Ananya Anand Arware1, Veena Nayak1 , Bharti Chogtu1*
, Bharti Chogtu1* , Vijetha Shenoy Belle2
, Vijetha Shenoy Belle2 and Seemitr Verma3
and Seemitr Verma3
1Department of Pharmacology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
2Department of Biochemistry, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
3Department of Pathology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
Corresponding Author E-mail:bharti.magazine@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2994
Abstract
Background: Platinum compounds like cisplatin, carboplatin used as anticancer drugs are known to cause nephrotoxicity. There is a need of drugs to prevent the drug induced nephrotoxicity. This experimental study was done to assess the nephroprotective role of rosuvastatin in carboplatin induced nephrotoxicity models and to compare it with melatonin. Methodology: A total of 36 albino rats were randomly divided into 6 groups (n=6). Group I was control group. In Groups II to VI nephrotoxicity was induced by intraperitoneal administration of a single dose of Carboplatin 128mg/kg on day 5. In Group II, no drug was administered. In group III and IV melatonin 5mg/kg and 10mg/kg was administered orally once a day for 10 days. In group V and VI, rosuvastatin 10mg/kg and 20mg/kg was administered orally once a day for 10 days. Blood was collected on day 0 for the baseline values and at an interval of 7 and 11 days for biochemical and antioxidant estimation. Kidneys were dissected at the end of study, weighed and renal tissue was subjected to histopathological analysis. Results: There was a significant decrease in urea, creatinine and uric acid in all the treatment groups. Also a significant decrease (p<0.05) was seen in MDA levels in treatment groups as compared to the negative control. A non-significant decrease was observed in IL-18 levels in the treatment groups. Also, histopathology of kidney tissues showed that in treatment groups, there were less changes in interstitium and vessels. Conclusion: Melatonin and rosuvastatin has shown a nephroprotective effect in carboplatin induced nephrotoxicity in terms of improved renal function tests, reduced IL-18 showing anti-inflammatory action, antioxidant action by decreasing MDA and increasing GSH and by histopathological studies of kidney tissue
Keywords
Antioxidant; Carboplatin induced nephropathy; Melatonin; Rosuvastatin
Download this article as:| Copy the following to cite this article: Arware A. A, Nayak V, Chogtu B, Belle V. S, Verma S. Antiinflammatory and Antioxidant Mediated Nephroprotection of Melatonin and Rosuvastatin in Carboplatin Induced Nephrotoxicity: An Experimental Study. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Arware A. A, Nayak V, Chogtu B, Belle V. S, Verma S. Antiinflammatory and Antioxidant Mediated Nephroprotection of Melatonin and Rosuvastatin in Carboplatin Induced Nephrotoxicity: An Experimental Study. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4cVYxUd |
Introduction
Carboplatin (cis-diammine-1,1-cyclobutanedicarboxylateplatinum II), a platinum coordination compound and alkylating agent is used in ovarian, head, neck and lung carcinomas. Carboplatin has been reported to cause hematological side effects like anemia, neutropenia leucopoenia, and minimal nephrotoxicity. 1The renal effects of carboplatin have been reported in high dose regimens and in the presence of concomitant risk factors.2 Though nephrotoxicity is dose related in both cisplatin- and carboplatin, carboplatin is less nephrotoxic but still leads to elevated serum creatinine in 10% of the patients.3 Cumulative doses of carboplatin have shown evidence of renal damage qualitatively like but less severe than that caused by cisplatin. Authors opine that proper monitoring of patients is required if high doses of carboplatin are used or carboplatin is combined with other nephrotoxic chemotherapy.4 However, there is a need for drugs that can prevent carboplatin-induced nephrotoxicity.
Melatonin is currently used in treatment of jet lag and readjustment of sleep wake cycle of shift workers. It gives protection against oxidative damage secondary to various factors like strenuous exercise, toxins primarily by scavenging the free radicals.5 Its role in attenuating the nephrotoxicity induced by drugs like vancomycin, amikacin, and cisplatin which cause oxidative stress in kidneys has been reported.6 Rosuvastatin, a hypolipidemic drug, decreases cholesterol levels by inhibiting the enzyme 3HMGCoA (3-hydroxy-3-methylglutaryl coenzyme) reductase. In addition, rosuvastatin has anti-inflammatory, immunomodulatory and antioxidant activities. Rosuvastatin like other statins has been reported to attenuate inflammation through inhibition of inflammatory cytokines as well as recruitment of inflammatory cells. Studies have shown the protective role of rosuvastatin in cisplatin-induced nephrotoxicity.7
In consideration of the above reports the present experimental study was done to assess and compare the nephroprotective effect of melatonin and rosuvastatin at low and high doses in carboplatin induced nephrotoxicity in rats.
