Rachana R* , Harshit Devtalla
, Harshit Devtalla , Arushi Agrawal
, Arushi Agrawal , Medha Agarwal
, Medha Agarwal and Shreya Kadyan
and Shreya Kadyan
Department of Biotechnology, JIIT Noida, A-10, Noida, UP, India.
Corresponding Author E-mail: rachana.dr@iitbombay.org
DOI : https://dx.doi.org/10.13005/bpj/3004
Abstract
Acute Respiratory Distress Syndrome (ARDS) is a dangerous lung condition characterised by non-cardiogenic pulmonary edoema caused by various factors, including inflammation and hypoxia. It is a more severe and evolved form of Acute Lung Injury (ALI) and requires the patient to be on mechanical ventilation for survival. Several medicinal plants, herbs, oils, and natural extracts have been studied for their anti-inflammatory properties and their targeted action on respiratory disorders. The target of the current study is to elaborate on the target-specific action of bioactive compounds from natural products by Molecular Docking and study their drug-likeness along with their other important pharmacokinetic properties. Bioactive compounds (total 71) from Zingiber officinale (ginger), Trifolium pratense (red clover), Curcuma longa (turmeric), Melaleuca alternifolia (tea tree), Ocimum tenuiflorum (Tulsi), Chlorophytum borivilianum (Safed Musli), Cinnamomum cassia (cinnamon), Elettaria cardamomum (cardamom), and Glycine max (soybean) were selected to be investigated and were screened against RhoA and VEGFR1. The ADMET properties and drug-likeness of the bioactive compounds were studied using Molinspiration and ADMETlab 2.0. Docking studies revealed that Hecogenin (-8.4 and -10.3 kcal/mol), Neotigogenin (-7.7 and -9.8 kcal/mol), and Neohecogenin (-7.6 and -9.7 kcal/mol) produced the best docking results, showing the lowest binding energies for RhoA and VEGFR1, respectively. These energies were found to be comparable to the standard ligands Fasudil (-7.3 kcal/mol for RhoA) and Pazopanib (-8.0 kcal/mol for VEGFR1) for the selected targets. Moreover, Stigmasterol (-7.6 kcal/mol) and Genistein (-8.4 kcal/mol) showed a good binding affinity with RhoA and VEGFR1, respectively. The ADME properties of these molecules were also studied. Thus, the best-docked ligands mentioned above can be used as potential novel compounds against these two targets to develop therapeutics against ARDS. Further in-vitro and in-vivo experiments are required to cement these claims and prepare next-generation natural therapeutics for ARDS.
Keywords
ARDS; Inflammation; Molecular Docking; Ligands; Targets; VEGFR1; RhoA
Download this article as:| Copy the following to cite this article: Rachana R, Devtalla H, Agrawal A, Agarwal M, Kadyan S. Bioactive Compounds from Natural Products as RHOA/ROCK and VEGFR1 Inhibitors: An In-silico Approach for Developing Therapeutics for ALI/ARDS. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Rachana R, Devtalla H, Agrawal A, Agarwal M, Kadyan S. Bioactive Compounds from Natural Products as RHOA/ROCK and VEGFR1 Inhibitors: An In-silico Approach for Developing Therapeutics for ALI/ARDS. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/47FHCnn |
Introduction
Laennec first used the term “idiopathic pulmonary oedema” to define ARDS in 1821. Since Ashbaugh and his colleagues were the first to use the term “ARDS” in 1967, its definition has changed over time to include more symptoms that help us understand the disease.1 Since then, controversies in defining ARDS have ranged from the “Murray Lung Injury Score” to the “The American-European Consensus (AECC)” definition to the “The Berlin definition”. While the Murray Lung Injury Score from 1988 incorporates the ‘scoring’ of hypoxemia, respiratory system compliance, chest radiographic findings, and level of end-expiratory positive pressure, the total score for the presence of ARDS in a patient must be above 2.5. 1, 2 The AECC definition from 1994 defines ARDS as having (i) an acute and sudden onset of respiratory distress; (ii) a chest X-ray with bilateral infiltrates; (iii) the presence of non-elevated left atrial pressure; and (iv) severe hypoxemia ≤ 200 mm Hg based on the PaO2/FiO2 ratio (regardless of PEEP). 3, 4 The 2012 Berlin definition considers patients suffering from ARDS under the following criteria: (i) the beginning of disease within one week of respiratory symptoms; (ii) acute respiratory failure along with unclear morbidities, including heart failure or oedema; (iii) a chest X-ray or CT scan result showing bilateral infiltrates and consistent pulmonary oedema; and (iv) severity defined according to oxygenation.3, 5
ARDS is a potentially fatal lung condition characterised by fluid accumulation in the alveoli (alveolar oedema), which results in decreased oxygen transfer to the organs (hypoxia) and affects their normal functioning. It used to be called “Adult Respiratory Distress Syndrome,” but it was changed to “Acute Respiratory Distress Syndrome” when it was seen in both children and adults. ARDS can be caused by polymorphisms in more than one gene, severe pneumonia, sepsis, inhaling dangerous substances (inhalation injury), or taking too much of a drug. 6 ARDS has a 30–40% mortality rate, with a mortality rate of 24% in patients aged 15–19 and 60% in patients aged 80 or more. Even though scientists have done a number of clinical studies, neither ventilatory support nor drug interventions have led to any improvements. Therefore, supportive therapies and treatment remain the primary approach for patients. Treatment is divided into pharmacologic and non-pharmacologic strategies to minimize the risk of harm from ventilation and gas exchange.7, 8 Drug treatments such as inhaled vasodilators and corticosteroids are considered the best treatments.
