Anu Altangerel1,2 , Chimedragchaa Chimedtseren2*
, Chimedragchaa Chimedtseren2* , Myadagbadam Urtnasan2
, Myadagbadam Urtnasan2 , Dejidmaa Buyantogtokh2
, Dejidmaa Buyantogtokh2 , Dagvatseren Begzsuren2
, Dagvatseren Begzsuren2 and Zulgerel Dandii3
and Zulgerel Dandii3
1International School of Mongolian Medicine, Mongolian National University of Medical Science, Ulaanbaatar, Mongolia.
2Institute of Traditional Medicine and Technology, Ulaanbaatar, Mongolia.
3School of Medicine, Mongolian National University of Medical Science, Ulaanbaatar, Mongolia.
Corresponding Author E-mail: ch.chimedragchaa@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2934
Abstract
Marchin-13 Tang is a traditional Mongolian medicine widely used to reduce blood pressure. The study aimed to evaluate the anti-hypertensive effect of Marchin-13 (M-13) Tang in the L-NAME-induced model of hypertension. The biological composition activity of M-13 Tang was examined using the UV spectrophotometric method. The experimental groups induced Hypertension models by L-NAME 40 mg/kg. The concentrations of serum nitric oxide (NO), Angiotensin-Converting Enzyme (ACE), superoxide dismutase (SOD), malondialdehyde (MDA), and catalase (CAT) were measured in M-13 Tang treatment groups by enzyme-linked immunosorbent assay (ELISA). The content of total phenolics was measured at 2.96±0.16%, while flavonoids were found to be present at a level of 1.81±0.1%. The reductions in mean arterial pressure (MAP) were statistically significant. They were observed from day 14 to day 21 after giving M-13 Tang at 90 mg/kg and 180 mg/kg (p<0.01). Moreover, treated with M-13 Tang 90 mg/kg and 180 mg/kg groups, serum levels of NO, SOD, and CAT were significantly (p<0.01) increased compared with the L-NAME (40 mg/kg) group. The levels of MDA and ACE showed a significant decrease in both the Marchin-13 Tang-treated groups compared to the L-NAME group (p<0.05). The results of our study illustrate that Marchin-13 effectively reduced blood pressure by mitigating oxidative stress, enhancing NO production, and decreasing ACE levels in hypertensive rats induced by L-NAME.
Keywords
Hypertensive; L-NAME; Marchin-13; Mongolian traditional medicine; Nitric oxide
Download this article as:| Copy the following to cite this article: Altangerel A, Chimedtseren C, Urtnasan M, Buyantogtokh D, Begzsuren D, Dandii Z. The Antihypertensive Effect of Marchin-13 Tang on L-NAME-induced Hypertension in Rats. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Altangerel A, Chimedtseren C, Urtnasan M, Buyantogtokh D, Begzsuren D, Dandii Z. The Antihypertensive Effect of Marchin-13 Tang on L-NAME-induced Hypertension in Rats. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3RLoEVR |
Introduction
The World Health Organization (WHO) has conveyed that around 1.28 billion adults across the globe experience hypertension1. In Mongolia, hypertension accounts for 50% of all cardiovascular ailments, making it the leading cause of mortality. This underscores the significant public health issue posed by hypertension in Mongolia, where it contributes to one of the world’s highest rates of death from hemorrhagic stroke2,3. Arterial hypertension is often treated with modern pharmaceuticals, but these medications usually lead to various side effects4. The WHO reports a rising trend in using herbal medicines in primary healthcare within developing countries, home to approximately 70 to 80 percent of the world’s population5. Consequently, there is a growing interest in researching inexpensive herbal medicines with few side effects6,7. Numerous traditional medicinal Tangs have been utilized in the treatment of hypertensive disease.According to research by Bin Dai, the traditional Chinese medicine Gao Zhi Yao contains herbs with various medicinal properties and can lower blood pressure and restore the cardiovascular system by nitric oxide (NO) and angiotensin II (Ang II) in the hypertension model8.
In traditional Mongolian medicine, Marchin-13 (M-13) Tang is used for treatment and has a history of use in traditional medicine for treating symptoms such as high blood pressure, stiff neck, and headaches9. Despite this, its effect action, and chemical composition have not been studied. Therefore, evaluating the antihypertensive effects of Marchin-13 Tang is important for further use in clinical practice. The M-13 Tang comprises C. tinctorius L, S. manshurica L, Fructus G. jasminoides J, R. cordifolia L, Trollius asiaticus L, Quercus robur L, Z. officinale Roscoe, P. incarnata (DC.) Freyn, Fructus T. bellirica Roxb, Radix I. helenium L, Radix S. alopecuroides L, Fructus T. chebula Retz, and A. guttata Bunge9. The pharmacological effects of these components are anti-inflammatory, antioxidant, neuroprotective, and antihypertensive. For example, the C. tinctorius L extract exhibits antihypertensive effects in hypertensive models induced by L-NAME10. The compound aloperine in S. alopecuroides L can improve nervous system function and lower blood pressure while exhibiting anti-inflammatory, pain-relieving, soothing, anti-tumor, and antibacterial properties11. Inula helenium L demonstrated anti-inflammatory, antioxidant, neuroprotective, and antiproliferative effects on cancer cell lines including antibacterial, antifungal, and prebiotic properties12.
