Lakshitha Niyatee Rao K1 , Abinaya Elango2
, Abinaya Elango2 , Padmaja Sugumar2
, Padmaja Sugumar2 , Vijayashree Raghavan3
, Vijayashree Raghavan3 , Pooja E Moorthy3
, Pooja E Moorthy3 , Sushil Chittrarasan2
, Sushil Chittrarasan2 , Srivignesh Ravi4
, Srivignesh Ravi4 and Arunkumar Radhakrishnan2*
and Arunkumar Radhakrishnan2*
1Department of Pharmacology, M. S. Ramaiah Medical College, Bengaluru, Karnataka, India.
2Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpattu, Tamil Nadu, India.
3Department of Pathology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpattu, Tamil Nadu, India
4Department of Pharmacology, PSP Medical College Hospital and Research Institute, Tambaram-Kanchipuram main road, Oragadam, Panruti, Sriperumbudur Taluk, Kancheepuram District, Tamil Nadu, India.
Corresponding Author E-mail: arunrmbbs1978@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2997
Abstract
Objective: To evaluate the antidiabetic and hypolipidemic effect of ethanolic seed extract of Prosopis juliflora in fructose induced hyperglycemia in wistar albino rats in comparison with Metformin. Materials and methods: 30 male wistar albino rats were divided equally into 5 groups. Group I and II were the normal and the disease control groups. While, groups III to V were the treatment groups. Animals in group I received regular drinking water; whereas, groups II to V received 20% fructose water for 8 weeks. After 8 weeks, animals in groups II to V had elevated fasting blood sugar, HOMA-IR, weight gain and dyslipidemia. From week 9 to 16 group I animals continued to receive regular drinking water, group II received 2ml of distilled water and groups III, IV and V received Metformin 200mg/kg, P.juliflora extract 400mg/kg and 600mg/kg respectively in addition to 20% fructose water. The animals were sacrificed at the end of 16 weeks and histopathological examination of pancreas was done. Biochemical and hematological assessments were done at baseline and at 16 weeks to assess safety of the interventions. Results: When compared to the disease control group, animals in group III treated with metformin and groups IV and V treated with P.juliflora extract at doses of 400mg/kg and 600mg/kg showed a significant decrease in Fasting blood glucose, HOMA-IR and improvement in lipid profile. Even though both the doses of the extract showed significant pharmacological activity, 600mg/kg showed better activity equivalent to metformin. Histopathological examination of pancreas showed regenerative changes in the metformin and P.juliflora 600mg/kg treated groups. No significant abnormality was observed in the biochemical and haematological parameters at the end of the study. Conclusion: P.juliflora seed extract in the dose 400 mg/kg and 600mg/kg exhibited antidiabetic and hypolipidemic activity with no significant adverse events, in this study. Both the doses were having anti dyslipidemic effect similar to metformin whereas 600 mg/kg dose of P.juliflora was having better antidiabetic effect comparable to Metformin. Keywords: Diabetes, Dyslipidemia, P.juliflora, Fructose, Metformin, Metabolic syndrome
Keywords
Diabetes; Dyslipidemia; Fructose; Metformin, Metabolic syndrome; P. juliflora
Download this article as:| Copy the following to cite this article: Rao K. L. N, Elango A, Sugumar P, Raghavan V, Moorthy E. M, Chittrarasan S, Ravi S, Radhakrishnan A. Antidiabetic and Hypolipidemic Effect of Ethanolic Seed Extract of Prosopis juliflora in Fructose Induced Hyperglycemia in Rats. Biomed Pharmacol J 2024;17(3). |
| Copy the following to cite this URL: Rao K. L. N, Elango A, Sugumar P, Raghavan V, Moorthy E. M, Chittrarasan S, Ravi S, Radhakrishnan A. Antidiabetic and Hypolipidemic Effect of Ethanolic Seed Extract of Prosopis juliflora in Fructose Induced Hyperglycemia in Rats. Biomed Pharmacol J 2024;17(3). Available from: https://bit.ly/4dQ8PFY |
Introduction
“Diabetes mellitus (DM) is a chronic heterogeneous metabolic disorder characterized by persistently elevated blood glucose levels (hyperglycemia) with disturbances in carbohydrate, protein and fat metabolism as a result of defect in insulin secretion, insulin action or both”. The chronic hyperglycemia in diabetes can cause damage, dysfunction and failure of various organs particularly the kidneys, eyes, heart, nerves and blood vessels.1
Diabetes can be classified based on its etiology into type I and type II. Type I diabetes mellitus (T1DM) occurs as a result of absolute insulin deficiency due to autoimmune destruction of pancreatic beta cells. It is commonly seen among the children and adolescents.2 Type II diabetes (T2DM) occurs as a result of impaired insulin secretion or increased insulin resistance due to beta cell dysfunction or insulin resistance due to reduced responsiveness of the target tissue like liver, skeletal muscle and adipose tissues towards insulin. It is commonly seen among the older individuals.3 Insulin resistance in T2DM causes increased production as well as reduced clearance of triglyceride rich lipoproteins thereby causing altered lipid metabolism and diabetic dyslipidemia.4
Diabetes can be managed with both pharmacological agents and non-pharmacological approaches such as like lifestyle modifications. Proper glycemic control helps in preventing and delaying complications due to diabetes. T1DM can be managed with insulin replacement therapy, whereas T2DM can be managed with a combination of oral hypoglycaemic agents, dietary changes and exercise. In spite of the availability of several groups of drugs, most of the patients do not respond to mono drug therapy and might require a combination of drugs for optimal glycemic control.5 Most of the conventional antidiabetic drugs are not devoid of adverse effects. Hence there is a constant search for newer agents including traditional medicines.