On the other hand, some non-drug strategies include choosing PEEP, setting the tidal volume, and lying down. 9 Many molecular mechanisms have also been discovered, which have significantly increased our understanding of the causes of ARDS. However, these mechanisms have not yet become ARDS treatments. 10 New therapeutic opportunities, such as gene therapy, mesenchymal stem cell therapy, lung ultrasound, specific and sensitive biomarkers, and the recent development of RALE (Radiographic Assessment of Lung Edema) are expected to improve the outcomes of ARDS in the future. 11
Pathogenesis and Targets
So far, studies have focused on finding risk factors linked to genes that code for cellular defense, vascular permeability, alveolar integrity, cell development and growth, coagulation, and oxidative stress. 12,13,14 Even though candidate gene association has been questioned in the past due to its difficult reproducibility and understanding, it has helped find a few genes that are linked to ARDS susceptibility or outcome. 15,16 Researchers have looked at many genes, including interleukins (IL-6 and IL-10), interleukin-1 receptor antagonist (IL-1RN), VEGFA, angiotensin-converting enzyme (ACE), soluble mannose-binding lectin 2 (MBL2), and visfatin (NAMPT). 14 As of 2015, 68 different case studies on people of European descent had linked a total of 81 candidate genes to ARDS susceptibility or outcomes. 14
VEGF Receptor 1 as a Therapeutic Target
Several preclinical studies have shown that the VEGF pathway is used for progressive signaling in ARDS. 17 Therefore, it can be assumed that VEGF signaling plays a role in the pathogenesis of ARDS. VEGF receptors are primarily expressed in cells, including alveolar type II epithelial cells, respiratory epithelial cells, and activated alveolar macrophages.18 Under normal conditions, VEGF promotes the formation and proliferation of vascular endothelial cells. However, it can increase vascular permeability during pathogenic conditions and cause inflammation. During the first GWAS of sepsis-associated ARDS, Beatriz et al. (2020) looked at people with European ancestry (n = 1935) and found a common variant (rs9508032) of the FLT1 gene (which codes for VEGFR1) that makes people less likely to get sepsis-associated ARDS. 19 Previously, the variation was neither associated with the susceptibility nor the outcome of ARDS. Even though a link between the FLT1 gene and pulmonary complications has been found,20 Hernadez-Pacheco and colleagues (2018) did a different study. Using information from rat models and ICU patients, an integrative multi-omics analysis was done, and a risk variant in the FLT1 gene was found. 21 They concluded that altered levels of VEGF receptors can either protect against or increase the severity of ARDS. In ARDS patients, having the right amount of VEGF receptors can protect the lungs from damage by reducing VEGF activity and vascular permeability. 21,22 Patients on ventilators with high VEGF receptor levels are more likely to get sepsis, alveolar abnormalities, cytokine storms, and eventually die. 23,24 Therefore, it can be concluded that the novel locus present in the FLT1 gene may serve as a potential diagnostic biomarker and VEGFR1 as a therapeutic marker in the diagnosis of ARDS.25
RhoA/ROCK as a Therapeutic Target
RhoA/ROCK cascade activation is one of the primary events that leads to the rigorous inflammatory response in the type 1 pneumocytes and vascular endothelium, causing lung injury and eventually resulting in the life-threatening condition of ARDS. 26 Rho-kinase activity is responsible for the increased expression of several pro-inflammatory cytokines, including IL-8, IL-1β, IL-6, and a transcription factor called NF-κB as discussed earlier. These pro-inflammatory cytokines are responsible for alleviating lung injury and respiratory distress. It has been observed that inhibition of RhoA eventually leads to reduced expression of these cytokines and further decreases the risk of vascular injury.
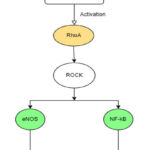 |
Figure 1: TNF-α mediated activation of RhoA |
Using a mouse model, the study found differential expression of IL-6 when treated with the standard Rho-kinase inhibitor Y-27632, which reduced inflammation, evidencing the above statement. 27 Another study found TNF-α and IL-1β to be involved in the inflammatory action in ventilator-induced ARDS, with Rho-kinase activity being the triggering factor. It was observed that the microvesicles shed from pulmonary cells during the disease progression were rich in TNF-α and IL-1β. The increased activity of Rho-kinase was the primary mediating factor. 28 Another study employed a standard Rho-A inhibitor, Fasudil, in studying the differential inflammatory response. Using a mouse as a model, researchers looked at how Fasudil was expressed in lung tissue. The results were confirmed using QT-PCR and western blotting. A significant decrease was observed in the inflammatory activity in the lung tissue when treated with the inhibitor.29
Another cytokine, TGF-β1, is known to exhibit increased expression in ALI. A study involving a Rho-A inhibitor has shown differential expression of TGF-β1, employing three in-vitro models. 30 These studies are essential to reinforce the hypothesis that Rho-kinase inhibitors have a significant regulatory immunomodulatory potential. These inhibitors can be further employed to curb inflammation and other comorbidities.