N(gamma)-nitro-L-arginine methyl ester (L-NAME) is commonly utilized internationally to induce a pathological model of hypertension in experimental animals, leading to a decrease in the production of nitric oxide (NO)13. The development of hypertension has been linked to oxidative stress, as evidenced by heightened levels of lipid peroxidation in individuals with high blood pressure. The surge in oxidative stress is known to diminish the availability of NO, a potent vasodilator, contributing to the onset of hypertension14. L-NAME causes an increase in angiotensin-converting enzyme (ACE) activity, and this elevated ACE activity subsequently promotes the transformation of Ang I (Angiotensin I) to Ang II (Angiotensin II). Angiotensin II (Ang II) directly causes constriction of blood vessels, resulting in increased blood pressure15.
Consequently, this research aims to assess the impact of Marchin-13 Tang on ACE activity, NO levels, antioxidant enzyme function, and malondialdehyde (MDA) in rats with hypertension induced by L-NAME. Captopril was used in the study as a positive control due to its ACE-inhibitory properties.
Materials and methods
Reagent and Drugs
N(gamma)-nitro-L-arginine methyl ester Hydrochloride (L-NAME, N0661; EC No. 257-116-1) and reference of gallic acid, rutin was picked up from Sigma-Aldrich Co.,(USA). Enzyme-linked immune sorbent assay (ELISA) kits purchased from MLBio Co. (China) were used in the study. The Marchin-13 Tang (serial number 951017), was purchased from the traditional pharmacy of the Institute of Traditional Medicine and Technology (ITMT) of Mongolia. Captopril (No: 10722A, Sopharma, Bulgaria) was purchased from a pharmacy in Ulaanbaatar, Mongolia.
Animals
We obtained 50 healthy male Wistar rats weighing between 210 and 250 grams from the experimental animal center at the ITMT of Mongolia. These rats were accommodated in controlled environment conditions: temperature at 20±1°C, humidity at 50-60%, a 12-hour light/dark cycle was established, and automatic ventilation was provided 8-15 times per hour. They were supplied with standard nutrients and water ad libitum.
Ethical Statement
We conducted the study per the animal experiment guidelines after obtaining approval from the MNUMS Ethics Committee (permit №2021/3-06).
Chemical analysis
Sample preparation
1 g of the powder of M-13 Tang was precisely weighed and extracted with 100 mL of 70% ethanol for 20 minutes at 700C, then cooled and filtered (Solution A).
Estimation of total flavonoid contents
3 mL of solution A was mixed with 6 mL of distilled H2O and 1 mL NaNO2 (5% w/v in water), shaken for 6 minutes, and 1 mL of 10% Al (NO3)3. After 6 minutes, 10 mL of 4% HCL was added and diluted up to 25 mL with distilled H2O, kept at room temperature for 15 min16. The absorbance was determined using Ultraviolet-visible Spectrophotometry at 500. Rutin equivalent was represented as the value of total flavonoids suggested in the M-13 Tang17.
Quantification of total phenolic compounds
The assessment of total phenolic compound content was conducted employing the Folin-Ciocalteu reagent16. Solution A had 99.6 mL of water added to it (Solution B). 10 mL of Solution B was mixed with 1 mL of 10% aqueous Folin-Ciocalteu solution and 14 ml aqueous Na2CO3 solution (10.75% w/v in water) stirred and left for 40 min. The absorbance value was measured in the test solution at 760 nm using a UV spectrophotometer (UV/VIS, Italy). The gallic acid equivalent was used to represent the content of the polyphenolic compound found in the M-13 Tang18.
Preparation of Marchin-13 Tang
Marchin-13 Tang was purchased from the traditional pharmacy of the Mongolian ITMT (Ulaanbaatar, Mongolia). The composition of the raw materials of Marchin-13 Tang is shown in Table 1. The decoction was prepared as a water extract with a concentration of 1:10 of M-13 powder according to the instructions of the National Pharmacopoeia of Mongolia (2011)19.