Currently many studies are being conducted on a large number of medicinal plants and some of these medicinal plants have proven their efficacy in treatment of DM. Prosopis species are commonly used in folk medicine for treatment of various diseases including DM.6
Prosopis juliflora (Sw) DC belongs to the family-fabacea and subfamily-mimosoideae. It is commonly called as mesquite.It is an aggressive invader and propagates through the seeds. Various parts of P.juliflora like the seeds, leaves and the fruits have been reported to have to have antibacterial7-10, antifungal11-14, antioxidant activity15, anticancer16-18 and antidiabetic properties.19,20 Though the effect of P.juliflora seeds on blood sugar levels has already been reported by literature, there are no data available regarding the effect of P.juliflora seeds on insulin levels and lipid profile, which usually get altered in patients with diabetes. In this study we used fructose to induce diabetes and diabetic dyslipidemia in wistar rats and planned to evaluate the anti-diabetic and hypolipidemic effect of P.juliflora seeds. Fructose is a lipogenic sugar. Increased consumption of fructose causes hepatic lipogenesis, which raises plasma triglyceride levels, lowers VLDL clearance, activates genes involved in hepatic de novo lipogenesis, and promotes triglyceride accumulation in hepatocytes and skeletal muscle. Ectopic triglyceride accumulation in tissues eventually leads to dyslipidaemia and insulin resistance.21
In our study we used fructose solution to induce T2DM in wistar albino rats as this model will induce features of T2DM along with metabolic symptoms like obesity, dyslipidemia and hyperinsulinemia. Also, patients with T2DM often present with obesity, reduced insulin sensitivity and beta cell compensatory mechanisms like excess basal insulin secretion and hyperinsulinemia. Diabetes and metabolic syndrome are often interrelated and many times, metabolic disorders may coexist along with T2DM.
Also, this model overcomes the disadvantages of chemically induced diabetic models by streptozotocin and alloxan as they cause selective loss of pancreatic beta cells and hyperglycemia occurs predominantly due to the cytotoxic effects on beta cells.22 Because of the spontaneous regeneration of beta cells, chemically induced diabetic models are often less stable and occasionally reversible. In addition to their cytotoxic effects on beta cells, these chemicals can also have toxic effects on other organs, especially the liver and the kidney. Despite being the most widely used model, chemical induction of T2DM has recently been criticized because it causes beta cell toxicity, which leads to rapid and accelerated destruction of beta cells causing insulin deficiency more than insulin resistance thus mimicking T1DM rather than T2DM.23 Due to the limitations of chemically induced diabetic models, we attempted to induce insulin resistance along with dyslipidemia in wistar rats by substituting the regular drinking water with 20% fructose solution and the model’s stability was determined by monitoring the fasting glucose and insulin levels. We further evaluated the antihyperglycemic and hypolipidemic effect of P.juliflora seed extract in wistar rats with fructose induced hyperglycemia as this model induced diabetic dyslipidemia along with insulin resistance.
Objectives
To assess the antidiabetic effect of ethanolic seed extract of Prosopis juliflora with the following outcome measures:
Fasting blood sugar (FBS), Homeostatis model assessment- estimated insulin resistance (HOMA-IR).
To assess the hypolipidemic effect of ethanolic seed extract of Prosopis juliflora with the following outcome measures:
Total cholesterol (TC), Triglycerides (TG), LDL cholesterol (low-density lipoprotein), VLDL cholesterol (very-low density lipoprotein) and HDL cholesterol (high-density lipoprotein).
Materials and Methods
Seeds collection and extract preparation
P.juliflora pods were collected near Madipakkam, Chennai, Tamil Nadu, and the seeds were separated. The plant and the seeds were identified and certified by an authorized botanist before preparation of the extract. Seeds of P.juliflora (500g) were washed, shade dried, powdered and soaked in 1 liter of ethanol (99.9 % v/v) for 72 hours with periodic shaking. The supernatant was filtered using whatman filter paper no 1. The filtrate obtained was evaporated using water bath for 48 hours. Finally, a brown sticky extract was obtained. The percentage yield obtained was 3.9%. Extract is stored in airtight container in refrigerator at 2-8for further use.
Phytochemical screening
Phytochemical analysis of the seed extract was performed to identify different phytochemicals such as saponins, alkaloids, glycosides, terpinoids, flavanoids and steroids using the methods described in literature. 24-28
Animals
This study was carried out with Institutional Animal Ethics Committee (IAEC) approval (Ref no: IAEC 1/Proposal:58/A.Lr:41/Dt:05.03.2021). 30 male Wistar albino rats weighing approximately 180-200g were housed in individual clean polypropylene cages. They were maintained at a temperature of 23-25 humidity 50-60% in alternate light and dark cycle with food and water.
Induction of hyperglycemia along with dyslipidemia
Hyperglycemia and dyslipidemia were induced by administering 20% fructose water daily for 16 weeks.29-33 Each rat was housed in a separate cage. 20% fructose solution was prepared daily by dissolving 2g of fructose powder in 10 ml of drinking water. Fructose water was provided daily through animal feeding bottles which were fit to the cage and the amount of fructose water consumed by the animals was monitored daily. If the animals exhausted all the fructose water, then regular drinking water was provided for the rest of the day. Animals with FBS level > 150mg/dl were included for the experiment.
Dosage selection of interventions
The dose of Metformin was calculated based on the formula given in FDA draft guidance for calculating animal dose from the human equivalent dose. The maximum recommended dose of Metformin is 2000 mg/day in humans. For a 60 kg human, the dose will be 33.3 mg/kg/day. The animal equivalent dose is calculated by multiplying the factor 6.2 for rats (FDA).34 The calculated animal dose of Metformin is 206.7 mg/kg/day. The value was rounded off to 200 mg/kg/day for Metformin in this study. The doses of the P.juliflora seed extract 400 mg/kg and 600 mg/kg was decided based on previous studies. 19,35 The interventions were be dissolved in 2 ml of distilled water and administered via orally using gavage.