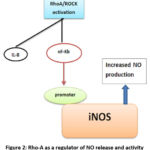 |
Figure 2: Rho-A as a regulator of NO release and activity |
Materials and Methods
Selection of Receptors and Ligands
The role of natural herbs in treating respiratory diseases has been well illustrated and deeply studied. The anti-inflammatory, anti-carcinogenic, and antioxidant properties of these herbs have been well exploited since medieval times. These properties can be attributed to several bioactive compounds present in these plants and their products. These compounds can act as promoters, inhibitors, or nutraceuticals, depending on their bioavailability and rate of absorption in the human system. Consumption of plant products, herbal extracts, and compounds extracted from plants has been known to exhibit therapeutic effects on affected tissues. This knowledge has been part of several ancient medical regimens worldwide. Moreover, these compounds have also been studied in-vitro and in-vivo for their pharmacological properties.
In this study, the authors have compiled a list of bioactive compounds from herbs that have been well explored for their anti-inflammatory effects. These herbs were selected from a vast research literature and database of pharmacological attributes. A library of compounds from these herbs was created, and their physical properties were examined. Compounds with a molecular weight of less than 500 were chosen. This is because compounds with a molecular weight of more than 500 can’t pass through the blood-brain barrier (BBB). The 3-D structures of the herbs that met these criteria were downloaded from the PubChem database (https://pubchem.ncbi. nlm.nih.gov/). All the downloaded structures were prepared using PyMol and further saved in .pdb format. These compounds served as ligands for future molecular docking experiments. Also, the 3D structures of FDA-approved inhibitors for the chosen targets were downloaded and used as a standard for the docking experiment. Also, the 3D structures of FDA-approved inhibitors for the selected targets were downloaded and used as a standard for the docking experiment.
3D structures of selected targets (RhoA and VEGFR1) were also downloaded (PDB id: 1A2B and 3HNG) from the PDB (Protein Data Bank) (https://www.rcsb.org/). These targets were selected via a thorough literature search and analysis of several databases, including KEGG and Reactome. The pathways primarily affected by the disorder were selected for target preparation. The intermediate molecules, promoters, co-factors, competitive inhibitors, and terminators were all analyzed to select the most suitable target. The selected targets were further checked for research status, including in-vitro, in-vivo, and clinical trials. These targets were not well explored and therefore selected for this study.
Preparation of Receptor and Ligand
PyMol was used to get the structure of the target protein ready for molecular docking by getting rid of water molecules, adding hydrogen, and making the structure neutral. Further, the prepared receptor was subjected to molecular docking using AutoDock Vina v1.1.2. The screened compounds were chosen as test ligands for the experiment, and a docking procedure was performed to understand the receptor-ligand interaction. A total of 71 compounds from anti-inflammatory herbs were used as ligands in this study.
Molecular Docking
The CASTp 3.0 (Computes Atlas of Surface Topography of Proteins) server (http://sts.bioe.uic.edu/castp/) was used to find the active sites on the target proteins. This analysis provided the most suitable position for the most rigid target-drug interaction throughout the target protein.31 The prepared receptor was selected as the macromolecule for the docking study. All the selected compounds were docked against the target protein to evaluate their binding energies using AutoDock Vina.32 The grid dimensions were set along the lines of the CASTp evaluation. Docking was performed on all of the test ligands, anti-diabetic drugs, and kinase inhibitors with a default exhaustiveness of 8. The binding energies were tabulated, and ligands exhibiting good binding affinity with the target were selected to evaluate detailed interactions with protein residues and ADMET analysis.
Drug-likeness Analysis and ADMET Evaluation of the Selected Phytocompounds
The drug-likeness attributes of the bioactive compounds showing good binding affinity with the target were studied using Molinspiration, which is an open-access web-based tool used for screening compounds based on several standard criteria (https://www.molinspiration.com/). Furthermore, the pharmacokinetic properties of the selected compounds were evaluated using ADMETlab 2.0 (https://admetmesh.scbdd.com/service/evaluation /index).
It calculates the drug-likeness of the compounds across the parameters of physicochemical properties, toxicity, medicinal chemistry, absorption, distribution, metabolism, and excretion.
Furthermore, two-dimensional visualization of the interaction between the target and the selected ligands was done using BIOVIA’s Discovery Studio Visualizer. This step would provide detailed insight into the interactions between the amino acid residues and the ligand sub-particles, with hydrogen bond interactions being the most preferred kind.
Results
Molecular Docking Results
The protein molecule was selected as the macromolecule, and the compounds were selected as ligands for the docking study. Firstly, both molecules were prepared for the experiment, and then the grid dimensions were set. The grid dimensions were set in accordance with the CASTp evaluations, and a config file was prepared to mention all the details as obtained in the grid box, as well as the energy range and exhaustiveness. Following that, a command for the docking process to start was executed, and results were available in 30–60 seconds.