Material and Methods
Animals
The study was conducted after approval by the Institutional Animal Ethics Committee (IAEC/KMC/109/2020). Animals were obtained from the Central Animal House of the institute in accordance with the committee for Control and supervision of experimentation on animal (CPCSEA) guidelines.Thirty-six female albino Wistar rats of weight ranging 150-250g, aged around 8-10 weeks were used for the study. The animals were housed under standard conditions, 12-hour light-dark cycle, 50% humidity, and 28°C temperature and provided with food and water ad libitum.
Drugs/Chemicals
Carboplatin (manufactured by Fresenius Kabi Oncology Ltd), Melatonin 3mg tab (manufactured in India by Sun Pharma Laboratories Ltd.), and Rosuvastatin 20mg tab (manufactured in India by Sun Pharma Laboratories Ltd.) was purchased from Manipal Hospital Pharmacy. All the other reagents used were purchased from Coral Clinical Systems and were of analytical grade. Dimethylsuphoxide (DMSO) was used as vehicle. The doses of drugs were selected as per previous studies.5,8,9
Experimental procedure
In the experiment, a total of 36 rats were randomly divided into 6 groups (n=6). The different groups received treatments as mentioned in Table 1. All groups other than control group were administered Carboplatin 128mg/kg intraperitoneal single dose on day 5 to induce nephrotoxicity.
The animals were weighed on day 0 and 11. Retro orbital blood collection from inner canthus of the eye using capillary tubes was done on day 0, 7 and 11 which was used for biochemical and antioxidant estimations. After 11th day, kidneys were dissected and weighed. The renal tissue was used for histopathological analysis.
Table 1: Treatment groups
|
Group1 (normal control) |
Dimethylsulfoxide (DMSO) as vehicle from day 0 to day 10 once a day |
|
Group II (Carboplatin control) |
DMSO: 1ml/kg/day p.o for 10 days from day 0 to day 10 once a day |
|
Group III (Carboplatin + Melatonin low dose) (Car +L.M) |
Melatonin: 5mg/kg p.o for 10 days from day 0 to day 10 once a day |
|
Group IV (Carboplatin + Melatonin high dose) (Car +H.M) |
Melatonin: 10mg/kg p.o for 10 days from day 0 to day 10 once a day |
|
Group V (Carboplatin + Rosuvastatin low dose) (Car +L.R) |
Rosuvastatin: 10mg/kg p.o for 10 days from day 0 to day 10 once a day |
|
Group VI (Carboplatin + Rosuvastatin high dose) (Car +H.R) |
Rosuvastatin: 20mg/kg p.o for 10 days from day 0 to day 10 once a day |
Biochemical estimations
Blood Urea was estimated usingGLDH kinetic method10 and serum creatinine was estimated by modified Jaffe’s Kinetic method.11Uric acid estimation was done by uricase/PAP method.12 Serum IL-18 was measured using ELISA.13 The antioxidants glutathione (GSH) and malondialdehyde (MDA) were assayed using methods described earlier.9
Histopathological Examination
The histological examination of kidney tissues was done using paraffin-embedded specimens. The specimens were cut into 6 mm thickness and stained with Hematoxylin-Eosin for light microscope examination (Olympus, BH-2, Tokyo, Japan). The characteristic histological changes in sections was observed and were analyzed semi-quantitatively using the technique of Houghton et al. 14 The lesions were graded as 0,1 or 2 if the histology showed normal structure, areas of focal granular-vacuolar epithelial cell degeneration or tubular epithelial necrosis respectively
Results
The effect of melatonin and Rosuvastatin on body weight and weight of kidneys in carboplatin induced nephrotoxicity is shown in table 2. No significant difference was observed in the weight of animals on day 0 and day 11. There was no significant change in the weight of kidneys in treatment groups as compared to control.