Drugs and Reagents
D-fructose was purchased from Rankem laboratory India ; Metformin was obtained from Intas Pharmaceuticals Ltd, India; Enzyme Linked Immunosorbent Assay kit for estimating rat fasting insulin levels was purchased from Bioassay technology Pvt. Ltd; Ethanol (99.9%) was purchased from Changshu Hongsheng Fine chemicals Co.Ltd; FBS levels were measured using glucometer- Contour plus, Ascensia Diabetes Care India Pvt Ltd; Lipid profile parameters (TC, TG, LDL, VLDL and HDL) were assessed using auto-analyser. Ketamine was purchased from Neo laboratories, India and Halothane was purchased from Raman & Weil Pvt Ltd, India.
Experimental design
30 male wistar albino rats divided into 5 groups and each group had 6 animals. The day of induction (starting fructose) was taken as day ‘0’ (baseline).
Table 1: Details of the grouping and study interventions
|
Groups |
No of rats |
Treatment |
|
|
0 to 8 weeks |
9 to 16 weeks |
||
|
I (control) |
6 |
Regular drinking water |
Regular drinking water |
|
II (Hyperglycemia control) |
6 |
20% fructose solution in drinking water |
20% fructose solution in drinking water + 2 ml distilled water |
|
III |
6 |
20% fructose solution in drinking water |
20% fructose solution in drinking water + Metformin 200 mg/kg |
|
IV |
6 |
20% fructose solution in drinking water |
20% fructose solution in drinking water + P.juliflora seed extract 400mg/kg |
|
V |
6 |
20% fructose solution in drinking water |
20% fructose solution in drinking water + P.juliflora seed extract 600mg/kg |
Study assessments
All the parameters were assessed at baseline, 8 weeks and 16 weeks. For assessing all the biochemical parameters mentioned below except FBS, blood was collected via retro orbital route after administering ketamine anesthesia intra peritoneally (50mg/kg). For measuring FBS level blood samples were obtained from tail tip.
FBS levels were measured after overnight fasting using glucometer.
For the assessment of beta cell function and insulin resistance, serum insulin levels were measured after overnight fasting with rat insulin ELISA kit and HOMA-IR was calculated using the formula (fasting insulin (micro U/L) * fasting glucose (nmol/L)/22.5
Lipid profile (TC, TG, LDL, VLDL and HDL) using auto-analyser.
Body weight was measured using automatic weight scale.
All the rats were sacrificed using high dose halothane anesthesia at the end of 16 weeks and histopathological examination (HPE) of pancreas was done.
Statistical analysis
Graphpad instat version 3.0 was used for performing statistical analysis. All the continuous variables were summarized as mean Standard deviation. For comparing the data within the groups and between the groups, inferential statistical tests such as paired t test& repeated measures ANOVA (for within group analysis) and one way ANOVA with Tukey’s post hoc test (for between group analysis) were used. P value less than 0.05 was considered as statistically significant.
Results
All the animals in the groups II to V developed features of T2DM and insulin resistance evidenced by increase in FBS levels and HOMA-IR. There was no mortality throughout the experimental duration.
Phytochemical screening
The phytochemical screening of the extract showed the presence of alkaloids, flavanoids and steroids.
Results of in vivo study
Body weight (BW)
All groups had similar
BW at baseline. At 8 weeks all groups gained significant weight. At 16 weeks,
the normal and disease control groups (I and II) gained significantly more
weight compared to the baseline and 8 weeks, whereas the treatment groups (III
to V) lost weight significantly when compared to 8 weeks (within group
analysis, P<0.0001). Intergroup comparison of BW showed that, although all
the groups gained weight after 8 weeks, animals in fructose supplemented groups
(II to V) had significantly higher weight gain than the normal control group
(I) (P<0.0001). At 16 weeks, Metformin and extract treated groups (400mg/kg
and 600mg/kg) lost weight significantly when compared to 8 weeks. On the other
hand, the normal and disease control groups (I and II) continued to gain
weight. Nevertheless, group II gained significantly more weight than group I
(P<0.001), and weight loss was similar and not statistically significant
among groups III to V (P>0.05). Data on BW is showed in figure 1.
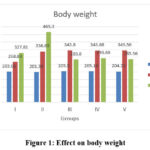 |
Figure 1: Effect on body weight. |
Fasting Blood sugar
All the groups (I-V) had similar FBS at baseline. In fructose fed groups (II to V) FBS increased at 8 weeks (all rats had FBS>150mg/dl). At 16 weeks, Metformin and extract treated groups (III to V) showed significantly reduced FBS compared to 8 weeks (within group analysis, P<0.0001) while the disease control group did not show any difference and the FBS remained elevated. When the reduction in FBS was compared among the groups, Metformin and extract (600mg/kg) showed similar reduction in FBS. Group IV (400mg/kg), though reduced the FBS levels before and after treatment, it was not comparable to Metformin and 600mg/kg extract. Although the FBS level decreased with treatment in the Metformin and extracts treated groups (III to V), their levels were still higher when compared to the normal control group (group I). The data of fasting blood sugar levels is shown in table 2.