Table 1: Molecular docking results showing the binding energies of best-docked compounds using AutoDock Vina
|
S.No. |
COMPOUNDS |
Binding energy (kcal/mol) |
|
|
RhoA (1A2B) |
VEGFR1 (3HNG) |
||
|
1 |
Stigmasterol |
-7.6 |
-7.6 |
|
2 |
Beta-Sitosterol |
-7.3 |
-7.2 |
|
3 |
Hecogenin |
-8.4 |
-10.3 |
|
4 |
Genistein |
-6.0 |
-8.4 |
|
5 |
9,10-Anthracenedione |
-7.1 |
-9.0 |
|
6 |
Neotigogenin |
-7.7 |
-9.8 |
|
7 |
Quercetin |
-7.0 |
-7.0 |
|
8 |
Apigenin |
-7.2 |
-7.9 |
|
9 |
Equol |
-6.6 |
-8.0 |
|
10 |
Neohecogenin |
-7.6 |
-9.7 |
|
11 |
Tokorogenin |
-7.5 |
-9.2 |
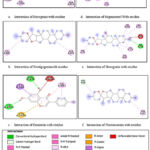 |
Figure 3: 2D protein-ligand interactions of best docked compounds using Biovia Discovery Studio (Figure 3a,3b and3c: Interaction of given compound with VEGFR1. Figure 3d,3e and 3f: Interaction |
Drug-Likeness and ADMET Properties of the Selected Phytocompounds
Binding energy provides an overview of the interaction between the macromolecule and the ligand. But there are several other criteria to evaluate the drug-likeness of any compound and further evaluate its potential in-vitro, in-vivo and in clinical trials. In this study, Molinspiration, a web-based platform to calculate molecular properties and bioactivity scores, was employed for different scoring criteria, including the number of hydrogen bond acceptors and donors (nON and nOHNH), number of atoms, partition coefficient (miLog P), number of violations to the Lipinski rule of five, and molecular volume. All the Lipinski parameters were calculated for the screened compounds and tabulated.
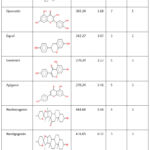 |
Table 2: Evaluation of drug-likeness of the best docked compounds using Molinspiration. |
The majority of the compounds chosen met the Lipinski rule of five. For a compound to be therapeutic, the rule states that its molecular mass must be less than 500 Da, it must have less than five hydrogen bond donors, less than ten hydrogen bond acceptors, and that the octanol-water coefficient must be less than five. Violation of more than two rules is not desirable for a therapeutic. Stigmasterol, Genistein, Apigenin, Tokorogenin, Hecogenin and Neotigogenin were the compounds with the best drug-likeness according to this criteria.
Furthermore, pharmacokinetic evaluation of the selected bioactive compounds was done using ADMETLAB 2.0. This web-based platform gives a detailed evaluation of the compounds based on all the ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) criteria. Absorption is the most important part of a compound’s bioavailability. A drug can be given in many ways, and its target areas can be reached through systemic circulation. So, the first step in pharmacokinetic evaluation is to study the parameters of how compounds are absorbed. Caco-2 and MDCK permeability, PGP interactions, HIA, and F30% scores provide a detailed account of compounds’ initial oral bioavailability and cellular surface adsorption.
Table 3: Pharmacokinetic scoring of selected bioactive compounds on their absorption parameters using ADMETlab 2.0
|
Phytocompounds |
Caco-2 permeability |
MDCK permeability (cm/s) |
PGP- inhibitor |
Pgp- substrate |
HIA |
F30% |
|
Stigmasterol |
High |
High |
High |
High |
High |
High |
|
Quercetin |
Low |
High |
High |
High |
High |
Low |
|
Equol |
High |
High |
High |
High |
High |
Low |
|
Genistein |
High |
High |
High |
Moderate |
High |
Low |
|
Apigenin |
High |
High |
High |
Low |
High |
Low |
|
Neohecogenin |
High |
High |
Low |
High |
High |
Moderate |
|
Neotigogenin |
High |
High |
Moderate |
High |
High |
Moderate |
|
9,10-Anthracenedione |
High |
High |
High |
High |
Low |
Low |
|
Hecogenin |
High |
High |
Low |
High |
High |
Moderate |
|
Tokorogenin |
High |
High |
High |
High |
High |
Moderate |
|
Beta-sitosterol |
High |
High |
Moderate |
High |
High |
High |
The drug’s distribution and metabolism in the body are important pharmacokinetic criteria for understanding how it works and how the interactions between ligands and the target moiety lead to the formation of new complexes. The drug’s ability to pass through the blood-brain barrier (BBB) and how well it binds to plasma proteins (PPB) are important to its distribution. The interaction with CYP isoforms also shows the rate of activation and deactivation, which is important for how the drug in question is broken down. Rapid metabolism and inactivation show a higher degree of drug activity in the host system.
