Mohammed A. Ezghayer 1* , Omar Hussein Ahmed 2
, Omar Hussein Ahmed 2 and Mostafa F. Tawfeeq 3
and Mostafa F. Tawfeeq 3
1Pharmacognosy Department, faculty of pharmacy, Tikrit university, Tikrit, Iraq.
2Pharmaceutical chemistry Department, faculty of pharmacy, Tikrit university, Tikrit, Iraq.
Corresponding Author E-mail: moh.cognosy@tu.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2920
Abstract
The work aimed to explore the phytochemicals of various fractions of Xanthium strumarium fruit extract since this plant is found to be toxic for both humans and animals in Iraq. The extracts underwent phytochemical screening, which indicated the occurrence of flavonoids, alkaloids, phenols, coumarin, saponin, tannins, and terpenoids. After extraction, fractionation was done using hexane, chloroform, and N-butanol respectively, for identifying the phytochemicals found in each fraction, UPLC-electrospray ionization–tandem mass spectroscopy was used which revealed the presence of 6 sterol and terpenes compound in hexane fraction, Nine compounds were detected in the chloroform fraction, among which 2-acetyl-atractyligenin and Artemisinin were the primary components. Additionally, this particular plant has been found to contain these compounds for the first time. Furthermore, Pungiolide C, dihydroartemisinin, and atractylenolide II were also detected for the first time in this plant. For the n-Butanol fraction, eight diterpene glycosides were recognized, with Atractyloside and Carboxyatractyloside and their desulphated derivatives as the major compounds responsible for the plant's toxicity. The butanol fraction also showed the presence of eight phenolic compounds, among which caffeoylquinic acid derivatives and flavonoid Syringetin were the major compounds in this fraction.
Keywords
Artemisinin; Carboxyatractyloside; UPLC-ESI-MS/MS; Xanthium strumarium
Download this article as:| Copy the following to cite this article: Ezghayer M. A, Ahmed O. H, Tawfeeq M. F. UPLC-ESI-MS\MS Phytochemicals Profiling of n-Butanol Chloroform and Hexane Fraction of Xanthium strumarium Fruit Extract. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Ezghayer M. A, Ahmed O. H, Tawfeeq M. F. UPLC-ESI-MS\MS Phytochemicals Profiling of n-Butanol Chloroform and Hexane Fraction of Xanthium strumarium Fruit Extract. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3Va7t2l |
Introduction
Xanthium strumarium L. is categorized as a type of weed which falls under the Asteraceae family. It is often encountered and seen commonly in roadside ditches, hedges, and rice fields.1 The plant’s aerial parts, which refer to the portions above the ground, are believed to contain different types of harmful alkaloids that haven’t been identified yet. These aerial parts also contain sesquiterpene lactones like xanthinin, xanthumin, and xanthatin (deacetylxanthinin), as well as toxic principles such as a sulphated glycoside called xanthostrumarin, atractyloside, and carboxyatractyloside, and phytosterols such as xanthanol2. Caffeoylquinic acids; and tocopherol.3 Carboxyatractyloside (CAT), a kaurene glycoside formerly known as xanthostrumarium, has been discovered as the principal poisonous component extracted from the plant 4.
CAT is water soluble, but it cannot be boiled away (decocted), and it is not simply rinsed away. Instead, eliminating the prickles—which can be partially done by stir-frying alone—is the greatest strategy to eliminate the poisonous component. 5. Xanthium fruits predominantly contain a group of derivatives known as caffeoylquinic acid, which belong to the phenolic acid class. The therapeutic applications of a fruit may depend on the type and level of caffeoylquinic acid derivatives present in it. Consequently, it is essential to verify the chemical composition and accurately identify each caffeoylquinic acid to evaluate the overall quality. The aforementioned factors are critical in determining the utility of Xanthium fruits for their medicinal properties6.
The aim of the study was to employ ultra-performance liquid chromatography equipped with electrospray ionization (ESI) tandem mass spectrometry, for the characterization of the toxic phytochemicals and chemical constituents present in the fruits of X. strumarium that grow naturally in Iraq.