Table 2: Fasting blood sugar
|
Groups
|
Fasting blood Sugar (mg/dl) mean SD |
P value |
||||
|
Baseline |
8 Weeks
|
16 Weeks
|
Within group comparison (Repeated measures ANOVA) |
Between (One way ANOVA) |
||
|
I |
Normal control |
86 ± 10.431 |
87.66 ± 10.78 |
87.5 ± 9.50 |
0.2771 |
< 0.0001 * |
|
II |
Disease control |
85.66 ± 5.95 |
158.16 ± 7.05 |
178.66 ± 7.36 |
< 0.0001* |
|
|
III |
Standard drug |
87 ± 5.17 |
159.66 ± 6.31 | 127.16 ± 5.52 |
< 0.0001* |
|
|
IV |
P.juliflora |
84.83 ± 5.45 |
163.33 ± 5.46 |
150.5 ± 6.38 |
< 0.0001* |
|
|
V |
P.juliflora |
84.66 ± 8.73 |
160.66 ± 5.46 |
131.66 ± 4.84 |
< 0.0001* |
|
|
Post hoc analysis:Group I (16weeks) Vs Groups III, IV and V (16 weeks)- P<0.001*, Group II (16 weeks) Vs groups III, IV and V (16 weeks)-P<0.001*, Group III (16weeks) Vs Group IV (16 weeks)-P<0.001*, Group III (16weeks) Vs Group V (16 weeks)- P>0.05, Group IV (16weeks) Vs Group V (16 weeks)-P<0.01* |
||||||
HOMA-IR
All the groups (I-V) had similar HOMA-IR levels at baseline. At 8 weeks, the HOMA-IR level increased significantly in the fructose supplemented groups (II to V). At 16 weeks, Metformin and extract treated groups (III to V) showed significant reduction in the HOMA-IR levels compared to 8 weeks (within group analysis, P<0.0001). However, HOMA-IR remained elevated in disease control group (II) till the end of the study (within group analysis, P< 0.0001). Intergroup comparison of the reduction in HOMA-IR levels showed that, group III and V treated with Metformin and extract (600mg/kg) showed similar reduction in HOMA-IR level. Even though group IV (400mg/kg) reduced the HOMA-IR levels before and after treatment, it was not comparable to Metformin and 600mg/kg extract. Although HOMA-IR levels decreased with treatment in the Metformin and extracts treated groups (III to V), it still remained higher than the normal control group (group I). Data is shown in figure 2.
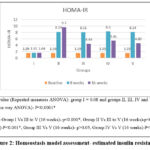 |
Figure 2: Homeostasis model assessment-estimated insulin resistance |
HDL cholesterol
HDL level in the normal control (group I) remained constant throughout the duration of the study. At 8 weeks, HDL levels decreased significantly in the fructose supplemented groups (II to V). At 16 weeks, HDL levels increased significantly in metformin and extract treated groups (III to V) when compared to 8 weeks due to the effect of the treatment, whereas it remained low in the disease control animals due to the progression of the disease (group II) (Within group analysis, P<0.0001). When the HDL levels were compared among the different groups, it was observed that the Metformin and extracts 400mg/kg and 600mg/kg treated groups (III to V) had significantly higher HDL levels at 16 weeks when compared to the disease control group (II). Also, the increase in the HDL levels in these groups were similar and comparable. However, when compared to the normal control group, HDL levels were lower in these groups. Data of HDL levels are shown in table 3.
Table 3: Effect on HDL cholesterol.
|
Groups |
HDL cholesterol (mg/dl) mean SD |
P value |
|||
|
Baseline |
8 Weeks |
16 Weeks |
Within group comparison (Repeated measures ANOVA) |
Intergroup comparison (One way ANOVA) |
|
|
I |
27.83±1.60 | 27.5±1.64 | 27.6±1.21 | 0.59 |
<0.0001* |
|
II |
27.5±1.87 | 22.5±1.37 | 19.5±1.37 |
<0.0001* |
|
|
III |
27.66±1.50 | 20.5±1.87 | 23.5±1.37 |
<0.0001* |
|
|
IV |
27.33±1.96 |
20.3±2.33 | 23±1.89 |
<0.0001* |
|
|
V |
27.5±1.64 |
20.1±2.04 | 23.3±1.36 |
<0.0001* |
|
|
Post hoc analysis: Group I (16weeks) Vs Groups III, IV and V (16 weeks)- P<0.01*, Group II (16 weeks) Vs Groups III, IV and V (16 weeks)- P<0.01*, <0.05* and <0.05* respectively. Group III (16 weeks) Vs Group IV and V (16 weeks)- P>0.05, Group IV (16 weeks) Vs Group V (16 weeks)- P>0.05 |
|||||
TC, TG, LDL and VLDL cholesterol
In the normal control animals (group I) TC, TG, LDL and VLDL levels remained constant throughout the experimental duration. These levels, on the other hand, were significantly elevated in the fructose fed groups (II to V) at the end of 8 weeks, and decreased after treatment in the metformin and extract treated groups (III to V) but remained elevated in the disease control animals (group II) (within group analysis, P<0.0001). Intergroups comparison showed that at the end of 16 weeks TC, TG, LDL and VLDL levels decreased significantly in the Metformin and extract treated (400mg/kg and 600mg/kg) groups when compared to the disease control animals (group II). But, when compared to the normal control animals (group I), the levels remained elevated. Furthermore, in the Metformin and extract treated (400mg/kg and 600mg/kg) groups the reduction in the levels of these parameters in these groups were almost similar and comparable. Data of TC and LDL cholesterol is shown in figure 3, 4 and data on TG and VLDL cholesterol is shown in table 4 and 5.