Table 1: Composition of M-13 Tang
|
Latin name |
Amount (g) |
Herb part |
Pharmacological effects |
|
Carthamus tinctorius L |
0.130 |
Herb |
Antihypertensive, antioxidant, anti-inflammatory and cardioprotective20 |
|
Sambucus manshurica L |
0.210 |
Caulis |
Antihypertensive, antioxidant, antimicrobial, antidiabetic, anti-inflammatory and antidepressant21 |
|
Gardenia jasminoides J. Ellis |
0.086 |
Fructus |
Antihyperglycemic, anti-atherosclerotic, anti-inflammatory, anti-arthritis, anti-apoptotic, antioxidant, anti-angiogenic, and antithrombotic22 |
|
Rubia cordifolia L |
0.080 |
Radix and rhizoma |
antibacterial, antioxidant, anticancer, anti-inflammatory, analgesic and hepatoprotective23 |
|
Trollius asiaticus L |
0.064 |
Herb |
Antiviral, antibacterial, anti-inflammatory and antioxidant24 |
|
Quercus robur L |
0.064 |
Fructus |
Antioxidant, antibacterial, antifungal, antidiabetic25 |
|
Z. officinale Roscoe |
0.064 |
Radix |
Antioxidant, anti-inflammatory, a blocker of voltage-dependent Ca2+ channels, a regulator of endothelial dysfunction and NO synthesis, an inhibitor of angiogenesis26 |
|
Pyrola incarnata (DC.) Freyn |
0.061 |
Folium |
Antioxidant27, Antimicrobial28 |
|
Terminalia bellirica (Gaertn.) Roxb |
0.061 |
Fructus |
Antihypertensive, Ca++ antagonist29 |
|
Inula helenium L |
0.052 |
Radix |
Anti-inflammatory, antioxidant, neuroprotective, antiproliferative30 |
|
Sophora alopecuroides L |
0.049 |
Radix |
Antihypertensive, anti-inflammatory, pain-relieving, soothing, anti-tumor, and antibacterial properties31 |
|
Terminalia chebula Retz |
0.049 |
Fructus |
Antihypertensive, ACE inhibitor32, antioxidant, anti-inflammatory, hepatoprotective, nephroprotective, antibacterial33 |
|
Arnebia guttata Bunge |
0.042 |
Radix |
Antibacterial, anti-inflammatory, hepatoprotective, antiviral34 |
Experimental Protocols
The experimental protocols involved randomizing the animals into five groups comprising 10 rats and subjecting them to various treatments over 21 days.
Group I served as the control and received distilled water orally.
Group II the hypertensive control, received L-NAME orally at 40 mg/kg/day35.
Group III received Captopril orally at 5 mg/kg/day35 and L-NAME at 40 mg/kg/day.
Group IV received oral administration of M-13 Tang at 90 mg/kg/day doses and L-NAME at 40 mg/kg/day.
Group IV received oral administration of M-13 Tang at 180 mg/kg/day doses and L-NAME at 40 mg/kg/day.
We assessed rat systolic and diastolic blood pressure using tail-cuff sensors on days 0, 7, 14, and 21.
Measurement of Systolic and Diastolic Blood Pressure
Noninvasive blood pressure measurements were conducted using a tail cuff on the rat’s tail. (Systole, Neurobotics, LLC, Moscow, Zelenograd). To perform noninvasive blood pressure measurements in rats, the animals were initially pre-warmed to a temperature of 28-32 degrees Celsius for 10-15 minutes. This step ensures adequate blood circulation in the tail and stabilizes blood flow36. A Phlogiston heating platform was utilized for heating (Phlogiston platform, Neurobotics, LLC, Moscow, Zelenograd)37. The Daniel DeMers method calculated the mean arterial pressure (MAP)38.
Enzyme-linked Immune sorbent assay (ELISA)
We collected blood samples 21 days later, let them stand for 15 minutes at room temperature, and then centrifuged them at 3000 rpm for 10 minutes to separate the serum. We measured the levels of Rat SOD (superoxide dismutase, sensitivity: 0.1 pg/ml), Rat CAT (catalase, sensitivity: 0.1 ng/L), Rat NO (nitric oxide, sensitivity: 0.1 μmol/L), Rat ACE (angiotensin converter enzyme, sensitivity: 0.1 ng/L), and Rat MDA (malondialdehyde, sensitivity: 0.01 nmol/L) using ELISA kits (Shanghai MLBIO Biotechnology Co.). According to the manufacturer’s instructions, an ELISA kit is made using a microplate reader (ChroMate-4300, Awareness Technology Co., USA).
Statistical analysis
The mean and standard deviation (SD) were computed for the observed values within each group, and the analysis used GraphPad Prism-9 software. A nonparametric Kruskal-Wallis test was used for the statistical analysis. The significance level was p < 0.05.