Materials and Methods
Collection of the plant material
Fruits of Xanthium strumarium were gained from Sinjar city, located near Mosul in Iraq, and were authenticated at Tikrit University’s College of Agriculture. Following cleaning, the fruits were dried at room temperature in a shaded area. They were then crushed into a powder and preserved for later use.
Method of Work
A quantity of 100 grams of fruit powder that has been air-dried is measured. Petroleum ether is used to defat the powder by overnight maceration to remove any waxy material and chlorophyll. The powder is then extracted for six hours using the Soxhlet, and the solvent used is (600 ml) 90% ethanol. The rotary evaporator is utilized to dry the extract. After the drying process, the weight of the extract is measured, and it is determined that the extraction yield is 31 grams. The extract is then dissolved in 100 milliliters of distilled water. Using a separatory funnel, the upper layers are partitioned with 100 milliliters of n-hexane three times, and then mixed and dried using rotary evaporators. To obtain a chloroform fraction, the remaining aqueous portion is partitioned with 100 ml of chloroform for three successive times, the lower chloroform layers are combined and dried. To obtain the butanol fraction, the aqueous portion from the previous step is partitioned with butanol three successive times then combined and dried by using a rotary evaporator.
Several qualitative phytochemical screening tests were conducted. These tests offer crucial information about the kinds of secondary metabolites present in the plant and help design an appropriate method for the isolation of these metabolites to determine the chemical composition of the crude extract. Crude fruit extracts were subjected to a preliminary phytochemical analysis, which identified the presence of flavonoids, alkaloids, phenols, coumarin, saponin, tannins, and terpenoids.
Method for UPLC
UPLC-ESI-MS/MS technique was employed in the experiment using a triple quadrupole instrument called XEVO TQD. The instrument was manufactured by Waters Corporation in Milford, MA01757 U.S.A. The ESI-MS was operated in negative ion acquisition mode and the Column used is ACQUITY UPLC- BEH C18 1.7 µm – 2.1 × 50 mm. The rate of flow was set at 0.2 mL/min. The solvent system was comprised of two solutions: water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B).
To prepare the sample solution, HPLC analytical grade methanol was used and then filtered using a membrane disc filter (0.2 m) before being subjected to LC-ESI-MS analysis. Before injecting into the UPLC apparatus, the sample mobile phase was prepared through sonication and filtering with a 0.2-m filter membrane disc. A sample injection quantity of 10 μL was used. Negative ion mode analysis was conducted using the following parameters: 150 °C as source temperature, 30 eV for the cone voltage, 3 k for the capillary voltage, 440 °C for the desolvation temperature, 50 L/h for the cone gas flow, and 900 L/h for the desolvation gas flow.
ESI mass spectrum showed m/z range of (100–1000). The peaks and spectra were analyzed using the software Maslynx 4.1, and the tentative identification of the peaks was made by comparing their retention times (Rt) and MS spectra with reported data 7.
Results
Butanol Fraction
By using the obtained data from UPLC-ESI-MS/MS that are mass fragmentations pattern, LC Rt, and molecular weights and comparing it with previous research and phytochemical mass libraries such as Respect, MONA, GNPS, CFM-id, and PubChem. sixteen phytochemicals have been tentatively identified in the butanol fraction. Figure 1 represents the total ion chromatograms (TICs) of the compounds extracted from the butanol fraction.
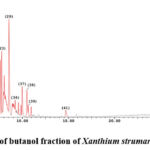 |
Figure 1: TIC of butanol fraction of Xanthium strumarium fruit extract. |
The fruit extract of Xanthium strumarium was analyzed using UPLC-ESI-MS/MS and the n-butanol fraction was found to contain a number of phytochemicals, as listed in Table 1. It was observed that the plant contained a significant amount of atractyloside derivatives, which could be a possible explanation for the plant high toxicity.