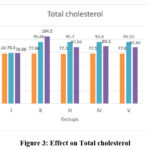 |
Figure 3: Effect on Total cholesterol |
Table 4: Effect on Triglycerides
|
Groups |
Triglycerides (mg/dl) mean SD |
P value |
|||
|
Baseline |
8 Weeks
|
16 Weeks
|
Within group (Repeated measures ANOVA) |
Between groups (One way ANOVA) |
|
|
I |
60.5±1.87 | 60.66±2.06 | 61.16±2.04 |
0.10 |
<0.0001* |
|
II |
60.16±3.06 | 73.5±3.45 | 78.83±2.92 |
<0.0001* |
|
|
III |
60.66±1.86 | 73.66±1.50 | 70.16±1.47 |
<0.0001* |
|
|
IV |
60.33±2.25 | 73.33±1.50 | 70±1.67 |
<0.0001* |
|
|
V |
60.5±1.37 | 74.16±1.16 | 70.1±1.16 |
<0.0001* |
|
|
Post hoc analysis: Group I (16 weeks) Vs Groups III, IV and V (16weeks)- P<0.001*, Group II (16 weeks) Vs Groups III, IV and V (16weeks)- P<0.001*, Group III (16 weeks) Vs Group IV and V (16 weeks)- P>0.05 and Group IV (16 weeks) Vs Group V (16 weeks)-P>0.05 |
|||||
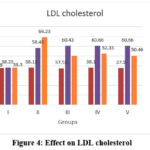 |
Figure 4: Effect on LDL cholesterol. |
Table 5: Effect on VLDL cholesterol
|
Groups |
VLDL (mg/dl) mean SD |
P value |
|||
|
Baseline |
8 Weeks
|
16 Weeks
|
Within group (Repeated measures ANOVA) |
Between groups (One way ANOVA) |
|
|
I |
12.1±0.37 | 12.13±0.41 | 12.23±0.40 |
0.10 |
<0.0001* |
|
II |
12.03±0.61 | 14.7±0.68 | 15.76±0.58 |
<0.0001* |
|
|
III |
12.13±0.37 | 14.73±0.30 | 14.03±0.29 |
<0.0001* |
|
|
IV |
12.06±0.45 | 14.66±0.30 | 14±0.33 |
<0.0001* |
|
|
V |
12.1±0.27 | 14.83±0.23 | 14.03±0.23 |
<0.0001* |
|
|
Post hoc analysis: Group I (16 weeks) Vs Groups III, IV and V (16weeks)- P<0.001*, Group II (16 weeks) Vs Groups III, IV and V (16weeks)- P<0.001*, Group III (16 weeks) Vs Groups IV and V (16 weeks)- P>0.05 and Group IV (16 weeks) Vs Group V (16 weeks)-P>0.05 |
|||||
Histopathology
HPE of pancreas showed the following changes:
In the normal control group (group I), HPE of pancreas did not show any significant changes (Figure 5). Whereas, in the disease control group (group II) islet cells were surrounded with inflammatory infiltrates such as lymphocytes, macrophages, and plasma cells (Figure 6). The metformin and P.juliflora extract 600mg/kg treated groups (group III and V) showed normal islets cells with large, pale and ovoid beta cells (Figure 7 and 9). While the P.juliflora extract 400mg/kg treated group showed some degenerative changes (Figure 8). These findings indicate that the islet cells may have recovered after treatment with P.juliflora extract 600mg/kg.
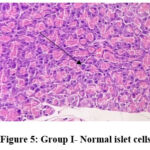 |
Figure 5: Group I- Normal islet cells |
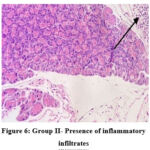 |
Figure 6: Group II- Presence of inflammatory infiltrates |
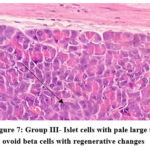 |
Figure 7: Group III- Islet cells with pale large to ovoid beta cells with regenerative changes |
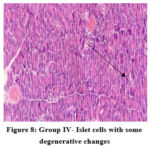 |
Figure 8: Group IV- Islet cells with some degenerative changes |
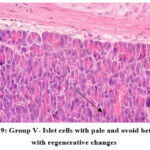 |
Figure 9: Group V- Islet cells with pale and ovoid beta cells with regenerative changes |
Discussion
Lipid abnormalities such hypertriglyceridemia and reduced HDL cholesterol levels as are commonly observed in patients with T2DM. Diabetic dyslipidemia can be treated with strict glycemic control and weight loss. Majority of the conventional anti-diabetic drugs are associated with unavoidable side effects.36An ideal anti-diabetic drug should be safe, effective in maintaining optimal blood glucose levels and also prevent long term complications. Alternative system of medicine is practiced by a large population for treatment of various diseases and ailments including DM.37 Herbal medicine & plant components have relatively low toxicity and fewer side effects and therefore, they can be considered as a therapeutic option for DM.38
In this study, male wistar albino rats were fed with 20% fructose water daily for a period of 8 weeks presented with features of T2DM like fasting hyperglycemia, insulin resistance. In addition to T2DM, fructose supplementation also induced some of the features of metabolic syndrome like weight gain and altered lipid profile (dyslipidemia).