Results
Polyphenolic compound and flavonoid contents
The polyphenolic compounds content of the ethanolic extract in M-13 Tang measured from the regression equation of calibration curve (y=0.084x – 0.001, r² =0.999) were 29.6±0.16 mg/g. The content of flavonoids in the ethanolic extract of M-13 tang, in rutin equivalent, measured using the calibration curve’s regression equation (y=0.041x + 0.007, r2 =0.9911) and are being represented in RuE were 18.1±0.1 mg/g. (Table 2).
Table 2: Polyphenolic and flavonoids in ethanol extract of the M-13 Tang
|
№ |
Bioactive compounds of M-13 Tang |
Standard reagent |
Content (mg/g) |
|
1. |
Total flavonoids |
Rutin |
18.1±0.1 mg/g |
|
2. |
Polyphenolic compounds |
Gallic acid |
29.6±0.16 mg/g |
Effects of M-13 Tang on blood pressure in hypertensive model
No significant differences were observed in MAP values among the five groups under investigation at the study’s commencement. However, from days 7 to 21, a notable increase in MAP was evident in the L-NAME group compared to the control group. In the comparative analysis, the group administered Captopril demonstrated significant reductions in MAP on days 7, 14, and 21 (p<0.01). Additionally, the administration of M-13 Tang (90 mg/kg and 180 mg/kg) significantly reduced MAP on days 14-21 (p<0.01) (Fig. 1).
 |
Figure 1: MAP in L-NAME induced hypertensive model |
SBP and DBP exhibited a significant increase in the L-NAME group compared to the control group (p<0.01). Animals treated with Captopril and M-13 Tang showed a substantially reduced SBP and DBP compared to the L-NAME group (p<0.01) (Tables 3 and 4).
Table 3: SBP in L-NAME induced hypertensive model
|
Time Point |
Control (mmHg) |
L-NAME (mmHg) |
Captopril 5 mg/kg (mmHg) |
M-13 90 mg/kg (mmHg) |
M-13 180 mg/kg (mmHg) |
|
0 day |
115.2±5.15 |
117.1±6.43 |
123.8±3.71 |
121.0±3.03 |
127.8±4.35 |
|
7 days |
95.6±4.31 |
142.5±6.93## |
109.5±9.05* |
133.0±6.48 |
126.6±6.40 |
|
14 days |
105.6±6.65 |
171.7±7.96## |
118.0±9.96** |
120.6±11.4** |
112.1±9.92** |
|
21 days |
114.4±4.99 |
166.2±9.42## |
112.7±6.74** |
116.0±8.65** |
113.6±10.1** |
Results are expressed as mean ± SD (n=8). ##p < 0.01, L-NAME group vs. control group; *p < 0.05, **p < 0.01, Captopril 5 mg/kg, M-13 90 mg/kg and M-13 180 mg/kg group vs. L-NAME group.
Table 4: DBP in L-NAME induced hypertensive rats
|
Time Point |
Control (mmHg) |
L-NAME (mmHg) |
Captopril 5 mg/kg (mmHg) |
M-13 90 mg/kg (mmHg) |
M-13 180 mg/kg (mmHg) |
|
0 day |
75.2±6.71 |
64.2±5.50 |
73.6±8.50 |
74.4±8.14 |
73.6±6.44 |
|
7 days |
76.6±5.08 |
118.1±6.39## |
76.5±9.50* |
88.3±12.2 |
104.6±8.74 |
|
14 days |
74.4±4.86 |
133.1±9.27## |
85.0±11.94** |
89.6±10.1** |
74.0±9.05** |
|
21 days |
73.6±3.85 |
136.6±7.58## |
83.1±8.20** |
90.5±5.0** |
88.6±11.9** |
Results are expressed as mean ± SD (n=8). ##p < 0.01, L-NAME group vs. control group; *p < 0.05, **p < 0.01, Captopril 5 mg/kg, M-13 90 mg/kg and M-13 180 mg/kg group vs. L-NAME group.
Effect of M-13 Tang on serum of NO levels in L-NAME-induced hypertensive model
NO synthesis was inhibited in the L-NAME-induced hypertension model. The captopril (5 mg/kg) group and the M-13 Tang (90 mg/kg) group exhibited significant increases in serum NO levels compared to the L-NAME group (p<0.05). There was a dose-dependent restoration of NO in the serum of the M-13 Tang groups (Figure 2).
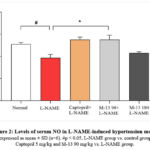 |
Figure 2: Levels of serum NO in L-NAME-induced hypertension model |
Effect of M-13 Tang on serum of ACE activity in L-NAME-induced hypertensive model
A significant increase in serum ACE levels was observed in the L-NAME group compared to the normal control group (p<0.05). Conversely, the ACE level exhibited a significant reduction in both the Captopril and M-13 Tang (90 mg/kg) treated groups compared to the L-NAME group (p<0.05). There was a dose-dependent reduction of ACE in the serum of the M-13 Tang groups (Figure 3).