Biochemical estimations
Blood urea
The blood urea in six groups is shown in Table 3. On day 11 Carboplatin showed a significant increase in blood urea levels as compared to control (p<0.05). Also on day 11, all treatment groups showed a significant decrease in blood urea levels as compared to the carboplatin group (Table 3)
Serum creatinine
The serum creatinine levels of different groups is shown in table 4. On day 11 carboplatin group showed a significant increase in serum creatinine levels as compared to control(p<0.05). There is a significant decrease in creatinine levels as compared to carboplatin in all treatment groups. On comparing day 11 and day 7 levels with day 0 in carboplatin group, a significant increase is observed on day 11 and day 7 (p<0.05).
Uric acid
On day 11 carboplatin group showed a significant increase in uric acid levels as compared to control(p<0.05). There was a significant decrease in uric acid levels as compared to carboplatin in rosuvastatin and high dose of melatonin treatment groups (table 5). On comparing day 11 and day 7 levels with day 0 in carboplatin group, a significant increase is observed on day 11 and day 7 (p<0.05).
IL-18 levels
The IL-18 levels were significantly more in carboplatin group on day11 as compared to control group (p<0.05). On day 11, all treatment groups showed a decrease in IL-18 levels as compared to carboplatin but the decrease was not statistically significant (p>0.05) as seen in table 6.
Antioxidant estimation
GSH assay
There is a non- significant decrease in GSH levels in carboplatin treated group as compared to control on day11 (table 7). GSH levels in the other treatment groups were comparable to control (p>0.05)
MDA assay
There was significant increase in MDA levels in carboplatin treated group in comparison to the control group. The MDA levels in the treatment groups were significantly reduced in comparison to the carboplatin treated group as shown in table 8.
Table 2: Body weight of rats on day 0 and day 11 and weight of kidney on day 11.
|
GROUPS |
Body weight in (g) DAY0 |
Body weight in (g) DAY11 |
Kidney weight (g) Day 11 |
Organ weight/Bodyweight |
|
1.Control |
153.33±8.16 |
182.00±16.92 |
0.62 ± 0.074 |
0.003 |
|
2.Carboplatin |
175.00±13.52 |
172.67±10.07 |
0.59 ± 0.028 |
0.003 |
|
3.Car+ L.M |
174.00±25.27 |
164.00±26.58 |
0.67 ± 0.079 |
0.003 |
|
4.Car +H.M |
177.33±20.20 |
182.67±32.40 |
0.65 ± 0 .096 |
0.003 |
|
5.Car +L.R |
184.17±19.33 |
182.67±32.40 |
0.70 ± 0.083 |
0.003 |
|
6.Car +H.R |
187.33±24.54 |
173.33±28.92 |
0.64 ± 0.062 |
0.003 |
All values are expressed as Mean±S.D
Table 3: Blood urea levels in different groups on day 0, 7 and 11.
|
GROUPS |
Urea at DAY 0 mg/dl |
Urea at DAY7 mg/dl |
Urea at DAY11 mg/dl |
|
1.Control |
1.06 ± 0.07 |
1.35 ± 0 .33 |
1.21± 0.13a |
|
2.Carboplatin |
1.23 ± 0.13 |
3.07 ±0.28a |
3.38± 0.13*a |
|
3.Car+ L.M |
1.17 ± 0.15 |
1.56 ±0 .36a |
1.22± 0.11+ |
|
4.Car +H.M |
1.01 ± 0.27 |
1.46 ± 0. 21a |
1.15 ± 0.19+ |
|
5.Car +L.R |
1.23 ± 0.03 |
1.73 ± 0.54 |
1.15 ± 0.20+ |
|
6.Car +H.R |
1.24 ± 0.03 |
1.25 ±0 .35 |
1.06 ± 0.32+ |
Repeated measures of ANOVA p<0.05, post hoc Bonferronis test *p<0.05 vs control, a vs day 0, One way ANOVA on day 11, post hoc Tukey test +p<0.05 vs carboplatin. All values are expressed as Mean±S.D
Table 4: Serum creatinine levels in different groups on day0, 7 and 11.