Table 1: The compounds discovered in the butanol fraction of the extract of Xanthium strumarium fruits are identified by their peak assignments. fragmentation pattern with Negative ion mode for the precursor ions and their diagnostic daughter ions are used for this purpose.
|
Peak no. |
Identified compound |
Precursor ion [M-H] |
MS\MS m\z ESI- |
Rt.min |
Ref. |
|
diterpene glycosides |
|||||
|
21 |
Carboxyatractyloside |
769 |
689,645,609 |
7.36 |
8,9 |
|
24 |
Atractyloside |
725 |
645,565,362,322 |
7.98 |
10,11 |
|
23 |
4-desulfated carboxyatractyloside |
689 |
645, 609, 344,362 |
7.76 |
12 |
|
25 |
3,4-didesulfated carboxyatractyloside |
609 |
459, 565, 362 |
8.05 |
12,13 |
|
32 |
Atractyloside III |
565 |
481, 463, 319 |
9.03 |
14 |
|
34 |
Atractyloside V |
659 |
481, 369,329 |
9.21 |
14 |
|
33 |
Atractyloside Methyl Ester |
739 |
659,565,369,329 |
9.10 |
10,15 |
|
34 |
Atractyligenin-2-O-β-D-glucoside |
481 |
369,329,317,301,145 |
9.21 |
16 |
|
Phenolic compounds |
|||||
|
29 |
Syringetin dimer |
691 |
346,330,316 |
8.55 |
17 |
|
14 |
Quercetin glucuronide |
477 |
301, 179, 151 |
6.46 |
17 |
|
13 |
Ononin |
429 |
267,252.224 |
6.16 |
18,19 |
|
18 |
dicaffeoylquinic acid |
515 |
353,299, 191, 173 |
6.80 |
6,20 |
|
3 |
caffeoylquinic acid |
353 |
191,179,173 |
2.95 |
21,22 |
|
4 |
caffeoylquinic acid dimer |
708 |
353,191,179 |
2.95 |
20,22 |
|
12 |
Methylchlorogenate |
368 |
191,163,295 |
5.79 |
23 |
|
20 |
Tricaffeoylquinic acid |
677 |
515,497,353 |
7.18 |
17,24 |
From the above data the major compounds in butanol fraction are Syringetin dimer in peak 29 and 4-desulfated carboxyatractyloside in peak 23 and various atractyloside derivatives.
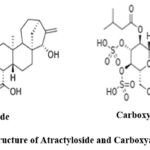 |
Figure 2: structure of Atractyloside and Carboxyatractyloside. |
For Carboxyatractyloside M-H is 769 and the product ions are [M-SO3-H] m/z 689, [M-SO3-COO-H] m\z 645, which is 4-desulfated atractyloside and [M-2SO3-H] m\z 609 which is 3,4-didesulfated carboxyatractyloside.
For atractyloside M-H is 725 and the product ions are [M-SO3-H] m\z 645 which is 4-desulfated atractyloside , [M-2SO3-H] m\z 564 corresponding to 3,4-didesulfated atractyloside, also the fragment [ATR − 2H]2 m\z 362 result from the presence of tow ionization site as shown in Fig.3.
4-desulfated carboxyatractyloside M-H 689 produced the same product ions as that of carboxyatractyloside and atractyloside which reflect they are derivatives 10
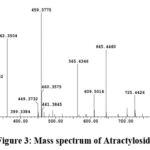 |
Figure 3: Mass spectrum of Atractyloside |
For Syringetin Loss of methyl groups from the 3-methoxy and 6-methoxy of the molecules result in the product ion of m\z 330 and m\z 315 respectively 25 as in Fig.5. Presence of caffeoylquinic acid derivatives is also common in Xanthium genus.
 |
Figure 4: mass1spectrum of Syringetin dimer |
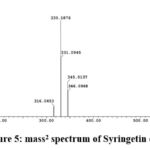 |
Figure 5: mass2 spectrum of Syringetin dimer |
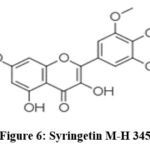 |
Figure 6: Syringetin M-H 345. |
Chloroform fraction
In chloroform fraction 9 compounds among which terpenoids and sterols were tentatively identified in the same manner in butanol fraction.