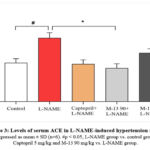 |
Figure 3: Levels of serum ACE in L-NAME-induced hypertension model |
Effects of M-13 Tang on serum of MDA and antioxidant enzyme levels
Table 4 shows the concentrations of MDA, SOD, and CAT in the serum of the experimental groups. L-NAME administration in rats significantly increased the level of MDA (a marker of oxidative stress) compared with control rats (p<0.05). However, when administered to hypertensive rats, captopril and M-13 Tang (90 and 180 mg/kg) significantly decreased MDA levels, indicating a reduction in L-NAME-induced oxidative stress. Furthermore, we observed that L-NAME markedly diminished the antioxidant enzymes SOD and CAT, indicating oxidative stress compared to the control group (p<0.01). Treatment with M-13 (90 and 180 mg/kg) notably decreased oxidative stress by restoring the beneficial antioxidant enzymes SOD and CAT (p<0.01).
Table 4: Levels of serum MDA and antioxidant enzymes in L-NAME-induced hypertension
|
Parameter |
Control |
L-NAME |
Captopril (5 mg/kg) |
M-13 (90 mg/kg) |
M-13 (180 mg/kg) |
|
MDA (nmol/L) |
11.11±4.50 |
24.44 ± 5.77# |
16.52 ± 2.51* |
15.13 ± 4.53* |
16.44 ± 5.00* |
|
SOD (pg/ml) |
99.60±11.8 |
39.64 ± 6.58## |
78.27 ± 8.16** |
62.36 ± 7.45** |
66.53 ± 8.42** |
|
CAT (ng/L) |
41.80±6.33 |
21.67 ± 2.12## |
35.56 ± 7.91* |
33.53 ± 3.93* |
32.21 ± 5.54* |
Results are expressed as mean ± SD (n=6). #p<0.05, ##p < 0.01, L-NAME group vs. control group; *p < 0.05, **p < 0.01, Captopril 5 mg/kg, M-13 90 mg/kg and M-13 180 mg/kg group vs. L-NAME group.
Discussion
Marchin-13 Tang is a traditional drug widely used in Mongolian traditional medicine to reduce blood pressure. An acute toxicity study of M-13 Tang, conducted using the by express method of Prozorovskii V.B39 determined the median lethal dose (LD50) to be 4.47 g/kg. Therefore, we selected 90 mg/kg and 180 mg/kg doses for this study. This study demonstrated the first experimental investigation to assess whether M-13 Tang exhibits an antihypertensive effect in L-NAME-induced hypertensive rat models. We observed that administering M-13 Tang in L-NAME-induced hypertensive rats reduced blood pressure and exerted antioxidant effects.
L-NAME is a synthetic substance that inhibits NO, and it is widely used internationally to create a model of hypertension in experimental animals13,40,41. NO produced by vascular endothelial cells is a potent vasodilator and plays an important role in vascular resistance and growth42. In this study, we found that administration of M-13 Tang (90 mg/kg) reduced blood pressure through vasodilation by restoring inhibited NO production. Campbell’s study reported increased oxidative stress, vascular inflammation, ACE activity, and expression following L-NAME administration13. ACE, pivotal in the RAAS, converts angiotensin I to angiotensin II, which is crucial in regulating blood pressure and fluid balance. Angiotensin II promotes vasoconstriction, leading to an increase in blood pressure14,15. In the current study, M-13 Tang (90 mg/kg) reduced serum ACE activity in rats receiving L-NAME. It was nearly as effective as the positive control captopril. Captopril inhibits the conversion of angiotensin I to angiotensin II, thereby reducing the processes that lead to high blood pressure and heart failure43. Captopril has been reported to have antioxidant properties with its thiol group44 and is widely used in clinical practice.
The M-13 Tang contains several herbs with antihypertensive properties. For instance, C. tinctorius L normalized the renin-angiotensin system (RAS), NO bioactivity, reduced oxidative stress markers, and improved endothelial function in L-NAME-induced hypertensive rats10. Manuela Ciocoiu’s study also demonstrated that the polyphenolic extract of S. manshurica L reduced SBP and DBP in the hypertension model45. Crocetin, a biologically active substance in G. jasminoides J, possesses antihypertensive and antithrombotic effects, prevents insulin resistance, and enhances sleep quality46. Also, there have been reports of the ACE activity inhibitory effects of Z. officinale Roscoe15. It has been utilized in traditional medicine to address hypertension and various cardiovascular ailments47.
This study demonstrates that L-NAME elevated serum lipid peroxidation decreased the level of antioxidant enzymes SOD, and CAT compared to the control group leading to severe tissue damage. This data is consistent with the results of other studies that L-NAME inhibits the activity of SOD and CAT48 whereas increases MDA in serum49 causing hypertension in rats. According to Harrison’s study, oxidative damage worsens as blood pressure increases49.