|
GROUPS |
Creatinine at DAY 0 mg/dl |
Creatinine at DAY7 mg/dl |
Creatinine at DAY11 mg/dl |
|
1.Control |
0.24 ± 0.03 |
0.23 ± 0.03 |
0.24 ± 0.03 |
|
2.Carboplatin |
0.24 ± 0.03 |
1.60 ±0 .66a |
1.86 ± 0.24*a |
|
3.Car+ L.M |
0.23 ± 0.03 |
0.64 ±0 .40 |
0.28 ± 0.09+ |
|
4.Car +H.M |
0.24 ±0. 03 |
0.37 ± 0.21 |
0.22 ± 0.11+ |
|
5.Car +L.R |
0.23 ± 0.03 |
0.38 ± 0.20 |
0.26 ± 0 .12+ |
|
6.Car +H.R |
0.24 ± 0.03 |
0.32 ± 0.27 |
0.22 ± 0.14+ |
Repeated measures of ANOVA, post hoc Bonferroni’s test p<0.05, *vs control, + vs carboplatin, One way ANOVA on day 11, post hoc Tukey test , a vs day 0. All values are expressed as Mean±S.D
Table 5: Uric acid levels in different groups on day 0, 7 and 11.
|
GROUPS |
Uric acid at DAY 0 mg/dl |
Uric acid at DAY7 mg/dl |
Uric acid at DAY11 mg/dl |
|
1.Control |
0.70 ± 0.21 |
0.82 ±0.34 |
0.96 ±0 .18 |
|
2.Carboplatin |
0.48 ±0.17 |
1.40 ±0.21a |
2.57 ± 1.07*a |
|
3.Car+ L.M |
0.82 ± 0.43 |
1.37 ±0.64 |
1.93 ± 0.54 |
|
4.Car +H.M |
0.89 ± 0.29 |
1.01 ±0.40 |
0.88 ±0 .38+ |
|
5.Car +L.R |
0.63 ± 0.23 |
0.82 ±0.43 |
1.01 ± 0.40+ |
|
6.Car +H.R |
0.75 ± 0.38 |
0.76 ±0.29 |
0.88 ± 0.45+ |
Repeated measure of ANOVA, post hoc Bonferroni’s test *p<0.05 vs day 0, + vs carboplatin. One way ANOVA on day 11, post hoc Tukey test ap<0.05 vs day 0. All values are expressed as Mean±S.D
Table 6: IL-18 levels on day0, 7 and 11.
|
GROUPS |
DAY0 ng/l |
DAY7 ng/l |
DAY11 ng/l |
|
1.Control |
11.83 ± 0.72 |
11.91 ± 0.79 |
11.89 ± 0.76 |
|
2.Carboplatin |
11.11 ± 0.84 |
14.06 ± 0.82 |
14.63 ± 0.82* |
|
3.Car+ L.M |
11.24 ± 0.76 |
12.95 ± 0.52 |
13.49 ± 0.94 |
|
4.Car +H.M |
10.91 ± 0.64 |
13.38 ± 0.75 |
12.78 ± 1.30 |
|
5.Car +L.R |
11.77 ± 0.53 |
12.84 ± 0.48 |
13.38 ± 1.11 |
|
6.Car +H.R |
11.76 ± 0.68 |
12.19 ± 0.94 |
12.86 ± 1.55 |
Repeated measures of ANOVA, post hoc Bonferroni’s test *p<0.05 vscarboplatin. Remaining groups were tested but not significantly comparable to control group. All values are expressed as Mean±S.D.
Table 7: GSH levels in kidney tissue of rats
|
GROUPS |
GSH levels in µM/mg tissue |
|
1.Control |
1.312 ± 0.13 |
|
2.Carboplatin |
1.235 ± 0.13 |
|
3.Car+ L.M |
1.463 ± 0.26 |
|
4.Car +H.M |
1.538 ± 0.46 |
|
5.Car +L.R |
1.354 ± 0.35 |
|
6.Car +H.R |
1.248 ± 0.19 |
All values are expressed as Mean±S.D
Table 8: MDA levels in kidney tissue of rats.
|
GROUPS |
MDA levels in nM/mg tissue |
|
1.Control |
51.15 ± 0.599 |
|
2.Carboplatin |
63.83 ± 1.23* |
|
3.Car+ L.M |
54.73±3.31+ |
|
4.Car +H.M |
48.61±5.89+ |
|
5.Car +L.R |
47.32±4.61+ |
|
6.Car +H.R |
47.33±5.19+ |
All values are expressed as Mean±S.D
One way ANOVA, p<0.05; post hoc Tukey test, *p<0.05 vs control, +p<0.05 vs carboplatin
Histopathological examinations
Microphotographs of rat kidneys stained by Hematoxylin and Eosin (200X) is shown in the figure 1.