Table 4: Pharmacokinetic scoring of selected bioactive compounds on their Distribution and Metabolism parameters using ADMETlab 2.0
|
Phytocompounds |
PPB (%) |
VD (L/kg) |
BBB penetration |
CYP inhibitors |
CYP Substrates |
|
Stigmasterol |
Low |
High |
High |
– |
2C19, 2D6 |
|
Quercetin |
Low |
High |
High |
1A2 |
– |
|
Equol |
Low |
High |
High |
1A2, 2C19, 2C9, 2D6 |
1A2, 2C9, 2D6 |
|
Genistein |
Low |
High |
High |
1A2, 2D6, 3A4 |
2C9, 2D6 |
|
Apigenin |
Low |
High |
High |
1A2, 2D6, 3A4 |
2C9, 2D6 |
|
Neohecogenin |
Low |
High |
Low |
– |
2C19, 2D6 |
|
Neotigogenin |
Low |
High |
Low |
– |
2C19, 2D6 |
|
Anthracenedione |
Low |
High |
High |
1A2 |
2C9 |
|
Hecogenin |
Low |
High |
Low |
– |
2D6, 2C19 |
|
Tokorogenin |
Low |
High |
Moderate |
– |
2D6, 2C19 |
|
Beta-Sitosterol |
Low |
High |
High |
– |
2C19, 2D6 |
Table 5: Pharmacokinetic scoring of selected bioactive compounds on their Excretion and Toxicity parameters using ADMETlab 2.0
|
Phytocompounds |
Clearance (mL/min/ kg) |
T1/2 |
hERG blockers |
H-HT |
Carcinogenicity |
Respiratory Toxicity |
|
Stigmasterol |
High |
High |
High |
High |
High |
High |
|
Quercetin |
High |
Long |
High |
High |
High |
High |
|
Equol |
High |
Long |
High |
High |
Low |
High |
|
Genistein |
High |
Long |
High |
High |
Moderate |
High |
|
Apigenin |
High |
Long |
High |
High |
High |
High |
|
Neohecogenin |
High |
Long |
High |
Moderate |
High |
Low |
|
Neotigogenin |
High |
Long |
High |
High |
High |
Moderate |
|
Anthracenedione |
High |
Long |
High |
High |
Low |
Low |
|
Hecogenin |
High |
Long |
High |
High |
High |
Low |
|
Tokorogenin |
High |
Long |
High |
High |
High |
Low |
|
Beta-sitosterol |
High |
High |
High |
High |
High |
High |
The analysis of all the ADMET properties (Absorption, Distribution, Metabolism, Excretion and Toxicity) put forward the pharmacokinetic front of the study. Stigmasterol, Hecogenin and Neotigogenin were the compounds with the best ADMET results and were considered for further molecular evaluation. These results also show that these molecules have excellent pharmacological potential and could be used as potential ligands in any other drug development study for their respective targets.
Interaction of Selected Phytochemicals with Target Proteins
Studying the interaction of ligand molecules with amino acid moieties present on the active site of the protein structure gives us insight into the nature and strength of the interaction between them. For studying the inhibitory nature of these phytochemicals, we need to measure their binding affinity with the target along with the probable interaction. These parameters are comparable to the natural ligands in the human system and the pre-existing standard inhibitors. If the research yields positive results, i.e., if the compound exhibits good binding and the nature of the interaction is strong and favorable, such compounds are selected for further research and drug development procedures.
The nature of interactions between ligands and targets was studied using a 2D model in Biovia Discovery Studio. There can be a number of interactions between these two moieties, including hydrogen bonds, ionic interactions, and C-H bonds, which are considered important for the purpose of drug discovery. Table 6 shows the results of the 2D analysis of the interactions obtained using Discovery Studio.
Table 6: Amino acid residues interacting with ligands; visualised using Biovia Discovery Studio
|
Target |
Compound |
Interacting AA residues |
No. of H-bonds |
No. of Other interactions |
|
RhoA |
Hecogenin |
Cys 1018 Arg 1021 Leu 1013 His 1020 Ala 874 |
2 |
3 |
|
Neotigogenin |
Leu 882 Ala 874 |
0 |
2 |
|
|
Genistein |
Glu 878 Asp 1040 Ile 881 Cys 1018 Ile 1038 Leu 882 |
2 |
4 |
|
|
VEGFR1 |
Hecogenin |
Lys 162 Leu121 Ala 161 Ser 160 Lys 118 Cys 20 Tyr 34 |
1 |
6 |
|
Neotigogenin |
Apg 68 Pro 101 |
0 |
2 |
|
|
Stigmasterol |
Ser 160 Asp 120 Ala 161 Lys 162 Lys 118 Cys 20 Ala 15 Tyr 34 |
2 |
6 |
Discussion
Ashbaugh and his colleagues first defined Acute Respiratory Distress Syndrome (ARDS) in 1967 based on a case report they wrote about. The patients included both children and adults who had symptoms such as increased lung stiffness, pulmonary edema, breathing difficulties, and hypoxaemia. Besides these symptoms, doctors also saw signs of sepsis, trauma, pneumonia, and aspiration. 1 In 1992, AECC came up with some criteria for diagnosing ARDS. 4 In 2012, these criteria were updated to become “The Berlin Definition.” 5 Clinical disorders associated with ARDS vary according to geographical location and healthcare systems in developing countries. For 50 years, there has been improvement in understanding the disease, its epidemiology, and its pathophysiology. It provides researchers with the knowledge to improve the treatment of the disease. Also, the treatment of ARDS has gotten better because of the many randomized trials that have been done to reduce the use of mechanical ventilation and fluid therapy.33
There are still challenges with screening ARDS patients because there is no strong evidence or agreement. Also, the disease only happens in a small number of people who have risk factors, which makes screening hard. 34 Moreover, the development of the disease is quickly leading to patients with such severity getting hospitalized within 12–14 hours. Therefore, clinical scores have been created to predict the patients at a high risk of developing the disease, such as the Lung Injury Prediction Score (LIPS).35,36,37 LIPS synthesise clinical data such as comorbidities, risk factors, and acute physiological variables. All these data generate a score, where a high score is an indication that the patient is at high risk of developing the disease.38
Another potential predictive instrument for ARDS is biomarkers. A study was conducted by Rubin et al. (1990). He came to the conclusion that VWF, a biomarker for endothelial damage, was high in people with non-pulmonary sepsis who were in the early stages of ARDS.39 In the same way, Ang-2 and IL-8 were also found to be higher during the early development of ARDS. So, finding the ARDS biomarkers has helped a lot with treating the disease in a more personalised way.35
In-silico molecular docking experiments, drug-likeness analysis, and drug-induced gene expression analysis of the selected bioactive compounds provide the computational background for the drug development process. In this study, 71 phytochemicals from a variety of sources were selected and analyzed for their therapeutic potential against sepsis-associated ARDS. Stigmasterol, Genistein, Beta-sitosterol, Apigenin, Hecogenin, Neo-hecogenin, Neotigogenin, 9,10-Anthrocenedione, Tokorogenin, Equol, and Quercetin were among the 11 compounds that demonstrated excellent binding affinity to the targets.
