisia campestris from Algeria. Turk J Pharm Sci. 2019;16(2):234–239.
CrossRef - Ochkur OV. Pharmacognostic research of the Artemisia L. genus species of Ukrainian flora. Thesis for the degree of candidate of pharmaceutical sciences, Kharkiv, 2014. 25 p. [in Ukrainian].
- Trifan A, Zengin G, Sinan KI, Sieniawska E, Sawicki R, Maciejewska-Turska M, Skalikca-Woźniak K, Luca SV. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants (Basel). 2022;11(5):1017.
CrossRef - Ickovski JD, Arsić BB, Mitić MN, Stojković MB, Đorđević MM, Stojanović GS. Chemometric Approach to the Composition of Flavonoid Compounds and Phenolic Acids and Antioxidant Potential of Artemisia Species from Different Habitats. Chem Biodivers. 2022;19(12):e202200365.
CrossRef - Guan H, Luo W, Bao B, Cao Y, Cheng F, Yu S, Fan Q, Zhang L, Wu Q, Shan MA. Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules. 2022;27(10):3292.
CrossRef - Shanaida M, Palamar O, Holembiovska O. Chromatographic Profile of Polyphenols in the Agastache foeniculum (Pursh) Kuntze Herb: Evaluation of Optimal Extraction Efficiency. Boimedical and Pharmacology Journal. 2024;17(1):63–69.
CrossRef - Stanković M, Ickovski JD, Ljupković RB, Stojanović GS. The effects of Artemisia methanol extracts and ferulic acid, rutin, rosmarinic acid, and quercetin on micronucleus distribution on human lymphocytes. Nat Prod Res. 2022;36(17):4536–4539.
CrossRef - Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, Lapkin AA. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract – possible synergistic and resistance mechanisms. PloS one. 2013;8(11):e80790.
CrossRef - Azmy AM, Abd Elbaki BT, Ali MA, Mahmoud AA. Effect of ozone versus naringin on testicular injury in experimentally induced ulcerative colitis in adult male albino rats. Ultrastruct Pathol. 2022;46(5):439–461.
CrossRef - Singh S, Garg G, Singh AK, Bissoyi A, Rizvi SI. Fisetin, a potential caloric restriction mimetic, attenuates senescence biomarkers in rat erythrocytes. Biochem Cell Biol. 2019;97(4):480–487.
CrossRef - Negahdari R, Bohlouli S, Sharifi S, Maleki Dizaj S, Rahbar Saadat Y, Khezri K, Jafari S, Ahmadian E, Gorbani Jahandizi N, Raeesi S. Therapeutic benefits of rutin and its nanoformulations. Phytother Res. 2021;35(4):1719–1738.
CrossRef - Marghich M, Daoudi NE, Amrani O, Addi M, Hano C, Chen JT, Mekhfi H, Ziyyat A, Bnouham M, Aziz M. Antioxidant Activity and Inhibition of Carbohydrate Digestive Enzymes Activities of Artemisia campestris L. Front Biosci. 2022;14(4):25.
CrossRef - Anaya-Eugenio GD, Rivero-Cruz I, Rivera-Chávez J, Mata R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J Ethnopharmacol. 2014;155(1):416–425.
CrossRef - Palacios-Espinosa JF, Núñez-Aragón PN, Gomez-Chang E, Linares E, Bye R, Romero I. Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin. Molecules. 2021;26(12):3654.
CrossRef - Bakchiche B, Gören AC, Aydoğmuş Z, Mataracikara E, Ghareeb M. Artemisia campestris and Artemisia herbaalba: LC-HRESI-MS profile alongside their antioxidant and antimicrobial evaluation. Acta Pharmaceutica Sciencia. 2022;60(2):131–152.
CrossRef - Limam I, Ghali R, Abdelkarim M, Ouni A, Araoud M, Abdelkarim M, Hedhili A, Ben-Aissa Fennira F. Tunisian Artemisia campestris L.: a potential therapeutic agent against myeloma – phytochemical and pharmacological insights. Plant Methods. 2024;20(1):59.
CrossRef - Nurlybekova A, Kudaibergen A, Kazymbetova A, Amangeld M, Baiseitova A, Ospanov M, Aisa H. A., Ye, Y., Ibrahim, M. A., & Jenis, J.. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules. 2022;27(16):5128.
CrossRef - Wang Z, Wang L, Huang H, Li Q, Wang X, Sun Q, Wang Q, Li N. In vitro antioxidant analysis of flavonoids extracted from Artemisia argyi stem and their anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chemistry. 2023;407:135198.
CrossRef