In control group (A) there are no changes in glomerulus, tubules and vessels, but there was mild small lymphocytic infiltration in interstitium. In carboplatin group (B) glomerular atrophy, vacuolar degeneration, hyaline casts were observed in the tubules. In car+ L.M group (C) glomerular atrophy, vacuolar degeneration, hyaline casts in tubules and moderate to severe small lymphocytic infiltration was seen in interstitium, but no changes were observed in the vessels. In car+ H.M (D) infiltration by small lymphoid cells are present. In car+ L.R (E) RBC casts seen inside the tubules. In Car + H.R (F) RBC casts seen inside the tubules along with glomerular atrophy and vacuolar degeneration but had no changes in interstitium and vessels.
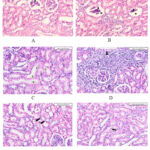 |
Figure 1: Histopathology of renal tissue in different treatment groups |
Discussion
In the present study, nephroprotective effects of melatonin and rosuvastatin in carboplatin induced nephrotoxic model were evaluated. Carboplatin 128mg/kg single dose i.p induced nephrotoxicity which was manifested as increase in blood urea, serum creatinine and serum uric acid levels. This was further confirmed by the glomerular atrophy, vacuolar degeneration and hyaline casts in kidney tubules seen in histopathology which was similar to a previous study. 15 Carboplatin caused dose-dependent renal injury in rats by depleting renal antioxidants, enhancing lipid peroxidation in addition to platinum content.16
Treatment with low and high doses of melatonin and carboplatin prevented the rise in blood urea, serum creatinine and uric acid levels on day 7 and day 11, which was suggestive of their nephroprotective property. This was confirmed by histopathological studies where there was only mild infiltration in interstitium and no changes in blood vessels.
Melatonin attenuates the nephrotoxicity induced by drugs by its antioxidant action as it strengthens the antioxidant enzymes and causes free radical scavenging directly. In addition, it modulates inflammatory cytokines which in turn reestablishes the balance of apoptosis and cell survival.17
Treatment with low and high doses of rosuvastatin and carboplatin prevented the rise in blood urea, serum creatinine and uric acid on day 7 and day 11 which is suggestive of their nephroprotective effect as well. Histopathology of rosuvastatin groups did not show any changes in interstitium and vessels. This effect was predominantly seen at the high dose of rosuvastatin. Statins in general produce nephroprotective effect by inhibiting receptor-mediated endocytosis that is responsible for the protein uptake in proximal tubular cells in experimental studies.18
Clinical studies have shown that statins produce nephroprotective effect secondary to pleiotropic effects like antithrombotic, anti-inflammatory and decreasing endothelial nitric oxide synthase.19
Though some studies report the superiority of lipophilic statins like atorvastatin over rosuvastatin in terms of nephroprotective effect20, other studies refute it and claim rosuvastatin to be better.
Comparison of melatonin and rosuvastatin did not show any significant difference in terms of nephroprotective activity and histopathological changes. This implicates that the nephroprotective action of melatonin is comparable to that of rosuvastatin. There was no significant difference in the nephroprotective activity of different doses of rosuvastatin and melatonin except that the low dose of melatonin did not show a significant decrease.