We found that M-13 Tang at a dose of 90 and 180 mg/kg reduced the serum MDA level compared to the L-NAME-induced hypertension group, indicating a protective effect against L-NAME-induced oxidative damage of vascular tissue. Moreover, M-13 Tang at a dose of 90 mg/kg increased significantly the levels of the antioxidant enzymes SOD and CAT compared to L-NAME induced hypertension group. These data indicate that M-13 Tang possesses antioxidant activity.
Our chemical analysis determined that M-13 Tang contains total phenolic (gallic acid 29.6±0.16 mg/g), and flavonoids (rutin, 18.1±0.1 mg/g). We hypothesize that this antioxidant activity is related to the high-content polyphenols of M-13 Tang. Researchers have discovered that phenolic and flavonoid compounds display anti-inflammatory50, antibacterial, and antioxidant effects, along with biologically active properties that help reduce blood pressure51 and decrease edema. Gallic acid demonstrates diuretic, anti-inflammatory, and soothing effects52. Li Jin and Xiao Yan’s study showed that gallic acid modulates the components of the renin-angiotensin-aldosterone system (RAAS), leading to a decrease in the expression of Ang II type I receptor (AT1) and angiotensin-converting enzyme inhibitor (ACEI) proteins within the aorta. Additionally, gallic acid relaxes vasoconstriction in both the aorta and mesenteric arteries and has been studied for antihypertensive effects53,54.
In the current study, M-13 Tang inhibited L-NAME induced serum ACE activity in rats . Also, our study demonstrated that treatment with M-13 Tang prevented the decrease in serum NO induced by L-NAME. Hence, M-13 Tang (90 and 180 mg/kg) showed antihypertensive effects against L-NAME induced hypertension in rats. There are many studies on antihypertensive traditional Chinese medicine. For example, GAO-ZI-YAO Tang has the effect of reducing blood pressure and restoring the cardiovascular system by regulating the production of NO and Ang II, as well as reducing inflammation8.
A limitation of our study is that we used only one model of hypertension. Further studies are also needed to isolate the biologically active substances of Marchin-13 Tang and investigate its antihypertensive effect in other models of hypertension. It would help to give more understanding of the mechanism of M-13 Tang.
Conclusion
In conclusion, we found that Marchin-13 Tang, significantly reduced MDA and ACE levels, while increasing NO, SOD, and CAT activities compared to L-NAME induced hypertension in rats. Consequently, these data indicate that the antioxidant activity of Marchin-13 Tang plays a crucial role in the antihypertension effect. We are pioneering the investigation into the antihypertensive effects of Marchin-13 Tang.
Acknowledgments
We thank the Institute of Traditional Medicine and Technology’s Research Center and the project team for their support during this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Source
Foundation of Science and Technology, Mongolia, Grant/Award number: Shut/Z-2017/05
References
- World Health Organization. Hypertension. Accessed 16 March 2023.
- Potts H., Baatarsuren U., Myanganbayar M. Hypertension prevalence and control in Ulaanbaatar, Mongolia. J Clin Hypertens. 2020;22(1):103-110.
CrossRef - Institute for Health Metrics and Evaluation. GBD Compare. It was accessed on 29 July 2019.
- Jackson RE., Bellamy MC. Antihypertensive drugs. BJA Education. 2015;15(6):280–285.
CrossRef - World Health Organization. Traditional medicine strategy: 2014-2023. Hong Kong SAR, China. 2013; 21-24
CrossRef - Rafieian K. M. Medicinal plants and the human needs. Journal of Herb Med Pharmacology. 2012;1(1):1-2.
CrossRef - Jargalsaikhan B., Ganbaatar N. Anti-Inflammatory Effect of Polyherbal Formulation (PHF) on Carrageenan and Lipopolysaccharide-Induced Acute Inflammation in Rats. Biomed Pharmacol J. 2019;12(4):1801-1809
CrossRef - Bin D., Zi-Zhang W., Hui Zh. Antihypertensive properties of a traditional Chinese medicine GAO-ZI-YAO in elderly spontaneous hypertensive rats, Biomedicine & Pharmacotherapy. 2020;131:110739
CrossRef - Jambalchoijidanzanperenlei. “Manag Rinchin Junai”. Traditional Medical Source Book. China: “Inner Mongolian medical treasurers” printing house; 1978:54-55
- Maneesai P., Prasarttong P. Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in L-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT₁R Expression. Nutrients. 2016;8(3):122.
CrossRef - Huang Y.X., Wang G. Traditional uses phytochemistry and pharmacological properties of Sophora alopecuroides L. European Journal of Inflammation. 2016;14(2):128-132
CrossRef - Buza V., Matei M.C., Ștefănuț L.C. Inula helenium: A literature review on ethnomedical uses, bioactive compounds, and pharmacological activities. Lucrări Ştiinţifice Seria Medicină Veterinară. 2020;63(1):53-59.