Stigmasterol, a phytosterol derived from soybean oil, demonstrated excellent binding energy with both targets and yielded promising results in protein-ligand interaction studies. The anti-inflammatory potential of stigmasterol has been well studied and documented. It reportedly suppressed the expression of pro-inflammatory cytokines, including TNF-α, IL-6, iNOS, and COX-2, and along with that increased the release of the pro-inflammatory cytokine IL-10 in a collagen- induced arthritic mice model, in a study demonstrating the inhibitory effects of stigmasterol on inflammatory factors and its overall impact on arthritis progression. 40 Another in-silico molecular docking study provided proof that stigmasterol is a down regulator of iNOS expression. iNOS is a crucial regulator of NO levels in the epithelial cells, which regulate the level of ALI (Acute Lung Injury).41 Another study provided evidence for its therapeutic role in cell-mediated lung injury by suppressing the VCAM-1 (vascular cell adhesion molecule-1) levels in ovalbumin induced asthmatic Guinea pigs. VCAM-1 is released by cytokine activated cells and its free circulatory forms lead to increased inflammatory response.42 Also, stigmasterol’s effect on the two targets chosen for this study has never been seen before, so it could act as a potential inhibitor against them.
Hecogenin is another compound that exhibited good binding affinity with the two targets and has been reported to have therapeutic effects on a number of disorders and long-term illnesses. Its anti-inflammatory potential has been demonstrated in a study with arthritic rats, where oral administration of this compound inhibited the production of pro-inflammatory cytokines like TNF-α and IL-12, both of which play important roles in the progression of rheumatoid arthritis and are also associated with alveolar damage that leads to ARDS.43 Furthermore, it has been reported to reduce NO levels in ulcerative colitis-ridden mice in a dose-dependent manner, further solidifying its status as a potential drug candidate for sepsis-associated ARDS.43
Genistein, an isoflavone, and a phytoestrogen made from red clover have also shown good binding energy with the targets, especially VEGFR1. Its anti-inflammatory role is well established. TNF-α-mediated NF-κB activity in epidermal keratinocyte cells is inhibited by genistein in a dose-dependent manner.44 This pathway is responsible for the increased iNOS activity in vascular epithelial cells, further causing alveolar damage, as discussed in previous sections. Genistein has also modulated the production of pro-inflammatory cytokines, including IL-1β and IL-6, to curb VEGF-A-mediated inflammation.45
Y. Q. He et al. (2021) compiled the action of 156 herbal bioactive compounds and tabulated their therapeutic potential. The compilation provided a multi-targeted in-vivo and in-vitro action of these compounds. 46 Interestingly, natural inhibitors for VEGFR1 and RhoA have not been explored yet; this is one of the first studies focusing on in-silico drug development against these two targets. After thorough research, the best-docked compounds and those that pass the pharmacokinetic criteria can be used as novel inhibitors for these two targets.
Conclusion
In this study, 71 bioactive compounds from natural sources were investigated for their activity against VEGFR1 and RhoA. 11 out of the 71 compounds were found to exhibit excellent binding energy against the targets. These compounds include Stigmasterol, Quercetin, Equol, Genistein, Apigenin, Neohecogenin, Neotigogenin, 9,10-Anthracenedione, Hecogenin, Tokorogenin and Beta-sitosterol. Three compounds, in particular, Hecogenin, Neotigogenin and Stigmasterol, showed the best in-silico results for both targets, and Genistein exhibited excellent affinity for VEGFR1. Well-established literature evidence and web-based database platforms have reinforced the hypothesis. Moreover, the herbs that comprise these compounds have been known for their therapeutic benefits. These compounds were then evaluated using a variety of criteria for pharmacokinetic evaluation and drug-likeness. Their interaction with the target moiety was also studied on a molecular level, and all the forces of attraction were well visualised and scored.
This is one of the first studies investigating natural products for these two targets. Best-performing ligands can be further tested in-vitro and in-vivo, and further clinical trials would cement these compounds as novel inhibitors for these targets.
Future Prospects
This study is unique in its focus on developing therapeutics for sepsis-associated disorders, specifically acute respiratory distress syndrome (ARDS), through the exploration of two targets and their mechanisms. These targets have not been extensively studied before, making this study a significant contribution to the field. Additionally, the results of this study are highly promising and warrant further investigation through wet experiments. The most stable ligands identified in this study should be used in the development of natural product-based therapeutics for ARDS.
In-vitro studies on cell lines like A549 and HUVEC would further solidify the potential of these ligands as pharmacological inhibitors for their targets. Different aspects of growing cells, like their viability, expression of target proteins, and NO levels in cells, can be monitored in order to fully cement the functionality of these compounds as potential inhibitors. Also, in-vivo experiments with animal models would help learn more about how these compounds are absorbed and broken down in the host system. Lastly, well-planned clinical trials with these compounds would finally help analyze their activity in the human system and would be the final step in a drug development regimen.