Studies have shown that different statins like atorvastatin and rosuvastatin have same efficacy in preventing contrast induced nephropathy.21
IL-18 is an inflammatory marker produced during acute kidney injury. IL-18, macrophage-derived cytokine, is expresses in cells like macrophages, dendritic cells, T cells, and B cells in addition to parenchymal kidney cells i.e.tubular epithelial cells, podocytes, and mesangial cells. 22 IL-18 plays an important role in processes that aggravate renal injury in extension phase of acute kidney injury. 23 Animal studies have shown that drugs that target IL-18–signaling axis can attenuate renal injury.24 In the present study, melatonin and rosuvastatin reduced the 1L-18 levels suggesting their anti- inflammatory action. The exact mechanism of IL-18 in renal injury is not clear but studies have shown that it has an important role in tubular injury and renal dysfunction and thus IL-18 as a target has a wide therapeutic potential.24
Melatonin has shown to ameliorate antibiotic induced nephrotoxicity by reducing oxidative stress in renal tissues. 25Melatonin and its metabolites scavenge free radicals. In addition to stimulating the synthesis of antioxidant enzymes it protects oxidative damage of antioxidant enzymes.26 Furthermore, melatonin also exerts an anti-apoptotic effect on various types of cells.27 The mechanism postulated by Dai W et al is that melatonin mediated cell apoptosis by inhibiting the Parkin1 signaling pathway induced by NLRP3 inflammasomes reduces the secretion of inflammatory cytokines like IL-18.28
Rosuvastatin produced significant renoprotective effect in gentamicin induced nephrotoxicity in Sprague Dolly rats by reducing kidney injury molecule-1 and interleukin-18.29 In addition to antioxidant action, the renoprotective effect of rosuvastatin can be mediated by lipid lowering action. prevention of lipid accumulation in renal tubules can also be contributing to renoprotective action of statins.7 Studies have shown that rosuvastatin produces nephroprotection by hemin-like effect on hemeoxygenase 1 which has a vital role to play in oxidative stress against antioxidants. 30 Also, when comparing between different statins, rosuvastatin showed a significant renoprotective effect as compared to simvastatin.7
As oxidative stress is a major mechanism implicated in platinum compounds induced nephrotoxicity, malondialdehyde and reduced glutathione assay was done in kidney tissue to measure lipid peroxidation and glutathione content. Carboplatin induced a significant increase in MDA and a decrease in GSH (though not significant). A significant decrease in MDA by both melatonin and rosuvastatin suggests their antioxidant mechanism.31 GSH is also a biomarker for oxidative stress and its increase in GSH in treatment groups was comparable to control group.
Both melatonin and rosuvastatin increased GSH levels most probably by inhibiting lipid peroxidation
This study has certain limitations. Urinary markers of kidney injury and other serum markers like beta 2 microglobulin which is an early marker of renal injury were not estimated.
Conclusion
To conclude, rosuvastatin and melatonin demonstrated an equi-efficacious nephroprotective effects mediated by anti-inflammatory and antioxidant properties. The anti-inflammatory action was primarily mediated by reducing IL-18 and antioxidant action by decreasing MDA and increasing GSH levels. Further long-term studies are warranted to substantiate these findings.
Acknowledgement
The authors acknowledge Kasturba Medical College, Manipal for the support and providing facilities for the conduct of research.
Conflict of Interest
The authors declare that there are no competing interests.
Funding Source
The study did not receive any external funding.
Ethics committee
The study was conducted after approval by the Institutional Animal Ethics Committee (IAEC/KMC/109/2020).
References
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic drugs. The Pharmacological basis of therapeutics. 13th ed. New York: McGraw Hill Education. 2018:1167-20.
- Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748-1756.
CrossRef - Goekkurt E, Al-Batran SE, Hartmann JT, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol. 2009;27(17):2863-2873.
CrossRef - English MW, Skinner R, Pearson AD, Price L, Wyllie R, Craft AW. Dose-related nephrotoxicity of carboplatin in children. Br J Cancer. 1999;81(2):336-341.
CrossRef - Gazi S, Altun A, Erdogan O. Contrast-induced nephropathy: preventive and protective effects of melatonin. J Pineal Res. 2006;41(1):53-57.
CrossRef - Sener G, Sehirli AO, Altunbas HZ, et al. Melatonin protects against gentamicin-induced nephrotoxicity in rats. J Pineal Res. 2002;32(4):231-236.
CrossRef - Maheshwari RA, Sailor GU, Patel L, Balaraman R. Amelioration of cisplatin-induced nephrotoxicity by statins. Indian J Pharmacol. 2013;45(4):354-358.
CrossRef - Husain K, Whitworth C, Rybak LP. Time response of carboplatin-induced nephrotoxicity in rats. Pharmacol Res. 2004;50(3):291-300.
CrossRef - Noori HY, Abd AH. Protective effect of Melatonin, Rosuvastatin and their combination against Amikacin induced nephrotoxicity in rats. Ann Trop Med Public Heal. 2019;22(5):98-107.