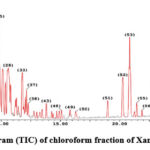 |
Figure 7: Total ion chromatogram (TIC) of chloroform fraction of Xanthium strumarium fruit extract. |
Table 2: Peaks assignments for compounds detected in the chloroform fraction of the extract of Xanthium strumarium fruits were made using the precursor ions fragmentation pattern and their daughter ions in positive ionization mode.
|
Peak no. |
Identified compound |
Precursor ion [M+H] |
MS\MS m\z ESI- |
Rt.min |
Ref. |
|
33 |
atractylenolide II |
233 |
215,187,175, |
11.78 |
26,27 |
|
19 |
4-epi-Xanthanol |
249 |
229,205,189 |
7.53 |
26,28 |
|
25 |
2-acetyl-atractyligenin |
363 |
345,328,303,297 |
9.43 |
29 |
|
53 |
Artemisinin dimer |
564 |
282,265,247,97 |
20.54 |
30,31,32 |
|
57 |
dihydroartemisinin |
285 |
267,249,144,88 |
23.05 |
33 |
|
33 |
ent-kaurene |
273 |
233,215,187 |
11.78 |
34 |
|
6 |
Carboxytractyligenin |
365 |
318,235,128 |
6.61 |
35,29 |
|
58 |
Pungiolide C |
512 |
493,449,343 |
24.28 |
36,37,38 |
|
51 |
Dihydroxyxanthatin |
281 |
263,245,128 |
18.98 |
37,38 |
In this fraction 2-acetyl-atractyligenin is the major compound and account for the toxic effect of this fraction along with xanthatin a sesquiterpene lactone. and its derivatives.
From the TIC of chloroform fraction in Fig.7 , Artemisinin dimer at peak 53 is major compound, and the mass spectrum of the compound Fig.8 the precursor ion [M+H] m/z 283 and [M+H]2 m/z 564 shows two ionization site and the fragment [M+H-H2O] m/z 265 and [M+H-2H2O] m/z 247.
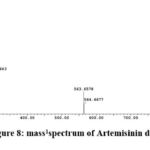 |
Figure 8: mass1spectrum of Artemisinin dimer |
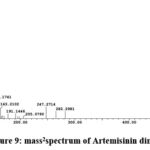 |
Figure 9: mass2spectrum of Artemisinin dimer |
Hexane fraction
Hexane fraction revealed the presence of 6 compounds (table 3) among which are sterols and terpenes. It is evident that hexane fraction does not contain toxic metabolites. It is also not uncommon to contain hexadecenoic acid since the fruit contains seeds inside that rich in fatty acid.
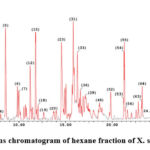 |
Figure 10: (TIC) Total ions chromatogram of hexane fraction of X. strumarium fruit extract. |
Table 3: The recognition of the compounds presents in hexane fraction of the extract obtained from Xanthium strumarium fruits was done by analyzing the fragmentation pattern of precursor ions and their diagnostic daughter ions. This analysis was performed in negative ion mode to determine the peak assignments accurately.
|
Peak no. |
Identified compound |
Precursor ion [M-H] |
MS\MS m\z ESI- |
Rt.min |
Ref. |
|
26 |
beta-Sitosterol |
414 |
396,297,273,133 |
15.5 |
18,2 |
|
55 |
β-amyrin |
425 |
407,393,218,191 |
21.30 |
26,18 |
|
61 |
Hexadecanoic acid |
255 |
239, 200, 127, 97 |
22.76 |
27 |
|
50 |
Kaur-16-ene |
271 |
255,239,187 |
18.9 |
28 |
|
46 |
stigamasterol |
411 |
367, 271, 255, |
18 |
29 |
|
15 |
Unknown sesquiterpene |
249 |
192,191,148,57 |
11.77 |
Discussion
This is the first study that identify eight toxic atractyloside derivatives in this species in the butanol fraction of the extract and since most of these derivatives are polar, they all eluted in the first ten minutes as in Fig.1.
The negative ionization mode for ESI gave more clear data for the butanol fraction than the positive ionization mode. As seen in Table 1 all the atractyloside derivatives differ only in loss of sulfated, carboxyl, and disulfated groups and are consistent with the fragmentation pattern of that derivative.