The use of herbal therapeutics and natural compounds in medication strategies has a rich history in various cultures worldwide, and it has become an increasingly popular area of scientific research in recent years. Hence, studies like the one mentioned in this context are vital to understanding the mechanism of action of these compounds as inhibitors. Additionally, the growing scientific interest in this field makes it a promising sector for the future, with potential for job opportunities in the field of herbal therapeutics.
Acknowledgement
The authors acknowledge Jaypee Institute of Information Technology, Noida, for providing the entire infrastructure to complete this project.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article
Conflict of Interests
The authors do not have any conflict of interest.
Data Availability
This statement does not apply to this article
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required
References
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet (London, England). 1967;2(7511):319-323.
CrossRef - Murray JF, Matthay MA, Luce JM, Flick MR. An Expanded Definition of the Adult Respiratory Distress Syndrome. American Review of Respiratory Disease. 1988;138(3):720-723.
CrossRef - Thille AW, Esteban A, Fernández-Segoviano P, Rodriguez JM, Aramburu JA, Peñuelas O, Cortés-Puch I, Cardinal-Fernández P, Lorente JA, Frutos-Vivar F. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med. 2013;187(7):761-767.
CrossRef - Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818-824.
CrossRef - Rubenfeld GD, Slutsky AS, Thompson BT, ARDS Definition Task Force for the. Definition of Acute Respiratory Distress Syndrome—Reply. JAMA. 2012;308(13):1321.
CrossRef - Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573-1582.
CrossRef - Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2021;47(11):1181-1247.
CrossRef - Chiumello D, Brochard L, Marini JJ, Slutsky AS, Mancebo J, Ranieri VM, Thompson BT, Papazian L, Schultz MJ, Amato M, Gattinoni L, Mercat A, Pesenti A, Talmor D, Vincent JL. Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Crit Care. 2017;21(1).
CrossRef - Umbrello M, Formenti P, Bolgiaghi L, Chiumello D. Current Concepts of ARDS: A Narrative Review. International Journal of Molecular Sciences. 2016;18(1):64.
CrossRef - Fanelli V, Montrucchio G, Sales G, Simonetti U, Bonetto C, Filippini C, Mengozzi G, Urbino R, Cappello P, Brazzi L. Immune response profile of SARS-CoV-2 associated AARDS patients during extracorporeal membrane oxygenation. SSRN Electron J. Published online 2020.
CrossRef - Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, Rice TW, Matthay MA, Calfee CS, Ware LB. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73(9):840-846.
CrossRef - Flores C, del Mar Pino-Yanes M, Villar J. A quality assessment of genetic association studies supporting susceptibility and outcome in acute lung injury. Critical Care. 2008;12(5):R130.
CrossRef - Acosta-Herrera M, Pino-Yanes M, Perez-Mendez L, Villar J, Flores C. Assessing the quality of studies supporting genetic susceptibility and outcomes of ARDS. Frontiers in Genetics. 2014;5.
CrossRef - Guillén‐Guío B, Acosta‐Herrera M, Villar J, Flores C. Genetics of Acute Respiratory Distress Syndrome. eLS. Published online April 15, 2016:1-9.
CrossRef - Clark MF, Baudouin SV. A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Medicine. 2006;32(11):1706-1712.
CrossRef - NCI-NHGRI Working Group on Replication in Association Studies, Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, Brooks LD, Cardon LR, Daly M, Donnelly P, Fraumeni JF Jr, Freimer NB, Gerhard DS, Gunter C, Guttmacher AE, Guyer MS, Harris EL, Hoh J, Hoover R, Kong CA, Merikangas KR, Morton CC, Palmer LJ, Phimister EG, Rice JP, Roberts J, Rotimi C, Tucker MA, Vogan KJ, Wacholder S, Wijsman EM, Winn DM, Collins FS. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655-660.
CrossRef - Yamashita M, Niisato M, Kawasaki Y. VEGF-C/VEGFR-3 signaling in macrophages ameliorates acute lung injury. European Respiratory Journal Published online. Published online 2021.
CrossRef - Medford ARL, Ibrahim NBN, Millar AB. Vascular endothelial growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. Journal of Critical Care. 2009;24(2):236-242.
CrossRef - Guillen-Guio B, Lorenzo-Salazar JM, Ma SF, Hou PC, Hernandez-Beeftink T, Corrales A, García-Laorden MI, Jou J, Espinosa E, Muriel A, Domínguez D, Lorente L, Martín MM, Rodríguez-Gallego C, Solé-Violán J, Ambrós A, Carriedo D, Blanco J, Añón JM, Reilly JP, Jones TK, Ittner CA, Feng R, Schöneweck F, Kiehntopf M, Noth I, Scholz M, Brunkhorst FM, Scherag A, Meyer NJ, Villar J, Flores C. Sepsis-associated acute respiratory distress syndrome in individuals of European ancestry: a genome-wide association study. Lancet Respir Med. 2020;8(3):258-266.
CrossRef - Kim JY, Hildebrandt MAT, Pu X, Ye Y, Correa AM, Vaporciyan AA, Wu X, Roth JA. Variations in the vascular endothelial growth factor pathway predict pulmonary complications. Ann Thorac Surg. 2012;94(4):1079-1084;
CrossRef - Hernandez-Pacheco N, Guillen-Guio B, Acosta-Herrera M, Pino-Yanes M, Corrales A, Ambrós A, Nogales L, Muriel A, González-Higueras E, Diaz-Dominguez FJ, Zavala E, Belda J, Ma SF, Villar J, Flores C, GEN-SEP Network. A vascular endothelial growth factor receptor gene variant is associated with susceptibility to acute respiratory distress syndrome. Intensive Care Med Exp. 2018;6(1):16.