- Campbell., Duncan J. L-NAME hypertension: trying to fit the pieces together. Journal of Hypertension. 2006;24(1):33-36.
CrossRef - Jaarin K., Foong W.D., Yeoh M.H. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics. 2015;70(11):751-757.
CrossRef - Ayodele J.A., Gustavo R.T., Vera M.M. Effect of dietary supplementation of ginger and turmeric rhizomes on angiotensin-1 converting enzyme (ACE) and arginase activities in L-NAME induced hypertensive rats. Journal of Functional Foods. 2015;17:792-801.
CrossRef - Food products quality and nutrition in relation to public. Balancing health and disease: Food safety & hygiene promotion. Progress in Nutrition. 2023; 25(1)
- Li G., Yu S., Zhou Y.H., Chen Q.F. Spectrophotometric determination of flavonoid content in leaves of Fagopyrum cymosum complex. Asian Journal of Chemistry. 2013; 25(13):7575.
CrossRef - Singleton V.L., Orthofer R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in enzymology. 1999;299:152-178.
CrossRef - National Pharmacopoeia of Mongolia. Infusum, Decoctum. Soyombo printing. 2011;451.
- Bunbupha, S., Pakdeechote, P., Maneesai, P. Carthamus Tinctorius L. extract attenuates cardiac remodeling in L-NAME-induced hypertensive rats by inhibiting the NADPH oxidase-mediated TGF-β1 and MMP-9 pathway. Annals of Anatomy – Anatomischer Anzeiger. 2019; 222:120–128.
CrossRef - Emmanuel N W., Jing L., Elijah M M., Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae), Journal of Ethnopharmacology. 2022; 292:115102.
CrossRef - Rohan S P., Phytochemistry, Pharmacological Activities and Intellectual Property Landscape of Gardenia jasminoides Ellis: a Review. Pharmacognosy Journal. 2015; 7 (5): 254-265.
CrossRef - Mohammad Abu B N., Md. Abdul M. Rubia cordifolia-phytochemical and Pharmacological evaluation of indigenous medicinal plant: A review. International Journal of Physiology, Nutrition and Physical Education. 2018; 3(1): 766-771.
- Witkowska-Banaszczak E. The genus Trollius-review of pharmacological and chemical research. Phytother Res. 2015 Apr;29(4):475-500.
CrossRef - Ștefănescu R, Ciurea CN, Mare AD. Quercus robur Older Bark—A Source of Polyphenolic Extracts with Biological Activities. Applied Sciences. 2022; 12(22):11738.
CrossRef - Li, C., Li, J., Jiang, F., Tzvetkov, N. T. Vasculoprotective effects of ginger (Zingiber officinale Roscoe) and underlying molecular mechanisms. Food & Function. 2021;12(5): 1897–1913.
CrossRef - Oyungerel Sh., Hari D B. Antioxidant Activity of Some Mongolian Plants. Mongolian Journal of Biological Sciences. 2014; 12(1-2): 27-32
CrossRef - Oyungerel, Sh., Yim, J H. Antimicrobial activity of some Mongolian plants. Mong. Journal of Biological Sciences. 2023; 21(2): 3−13.
CrossRef - Khan A.-U., Gilani A H. Pharmacodynamic evaluation of Terminalia bellerica for its antihypertensive effect. Journal of Food and Drug Analysis. 2008; 16(3): 6-14
CrossRef - Buza V., Matei M.C., Ștefănuț L.C. Inula helenium: A literature review on ethnomedical uses, bioactive compounds, and pharmacological activities. Lucrări Ştiinţifice Seria Medicină Veterinară. 2020;63(1):53-59.
- Huang Y.X., Wang G. Traditional uses phytochemistry and pharmacological properties of Sophora alopecuroides L. European Journal of Inflammation. 2016;14(2):128-132
CrossRef - Sornwatana T., Bangphoomi K., Roytrakul S. Chebulin: Terminalia chebula Retz. fruit-derived peptide with angiotensin-I-converting enzyme inhibitory activity. Biotechnol Appl Biochem. 2015; 62(6):746-53.