The anti-diabetic and hypolipidemic activity of the seed extract was compared with metformin. It was observed that both the doses of extract (400mg/kg and 600mg/kg) reduced the blood glucose levels. However, the dose 600mg/kg showed maximum reduction which was comparable to the effect produced by metformin. Therefore, it is clear that the ethanolic seed extract of P.juliflora significantly reduced the blood glucose levels in a dose-dependent manner. Similar findings were observed in another study where methanolic seed extract of P.juliflora in the dose 600mg/kg reduced the blood sugar levels in streptozotocin induced diabetic rat model.19
Phytochemical analysis showed the presence of alkaloids, flavonoids, terpenoids &steroids. Based on this, it is proved that P.juliflora seeds are rich in polyphenolic compounds like flavonoids. Flavonoids are biologically active secondary metabolites in the plants.39 They have numerous beneficial effects on metabolic disorders such as cardiovascular disease, obesity, cancer and DM.40Chronic hyperglycemia in T2DM raises inflammatory cytokine levels which may cause endoplasmic reticulum stress, oxidative stress and lysosomal destabilization, which ultimately leads to beta cell death through apoptosis.41 Flavonoids promote beta cell proliferation and decrease apoptosis, regulate glucose metabolism in the liver, reduce the hepatic glucose output, regulates the enzymes involved in the carbohydrate metabolism (inhibits -glucosidase), exhances the expression of glucose transporters, promote insulin secretion and reduce insulin resistance.42,43 According to many studies, flavonoids also act as insulin mimetics and insulin secretagogues.44-46 Therefore, the hypoglycaemic effect of ethanolic seed extract of P.juliflora might be due to the presence of bioactive phytoconstituents like flavonoids that act separately or synergistically and either stimulates or mimics the action of insulin.
In our study, we discovered that animals in the fructose fed groups gained significantly more weight at the end of 8 weeks, when compared to the animals in the normal control group. Similar findings were observed in another study where wistar rats fed on 20% fructose water for 8 weeks had significantly higher body weight when compared to the control group.47 Fructose promotes de novo lipogenesis in animals, resulting in increased body weight and adipocity. Obesity develops as a result of the dyslipidemia and increased body fat stores. Insulin resistance and obesity frequently coexist. This could explain the increased body weight and insulin resistance observed in our study48-50
We also observed that the levels of TG, LDL, VLDL and TC increased while HDL cholesterol decreased after the induction of diabetes at 8 weeks. Similar findings were reported in some of the previous studies where wistar rats fed on 20% fructose water for 8 weeks and 21 weeks respectively, developed signs of dyslipidemia as well as insulin resistance.29,30
“Dyslipidemia is characterized by elevated levels of TC, LDL, VLDL and TG, as well as a decrease in HDL cholesterol”51. In our study, the lipid parameters remained unchanged in the disease control group at the end of treatment. In contrast, there was a significant increase in HDL levels and a decrease in the levels of TG, LDL, VLDL and TC in the metformin and extracts treatment groups (400mg/kg and 600mg/kg). Moreover, both the doses of extract were having anti dyslipidemic effect similar to metformin
Hyperglycemia is frequently associated with dyslipidemia in T2DM. Insulin activates the enzyme lipoprotein lipase, which causes triglyceride hydrolysis. Lipoprotein lipase is not activated in T2DM due to insulin resistance, and catabolism of triglyceride-rich lipoproteins is decreased, resulting in hyper-triglyceridaemia and other lipoprotein alterations.52 Furthermore, the hypolipidemic effect of the P.juliflora seed extract can be attributed to the presence flavonoids and terpenoids which might cause inhibition of the enzyme lipoprotein lipase and thereby decreasing the levels of triglycerides, LDL, VLDL and total cholesterol.53,54
Biochemical and haematological assessments were done at baseline and at 16 weeks to assess safety of the interventions and no significant abnormality was observed.
HPE of pancreas showed regenerative changes in the metformin and extract 600mg/kg treated groups. These findings indicate that the damaged islet cells may have recovered after treatment with the plant extract.
One of the major goals in the management of diabetes is to maintain optimal blood glucose levels to prevent complications.55 considering the current study’s findings as well as the available evidence for anti-diabetic and hypolipidemic activity, P.juliflora seed extract can be used to treat hyperglycemia and dyslipidemia associated with T2DM. However, further studies are needed to determine the exact mechanism of such activity by isolating bioactive compounds and assessing their effect.
Conclusion
P.juliflora seed extract in the doses 400 mg/kg and 600mg/kg exhibited antidiabetic and hypolipidemic activity in terms of reduction in the fasting blood sugar, HOMA-IR and lipid levels. Both the doses, 400 and 600 mg/kg showed hypolipidemic activity similar to Metformin whereas the antidiabetic activity showed a dose dependent response i.e., 600 mg/ kg was having better antidiabetic effect compared to 400 mg/ kg and the reduction in blood sugar was similar to that of Metformin. Both the doses of the extract were safe as there were no significant abnormality observed in the hematological and biochemical parameters. Further, regenerative changes were noted in the pancreas of the animals treated with Metformin and 600 mg/kg of the extract. All these findings suggest that the seed extract of P.juliflora has a potential therapeutic benefit in diabetes and hyperlipidemia.
Acknowledgement
We thank Chettinad Academy of Research and Education for permitting and supporting us to conduct this study.
Conflict of Interest
The author(s) declares no conflict of interest
Funding source
The author(s) received no financial support for the research, authorship, and/or publication of this article
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetescare. 2014 Jan 1;37(Supplement 1):S81-90.
- Baynes HW. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J diabetes metab. 2015 May 1;6(5):1-9.5.
- KA. Pathophysiology of type 2 diabetes and its treatment policy. JMAJ. 2010 Feb;53(1):41-6.
- Sugden M, Holness M. Pathophysiology of diabetic dyslipidemia: implications for atherogenesis and treatment. Clinical Lipidology. 2011 Aug 1;6(4):401-11.
- Feyzmand S, Shahbazi B, Marami M, Bahrami G, Fattahi A, Shokoohinia Y. Mechanistic in vitro evaluation of Prosopis farcta roots potential as an antidiabetic folk medicinal plant. Pharmacognosy Magazine. 2017 Oct;13(Suppl 4):S852..
- Ukande MD, Shaikh S, Murthy K, Shete R. Review on Pharmacological potentials of Prosopis juliflora. Journal of Drug Delivery and Therapeutics. 2019 Aug 22;9(4-s):755-60.