It is not uncommon for the plant extract to contain dimers of certain phytochemicals, and this can be seen in the first mass chart as a doubling of m/z as it contains two ionization sites while in the second mass normal fragments are seen and consistent with that compound as in Fig. 4 and Fig.5 of Syringetin dimer.
In chloroform fraction, we can see that most of the identified compounds are aglycone derivatives of compounds in butanol fraction, and this further supports the identification of these phytochemicals.
Artemisinin is identified for the first time in this plant and being a major compound may facilitate the utilization of this plant as source for Artemisinin.
Hexane fraction contains some major unidentified peaks ( peak 5,15,23,31 )which may reflect new compounds that need to be isolated and characterized. So this type of research allows researchers to focus on compounds that are not being identified yet instead of separating already-known compounds.
Conclusion
This study highlights the significance of using UPLC-ESI MS/MS for the provisional identification of unidentified chemical compounds. although this type of identification is not sufficient it gives important information before isolation of the required compounds, it also plays a role in confirmation of known compounds.
The toxicity of the fruit is due to atractyloside derivatives identified in N-butanol and chloroform fractions with 4-desulfated carboxyatractyloside being the major one in butanol fraction and 2-acetyl-atractyligenin in chloroform fraction. with no identified toxic compounds in hexane fraction. There are many of unidentified compounds in hexane fraction that needed to be identified.
Acknowledgement
We need to thank Central laboratory staff of Tikrit university for their valuable support.
Conflict of Interests
The writers state that they do not have any competing interests to declare.
Funding Sources
The work is self-funded.
References
- Sastry, S. K., Mandal, B., Sano, T. & Hammond, J. Xanthium strumarium (Cocklebur). in Encyclopedia of Plant Viruses and Viroids 2809–2811 (Springer, 2019).
CrossRef - Fan, W. et al. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 24, 359 (2019).
CrossRef - Vladymyrova, I. V, Georgiyants, V. A., Tishakova, T. S. & Shumova, H. S. Investigation of the effect of xanthium strumarium l. extract on the level of thyroid-stimulating hormones and mass coefficient of rat thyroid gland. Научный журнал «Вестник НАН РК» 15–22 (2019).
CrossRef - Han, T. et al. Authentication and quantitative analysis on the chemical profile of Xanthium fruit (Cang-Er-Zi) by high-performance liquid chromatography-diode-array detection tandem mass spectrometry method. Anal Chim Acta 634, 272–278 (2009).
CrossRef - Juan-xiu, L. I. U. et al. Differences of Chemical Compositions in Xanthii Fructus before and after Stir-Frying by UPLC-Triple TOF MS/MS. Journal of Chinese Mass Spectrometry Society 38, 157 (2017).
- Han, T. et al. Bioactivity-guided fractionation for anti-inflammatory and analgesic properties and constituents of Xanthium strumarium L. Phytomedicine 14, 825–829 (2007).
CrossRef - Al-Saleem, M. S. M. et al. Metabolic Profiling and In Vitro Assessment of the Biological Activities of the Ethyl Acetate Extract of Penicillium chrysogenum “Endozoic of Cliona sp. Marine Sponge” from the Red Sea (Egypt). Mar Drugs 20, 326 (2022).
CrossRef - van Kiem, P., Hoang, N. H., Thu, V. K., Tai, B. H. & Nhiem, N. X. Diterpene glycosides and phenolic compounds from the fruits of Xanthium strumarium. Vietnam Journal of Chemistry 58, 648–653 (2020).
CrossRef - Botha, C. J., Lessing, D., Rösemann, M., van Wilpe, E. & Williams, J. H. Analytical confirmation of Xanthium strumarium poisoning in cattle. Journal of Veterinary Diagnostic Investigation 26, 640–645 (2014).
CrossRef - Brucoli, F., Borrello, M. T., Stapleton, P., Parkinson, G. N. & Gibbons, S. Structural characterization and antimicrobial evaluation of atractyloside, atractyligenin, and 15-didehydroatractyligenin methyl ester. J Nat Prod 75, 1070–1075 (2012).
CrossRef - Yang, L. et al. RRLC-MS/MS method for the quantitation of atractyloside in Fructus Xanthii (Xanthium sibiricum). Analytical Methods 5, 2093–2097 (2013).