CrossRef - Acosta-Herrera M, Lorenzo-Diaz F, Pino-Yanes M, Corrales A, Valladares F, Klassert TE, Valladares B, Slevogt H, Ma SF, Villar J, Flores C. Lung transcriptomics during protective ventilatory support in sepsis-induced acute lung injury. PLoS One. 2015;10(7):e0132296.
CrossRef - Shapiro NI, Schuetz P, Yano K, Sorasaki M, Parikh SM, Jones AE, Trzeciak S, Ngo L, Aird WC. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care. 2010;14(5):R182.
CrossRef - Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, Schuetz P, Aird WC, Shapiro NI. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39(5):427-432.
CrossRef - Hou PC, Filbin MR, Wang H, Ngo L, Huang DT, Aird WC, Yealy DM, Angus DC, Kellum JA, Shapiro NI. Endothelial permeability and hemostasis in septic shock. Chest. 2017;152(1):22-31.
CrossRef - Ding R, Zhao D, Li X, Liu B, Ma X. Rho-kinase inhibitor treatment prevents pulmonary inflammation and coagulation in lipopolysaccharide-induced lung injury. Thrombosis Research. 2017;150:59-64.
CrossRef - Birukova AA, Tian Y, Meliton A, Leff A, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2012;302(9):L965-L975.
CrossRef - Zhang S, Dai H, Zhu L, Lin F, Hu Z, Jing R, Zhang W, Zhao C, Hong X, Zhong JH, Pan L. Microvesicles packaging IL-1β and TNF-α enhance lung inflammatory response to mechanical ventilation in part by induction of cofilin signaling. Int Immunopharmacol. 2018;63:74-83
CrossRef - Wang J, Kong H, Xu J, Wang Y, Wang H, Xie W. Fasudil alleviates LPS-induced lung injury by restoring aquaporin 5 expression and inhibiting inflammation in lungs. Journal of Biomedical Research. 2019;33(3):156-163.
CrossRef - Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-β1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. Journal of Applied Physiology. 2006;101(2):375-384.
CrossRef - Tian W, Chen C, Lei X, Zhao J, Liang J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Research. 2018;46(W1):W363-W367.
CrossRef - Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry. 2009;31(2):NA-NA.
CrossRef - Arroliga AC, Matthay MA, Wiedemann HP. Preface. Clinics in Chest Medicine. 2006;27(4):xiii-xiv.
CrossRef - Ferguson ND, Frutos-Vivar F, Esteban A, Gordo F, Honrubia T, Peñuelas O, Algora A, García G, Bustos A, Rodríguez I. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care. 2007;11(5):R96.
CrossRef - Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736-742.
CrossRef - Matthay MA. Challenges in predicting which patients will develop ARDS. The Lancet Respiratory Medicine. 2016;4(11):847-848.
CrossRef - Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early Acute Lung Injury. Critical Care Medicine. 2013;41(8):1929-1937.
CrossRef - Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, Talmor D, Gajic O, Ware LB, Gong MN, US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A). Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: The LIPS-A randomized clinical trial. JAMA. 2016;315(22):2406-2414.
CrossRef - Rubin DB, Wiener-Kronish JP, Murray JF. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in non pulmonary sepsis syndrome. Journal of Clinical Investigation. 1990;86(2):474-480.
CrossRef - Ahmad Khan M, Sarwar AHMdG, Rahat R, Ahmed RS, Umar S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. International Immunopharmacology. 2020;85:106642.
CrossRef - Kumar G, Mukherjee S, Patnaik R. Identification of Withanolide-M and Stigmasterol as Potent neuroprotectant and Dual inhibitor of Inducible/Neuronal Nitric Oxide Synthase by Structure-Based Virtual Screening Identification of Withanolide-M and Stigmasterol as Potent neuroprotectant and Dual inhibitor of Inducible/ Neuronal Nitric Oxide Synthase by Structure-Based Virtual. Screening Journal of Biological Engineering Research and Review. 2017;4(1):9-13.
- Antwi AO, Obiri DD, Osafo N. Stigmasterol Modulates Allergic Airway Inflammation in Guinea Pig Model of Ovalbumin-Induced Asthma. Mediators of Inflammation. 2017;2017:1-11.
CrossRef - Ingawale DK, Patel SS. Hecogenin exhibits anti-arthritic activity in rats through suppression of pro-inflammatory cytokines in Complete Freund’s adjuvant-induced arthritis. Immunopharmacology and Immunotoxicology. 2017;40(1):59-71.
CrossRef - Goh YX, Jalil J, Lam KW, Husain K, Premakumar CM. Genistein: A Review on its Anti-Inflammatory Properties. Frontiers in Pharmacology. 2022;13.
CrossRef - Sutrisno S, Mariyani M, Herawati I, Rinata E, Jehanara J, Yueniwati Y, Nurdiana N, Noorhamdani N, Santoso S. The effects of genistein as antiinflammatory and antiangiogenesis in primary endometriosis cell culture. Asian Pac J Reprod. 2014;3(4):299-304.
CrossRef - He YQ, Zhou CC, Yu LY, Wang L, Deng JL, Tao YL, Zhang F, Chen WS. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol Res. 2021;163(105224):105224.
CrossRef