- Ahmad, Aqeel & Khan, K. & Ahmad, Viqar& Qazi, Sabiha. (1986). Antibacterial Activity of Juliflorine Isolated from Prosopis juliflora. Planta medica. 52. 285-8. 10.1055/s-2007-969153.
- Saleh I, Abu-Dieyeh MH. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Scientific Reports. 2021 Apr 12;11(1):1-7.
- Sathiya M, Muthuchelian K. Investigation of Phytochemical Profile and Antibacterial Potential of Ethanolic Leaf Extract of Prosopis juliflora DC. Ethnobotanical leaflets. 2008;2008(1):167
- Youssef AS. Phytochemistry and Antibacterial Activity of Prosopis juliflora (SW.) DC. Saudi J PatholMicrobiol. 2021;6(11):427-33
- Vendan KT, Nidoni U. Evaluation on Antifungal Property of Supercritical Carbon Dioxide Extract of Prosopis juliflora Leaves against Plant Pathogens. Int. J. Curr. Microbiol. App. Sci. 2018;7(8):1-9
- Valli S, Gokulshankar S, Mohanty BK, Ranjith MS, Ashutosh SR, Remya V. Anticryptococcal Activity of Alkaloid Rich Fraction of Leaves of Prosopis juliflora: A future promising supplementary therapy for cryptococcosis and cryptococcal meningitis. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6:491-5.
- Bazie S, Ayalew A, Woldetsadik K. Antifungal activity of some plant extracts against Colletotrichum musae the cause of postharvest banana anthracnose. J Plant Path Microb2014;5:1e4.
- Abdul AN, Hadi B, Muhammad AZ, Muhammad ZA, Arshad I, Sohaib R, Izhar M, Sabir HS. Antimicrobial and antioxidant activities of Mimosaceae plants; Acacia modesta Wall (Phulai), Prosopis cineraria (Linn.) and Prosopis juliflora (Swartz). Journal of medicinal plants research. 2012 Apr 23;6(15):2962-70.
- Lakshmibai R, Amirtham D, Radhika S. Preliminary phytochemical analysis and antioxidant activities of Prosopis juliflora and Mimosa pudica leaves. Int J Sci EngTechnol Res. 2015 Aug;4(30):5766-70.
- Utage BG, Patole MS, Nagvenkar PV, Kamble SS, Gacche RN. Prosopis juliflora (Sw.), DC induces apoptosis and cell cycle arrest in triple negative breast cancer cells: in vitro and in vivo investigations. Oncotarget. 2018 Jul 7;9(54):30304.
- Elbehairi SE, Ahmed AE, Alshati AA, Al-Kahtani MA, Alfaifi MY, Alsyaad KM, Alalmie AY, Ahamed MM, Moustafa MF, Alhag SK, Al-Abd AM. Prosopis juliflora leave extracts induce cell death of MCF-7, HepG2, and LS-174T cancer cell lines. EXCLI journal. 2020;19:1282.
- Arya G, Kumari RM, Gupta N, Kumar A, Chandra R, Nimesh S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: reaction optimization, antimicrobial and catalytic activities. Artificial cells, nanomedicine, and biotechnology. 2018 Jul 4;46(5):985-93.
- Anti-diabetic activity of methanolic (seed) extract of prosopisjuliflora (sw.) dc in streptozotocin induced diabetic rats (shobhitprakashsrivastava* and ashutoshmishra in Plant Archives Vol. 20 Supplement 1, 2020 pp. 1046-1050)
- Ukande, M. & Murthy, Krishna &Shete, R. &Solunkhe, R.. (2019). EVALUATION OF HYPOGLYCEMIC ACTIVITY OF PROSOPIS JULIFLORA ON ALLOXAN INDUCED DIABETIC RAT MODEL. INDIAN DRUGS. 56. 33-41. 10.53879/id.56.10.12055.
- du Toit EF, Donner DG. Myocardial insulin resistance: an overview of its causes, effects, and potential therapy. Insulin resistance. 2012 Dec 12.
- Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO. Challenges and issues with streptozotocin-induced diabetes–a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chemico-biological interactions. 2016 Jan 25;244:49-63.
- pal singh, Manish & Pathak, Kamla. (2015). Animal models for biological screening of anti-diabetic drugs: An overview. Euro J Exp Biol. 5. 37-48.
- Nigussie D, Davey G, Legesse BA, Fekadu A, Makonnen E. Antibacterial activity of methanol extracts of the leaves of three medicinal plants against selected bacteria isolated from wounds of lymphoedema patients. BMC Complementary Medicine and Therapies. 2021 Dec;21(1):1-0.)
- Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB. Preliminary Analysis of Phytoconstituents and Evaluation of Anthelminthic Property of Cayratia auriculata (In Vitro). Maedica. 2019 Dec;14(4):350.)
- Das BK, Al-Amin MM, Russel SM, Kabir S, Bhattacherjee R, Hannan JM. Phytochemical screening and evaluation of analgesic activity of Oroxylum indicum. Indian journal of pharmaceutical sciences. 2014 Nov;76(6):571.
- Junaid RS, Patil MK. Qualitative test for preliminary phytochemical screening. International Journal of Chemical Studies. 2020;8(2):603-8.)
- Panchal P, Parvez N. Phytochemical analysis of medicinal herb (Ocimum sanctum). International Journal of Nanomaterials, Nanotechnology and Nanomedicine. 2019 Jul 22;5(2):008-1
- Norshalizah Mamikutty NM, Zar Chi Thent ZC, Shaiful Ridzwan Sapri SR, Natasya Nadia Sahruddin NN, Mohd Rafizul MY, Farihah Haji Suhaimi FH. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats.