CrossRef - Kedar K, Patil J, Nimkar S. Urea and creatinine levels in vaginal fluid-a reliable marker for prelabour rupture of membranes. Journal of Evolution of Medical and Dental Sciences. 2018 ;7(20):2456-60.
CrossRef - Marakala V, Avinash SS, Shivashankara AR, Malathi M, Kumar A. Serum creatinine assay: enzymatic vs kinetic Jaffe’s method. J Evol Med Dent Sci. 2012;1(4):328-34.
CrossRef - DETURK WE. The adaptive formation of urease by washed suspensions of Pseudomonas aeruginosa. J Bacteriol. 1955;70(2):187-191.
CrossRef - Nozaki Y, Kinoshita K, Yano T, et al. Signaling through the interleukin-18 receptor α attenuates inflammation in cisplatin-induced acute kidney injury. Kidney Int. 2012;82(8):892-902.
CrossRef - Houghton DC, Plamp CE 3rd, DeFehr JM, Bennett WM, Porter G, Gilbert D. Gentamicin and tobramycin nephrotoxicity. A morphologic and functional comparison in the rat. Am J Pathol. 1978;93(1):137-152.
- Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by Cisplatin. J Toxicol Pathol. 2013;26(3):293-299.
CrossRef - Husai K, Jagannathan R, Hasan Z, et al. Dose response of carboplatin-induced nephrotoxicity in rats. Pharmacol Toxicol. 2002;91(2):83-89.
CrossRef - Raza Z, Naureen Z. Melatonin ameliorates the drug induced nephrotoxicity: Molecular insights. Nefrologia (Engl Ed). 2020;40(1):12-25.
CrossRef - Agarwal R. Statin induced proteinuria: renal injury or renoprotection? J Am Soc Nephrol. 2004;15(9):2502-2503.
CrossRef - Zhang J, Guo Y, Jin Q, Bian L, Lin P. Meta-analysis of rosuvastatin efficacy in prevention of contrast-induced acute kidney injury. Drug Des Devel Ther. 2018;12: 3685-3690.
CrossRef - Jabarpour M, Rashtchizadeh N, Ghorbani Haghjo A, et al. Protection of renal damage by HMG-CoA inhibitors: A comparative study between atorvastatin and rosuvastatin. Iran J Basic Med Sci. 2020;23(2):206-213.
- Firouzi A, Kazem Moussavi A, Mohebbi A, et al. Comparison between rosuvastatin and atorvastatin for the prevention of contrast-induced nephropathy in patients with STEMI undergoing primary percutaneous coronary intervention. J Cardiovasc Thorac Res. 2018;10(3):149-152.
CrossRef - Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213-224.
CrossRef - Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10(1):147-155.
CrossRef - Wu H, Craft ML, Wang P, et al. IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol. 2008;19(12):2331-2341.
CrossRef - Rastogi S, Gupta S, Halda C, Chandra D. Nephroprotective effect of melatonin and L-Ascorbic acid (Vitamin-C) against ampicillin- induced toxicity in Funambulus pennant. Egyptian Journal of Basic and Applied Sciences 2020 ;7(1)8-12.
CrossRef - Gitto E, Tan DX, Reiter RJ, et al. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol. 2001;53(10):1393-1401.
CrossRef - Zhai M, Li B, Duan W, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63(2):10.1111/jpi.12419.
CrossRef - Dai W, Huang H, Si L, et al. Melatonin prevents sepsis-induced renal injury via the PINK1/Parkin1 signaling pathway. Int J Mol Med. 2019;44(4):1197-1204.
CrossRef - Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Rosuvastatin Attenuates acute nephrotoxicity through modulation of oxidative stress in Sprague Dawley rats. J Pak Med Assoc. 2019;69(Suppl 3)(8):S98-S102.
- Heeba GH, Ali MAM, El-Sheikh AAK. Rosuvastatin Induces Renal HO-1 Activity and Expression Levels as a Main Protective Mechanism against STZ-Induced Diabetic Nephropathy. Medicina (Kaunas). 2022;58(3):425.
CrossRef - Ozbek E, Cekmen M, Ilbey YO, Simsek A, Polat EC, Somay A. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kappaB pathways. Ren Fail. 2009;31(5):382-392.
CrossRef