CrossRef - Varga, E., Domokos, E., Kelemen, H., Fulop, I. & Kursinszki, L. HPLC-ESI-MS/MS Profiling of Phenolic Acids, Flavonoids And Sesquiterpene Lactones from Xanthium spinosum. Rev. Chim 71, 558–564.
CrossRef - Jiang, H. et al. The fruits of Xanthium sibiricum Patr: A review on phytochemistry, pharmacological activities, and toxicity. World Journal of Traditional Chinese Medicine vol. 6 408–422 Preprint at https://doi.org/10.4103/wjtcm.wjtcm_49_20 (2020).
CrossRef - Tsukui, A. et al. Direct-infusion electrospray ionization-mass spectrometry analysis reveals atractyligenin derivatives as potential markers for green coffee postharvest discrimination. LWT 103, 205–211 (2019).
CrossRef - Cheng, Y., Fu, J., Chen, L., Li, L. L. & Qu, J. Diterpenoid glycosides and monoterpenoid glycosides from the fruits of Xanthium chinense. J Asian Nat Prod Res 21, 207–216 (2019).
CrossRef - Lang, R., Beusch, A. & Dirndorfer, S. Metabolites of dietary atractyligenin glucoside in coffee drinkers’ urine. Food Chem 405, 135026 (2023).
CrossRef - Poljuha, D. et al. LC–DAD–MS phenolic characterisation of six invasive plant species in Croatia and determination of their antimicrobial and cytotoxic activity. Plants 11, 596 (2022).
CrossRef - Amina_Sultana_Chemistry_2015_FUUAST_Karachi_22.06.2016.
- Han, T., Li, H., Zhang, Q., Zheng, H. & Qin, L. New thiazinediones and other components from Xanthium strumarium. Chem Nat Compd 42, 567–570 (2006).
CrossRef - Kamboj, A. & Saluja, A. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). International Journal of Green Pharmacy vol. 4 129–139 Preprint at https://doi.org/10.4103/0973-8258.69154 (2010).
CrossRef - Wollenweber, E., Dörr, M., Fritz, H. & Valant-Vetschera, K. M. Exudate flavonoids in several Asteroideae and Cichorioideae (Asteraceae). Zeitschrift für Naturforschung C 52, 137–143 (1997).
CrossRef - Zhang, Y. et al. Qualitative and quantitative determination of Atractylodes rhizome using ultra-performance liquid chromatography coupled with linear ion trap–Orbitrap mass spectrometry with data-dependent processing. Biomedical Chromatography 33, (2019).
CrossRef - Pandey, D. P. & Rather, M. A. Isolation and Identification of Phytochemicals from Xanthium strumarium. Int J Chemtech Res 4, 266–271 (2012).
CrossRef - Varga, E., Domokos, E., Kelemen, H., Fulop, I. & Kursinszki, L. HPLC-ESI-MS/MS Profiling of Phenolic Acids, Flavonoids And Sesquiterpene Lactones from Xanthium spinosum. Rev. Chim 71, (2020).
CrossRef - Favre, G. et al. New acylated flavonols identified in Vitis vinifera grapes and wines. Food Research International 112, 98–107 (2018).
CrossRef - Opiyo, S. A. Triterpenes and Sterols from Ocimum suave. (2022).
- Kaur, M., Kamboj, A., Rathour, A. & Saluja, A. K. Isolation and Characterization of Constituents from the Leaves of Xanthium strumarium and their Evaluation for Antioxidant and Antimicrobial Potential. Nat Prod Chem Res 3, 2 (2015).
CrossRef - Silva, M. L. et al. Selective cytotoxicity of ent‐kaurene diterpenoids isolated from Baccharis lateralis and Baccharis retusa (Asteraceae). Arch Pharm (Weinheim) 355, 2200083 (2022).
CrossRef - Kamboj, A., Pooja, A. & Saluja, A. K. Isolation and Characterization of Bioactive Compounds from the Petroleum Ether Extracts of Leaves of Xanthium Strumarium Linn. BioMedRx 1, 235–238 (2013).