- Dupas J, Goanvec C, Feray A, Guernec A, Alain C, Guerrero F, Mansourati J. Progressive induction of type 2 diabetes: effects of a reality–like fructose enriched diet in young Wistar rats. PLoS One. 2016 Jan 22;11(1):e0146821.
- Zarfeshani A, Mutalib MS, Khaza’ai H. Evaluating of high fructose diet to induce hyperglycemia and its inflammatory complications in rats. Pak J Nutr. 2012;11(1):21.
- Kubacka M, Kotańska M, Szafarz M, Pociecha K, Waszkielewicz AM, Marona H, Filipek B, Mogilski S. Beneficial effects of non-quinazoline α1-adrenolytics on hypertension and altered metabolism in fructose-fed rats. A comparison with prazosin. Nutrition, Metabolism and Cardiovascular Diseases. 2019 Jul 1;29(7):751-60.
- Gunawan S, Aulia A, Soetikno V. Development of rat metabolic syndrome models: A review. Veterinary World. 2021 Jul;14(7):1774.
- Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers Center for Drug Evaluation and Research (CDER). 2005 Jul:7.
- 35.Wamburu RW, Kareru PG, Mbaria JM, Njonge FK, Nyaga G, Rechab SO. Acute and sub-acute toxicological evaluation of ethanolic leaves extract of Prosopis juliflora (Fabaceae). Journal of Natural Sciences Research. 2013;3(1):8-15.
- Piero NM, Mwaniki NE, Murugi NJ, Agyirifo SD, Gathumbi KP, Muchugi NA, Mwangi MJ. Antidiabetic effects of aqueous leaf extracts of acacia nilotica in alloxan induced diabetic mice.
- Rosalie IO, Ekype EL. Antidiabetic potentials of common herbal plants and plant products: A glance. International Journal of Herbal Medicine. 2016;4(4):90-7.
- Gupta P., De A. Diabetes mellitus and its herbal treatment. Int. J. Res. Pharm. Biomed. Sci. 2012;3:706–721.
- Mathesius U. Flavonoid functions in plants and their interactions with other organisms. Plants. 2018 Apr 3;7(2):30
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacological reviews. 2000 Dec 1;52(4):673-751.
- Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomedicine & Pharmacotherapy. 2019 Mar 1;111:947-57.
- Mathesius U. Flavonoid functions in plants and their interactions with other organisms. Plants. 2018 Apr 3;7(2):30.
- Mohan SC, Jain N, Sumathi S. Mechanisms of Action of Flavonoids in the Management of Diabetes mellitus. Journal of Drug Delivery and Therapeutics. 2021 Oct 15;11(5-S):194-202.
- Soares JM, Leal AE, Silva JC, Almeida JR, de Oliveira HP. Influence of flavonoids on mechanism of modulation of insulin secretion. Pharmacognosy Magazine. 2017 Oct;13(52):639.
- Soares JM, Leal AE, Silva JC, Almeida JR, de Oliveira HP. Influence of flavonoids on mechanism of modulation of insulin secretion. Pharmacognosy Magazine. 2017 Oct;13(52):639.
- Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomedicine & Pharmacotherapy. 2019 Mar 1;111:947-57.
- Kumar SR, Mohd Ramli ES, Abdul Nasir NA, Mohd Ismail N, MohdFahami NA. Methanolic extract of Piper sarmentosum attenuates obesity and hyperlipidemia in fructose-induced metabolic syndrome rats. Molecules. 2021 Jun 29;26(13):3985.
- Kasim Karakas SE, Vriend H, Almario R, Chow LC, GoodmanMN. Effects of dietary carbohydrates on glucose and lipidmetabolism in golden Syrian hamsters. J Lab Ciln Med 1996;128:208-213.
- Reddy SS, Karuna R, SaralakumariD.Prevention of insulinresistance by ingesting aqueous extract of Ocimum sanctum tofructose fed rats. HormMetab Res 2008; 40: 44-49
- Kok N, Robertroid M, Deizenne N. Dietary oligo fructosemodifies the impact of fructose on hepatic triacylglycerolmetabolism. Metabolism 1996; 45: 1547-50.
- Shivashankara AR, Prabhu AN, Dsouza PP, Baliga BR, Baliga MS, Palatty PL. Antidiabetic and hypoglycemic effects of Syzygiumcumini (Black Plum). Bioactive food as dietary interventions for diabetes. 2013 Jan 1:537-54.
- Kalmar T, Seres I, Balogh Z, Kaplar M, Winkler G, Paragh G. Correlation between the activities of lipoprotein lipase and paraoxonase in type 2 diabetes mellitus. Diabetes & metabolism. 2005 Dec 1;31(6):574-80.
- Gudise V, Chowdhury B, Manjappa AS. Antidiabetic and antihyperlipidemic effects of Argyreiapierreana and Mateleadenticulata: Higher activity of the micellar nanoformulation over the crude extract. Journal of traditional and complementary medicine. 2021 May 1;11(3):259-67.
- Ashfaq A, Khan AU, Minhas AM, Aqeel T, Assiri AM, Bukhari IA. Anti-hyperlipidemic effects of Caralluma edulis (Asclepiadaceae) and Verbena officinalis (Verbenaceae) whole plants against high-fat diet-induced hyperlipidemia in mice. Tropical Journal of Pharmaceutical Research. 2017 Nov 14;16(10):2417-23.
- Ruckmani A, Rajasekaran D, Abinaya E, Arunkumar R. Assessment of Safety and Efficacy of Bromocriptine in Comparison with Teneligliptin in Newly Diagnosed Type 2 Diabetes Mellitus. Biomedical and Pharmacology Journal. 2020 Mar 28;13(1):269-80.