CrossRef - Bulbul Md R H., Chowdhury Mohammad N U. A comprehensive review on the diverse pharmacological perspectives of Terminalia chebula Retz. Heliyon. 2022; 8: e10220.Bin D., Zi-Zhang W., Hui Zh. Antihypertensive properties of a traditional Chinese medicine GAO-ZI-YAO in elderly spontaneous hypertensive rats, Biomedicine & Pharmacotherapy. 2020;131:110739
CrossRef - Kanika D., Gurpreet K., Keshav K. Species of Arnebia Genus Found in the Western Himalayas: Arnebia euchroma (Royle ex Benth.), Arnebia benthamii (Wall. Ex G Don) Johnston, Arnebia guttata Bunge. Immunity Boosting Medicinal Plants of the Western Himalayas. 2023; 77–105
CrossRef - Sung J.H., Jo Y.S., Kim S.J. Effect of Lutein on L-NAME-Induced Hypertensive Rats. Korean J Physiol Pharmacol. 2013;17(4):339-45.
CrossRef - Aljerf, L., Williams, M., Ajong, A.B., Comparative Study of the Biochemical Response Behavior of Some Highly Toxic Minerals on Selenosis in Rats. Revista de Chimie. 2021; 72(2): 9-18
CrossRef - Belemnaba L., Nitiéma M., Ilboudo S. Preclinical Evaluation of the Antihypertensive Effect of an Aqueous Extract of Anogeissus leiocarpa (DC) Guill et Perr. Bark of Trunk in L-NAME-Induced Hypertensive Rat. J Exp Pharmacol. 2021;13:739-754
CrossRef - DeMers D., Wachs D. Physiology, Mean Arterial Pressure. StatPearls. 2023.
- Prozorovskii VB, Prozorovskaya MP, Demchenko VM. Express method of determining the median effective dose and its error. Pharmacol Toxicol. 1978;4:497–500.
- Vishal R.M., Mohan V., Subhash L.B. Antihypertensive and cardioprotective effects of the Lagenaria siceraria fruit in NG-nitro-L-arginine methyl ester (L-NAME) induced hypertensive rats, Pharmaceutical Biology, 2012;50(11):1428-1435.
CrossRef - Kanthlal S.K., Jipnomon J., Bindhu P. Antioxidant and vasorelaxant effects of aqueous extract of large cardamom in L-NAME induced hypertensive rats. Clinical and Experimental Hypertension. 2020;42(7):581-589.
CrossRef - Shin W, Cuong TD, Lee JH. Arginase inhibition by ethylacetate extract of Caesalpinia sappan lignum contributes to activation of endothelial nitric oxide synthase. Korean J Physiol Pharmacol. 2011; 15:123–128.
CrossRef - Sornwatana T., Bangphoomi K., Roytrakul S. Chebulin: Terminalia chebula Retz. fruit-derived peptide with angiotensin-I-converting enzyme inhibitory activity. Biotechnol Appl Biochem. 2015; 62(6):746-53.
CrossRef - Pechanova O. Contribution of captopril thiol group to the prevention of spontaneous hypertension. Physiological research. 2007; 56 (1): 41-48
CrossRef - Ciocoiu M., Badescu M., Badulescu O., Badescu L. The beneficial effects on blood pressure, dyslipidemia, and oxidative stress of Sambucus nigra extract associated with renin inhibitors. Pharm Biol. 2016;54(12):3063-3067.
CrossRef - Wenping X., Shiming L., Siyu W., Chi-Tang H. Chemistry and bioactivity of Gardenia jasminoides. Journal of Food and Drug Analysis. 2017;25(1):43-61.
CrossRef - Duke JA. Handbook of medicinal herbs. CRC Press; 2002 Jun 27.
CrossRef - Cardoso A.M., Martins C.C., Fiorin F.S. Physical training prevents oxidative stress in L-NAME-induced hypertension rats. Cell Biochem Funct. 2013;31(2):136-51.
CrossRef - Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. JASH 2007; 1: 30–44.
CrossRef - Al-Khayri J.M., Sahana G.R., Nagella P. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules. 2022;27(9):2901.
CrossRef - José Luiz de B.A., Jéssica Maria A.B. Phenolic compounds in hypertension: Targeting gut-brain interactions and endothelial dysfunction, Journal of Functional Foods. 2023;104: 105531.
CrossRef - Erdenechimeg Ch., Guiqide A., Dejidmaa B., Ch. Total phenolic, flavonoid, alkaloid, and iridoid content and preventive effect of Lider-7-tang on lipopolysaccharide-induced acute lung injury in rats. Braz. J. Med. Biol. Res. 2017;50(12):1-6.
CrossRef - Jin L, Piao Z.H, Sun S. Gallic Acid Reduces Blood Pressure and Attenuates Oxidative Stress and Cardiac Hypertrophy in Spontaneously Hypertensive Rats. Sci Rep. 2017;7(1):15607.
CrossRef - Yan X, Zhang Q.Y, Zhang Y.L, Han X. Gallic Acid Attenuates Angiotensin II-induced hypertension and Vascular Dysfunction by Inhibiting the Degradation of Endothelial Nitric Oxide Synthase. Front. Pharmacol. 2020;11:1121.
CrossRef








