Prasad Arvind Thakurdesai1* , Savita Raju Nimse1
, Savita Raju Nimse1 , Padmaja Santosh Kore2
, Padmaja Santosh Kore2 and Urmila Manoj Aswar3
and Urmila Manoj Aswar3
1Department of Scientific Affairs, Indus Biotech Limited, Pune, India.
2Department of Pharmacology, Modern College of Pharmacy, Nigdi, Pune, India.
3Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India.
Corresponding Author E-mail: prasad@indusbiotech.com
DOI : https://dx.doi.org/10.13005/bpj/2896
Abstract
The leaves of Centella asiatica L. Urban (C. asiatica) and their bioactive compounds, triterpenoids asiaticoside (AS) and madecassoside (MA), are effective in reducing psychological stress and associated behavioral disorders in the conducted in vivo research. The present study evaluated AS+MA-based standardized C. asiatica leaves extract (INDCA) on SIS-induced suicidal behavior-related traits in laboratory rats. Male rats (n=6) were randomized, grouped, and individually caged for seven days for stress induction. For the next seven days (D7 to D14), rats were orally administered vehicle (stress control), positive control (fluoxetine, 30 mg/kg), and or INDCA (3, 10, 30 mg/kg) once a day. A separate group of rats without isolation stress (normal rats) was maintained. The scores for suicidal behavior-related traits, such as aggression, impulsivity, irritability, learned helplessness, and plasma cortisol, were measured after 14-day treatment. The stress control group showed a significant increase in aggression, irritability (total score), learned helplessness (escape latency, escape failure, and recovery attempts), and plasma cortisol levels, which confirmed suicidal behavior-related traits. The INDCA-treated rats showed a dose-dependent reduction in stress-induced behavioral traits and elevated plasma cortisol levels. In conclusion, subacute administration of INDCA showed amelioration of suicidal behavior in social-isolation-induced stress in laboratory rats and suggested a promising natural and safe option for the management of stress-induced behavioral disorders, including suicidal behavior.
Keywords
Cortisol; Gotu kola leaves; Social Isolation; Stress Disorders; Suicidal Ideation
Download this article as:| Copy the following to cite this article: Thakurdesai P. A, Nimse S. R, Kore P. S, Aswar U. M. Standardized Extract from the Gotu Kola Leaves Improves Suicidal Behavior in Stressed Rats Subjected to Social Isolation. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Thakurdesai P. A, Nimse S. R, Kore P. S, Aswar U. M. Standardized Extract from the Gotu Kola Leaves Improves Suicidal Behavior in Stressed Rats Subjected to Social Isolation. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3XeYVsn |
Introduction
The issue of suicide in the young population is a matter of global concern, and many countries have recognized the need for effective strategies to prevent it1. Increasing evidence suggests that social surroundings play a crucial role in anxiety behavior 2, 3 and suicidal behaviors 4, 5. However, biomarkers or specific treatment options for managing suicidal behaviors are lacking6. At the same time, major depressive disorder (MDD) remains among the most debilitating psychiatric disorders7. Selective serotonin reuptake inhibitors (SSRIs), a class of antidepressants, have been the primary treatment for major depressive disorder (MDD) and related conditions8-10 but have limitations in children and young adults due to suicidal ideation11, 12.
Many psychological disorders are stress-related and mediated through hypothalamic–pituitary–adrenal (HPA) axis 13-15. The brain serotonergic system, which is responsible for stress-related functions, is strongly correlated with mood and anxiety16. Therefore, antidepressant agents with stress-relieving properties but without the side effects of suicidal traits are required.
Social isolation is a factor in suicidal behaviors4. In addition, social connections in the community may protect stressed individuals from developing suicidal desires 17, 18. Consistent with the “stress reactivity” concept, cortisol has been reported as a dependable indicator of HPA axis function in MDD19, 20. Therefore, stress and cortisol reduction are essential targets for the management of many psychological disorders, including suicidal ideation.
Plant-derived natural products with evidence of brain tonic and stress-relieving properties have been explored to manage psychological disorders21-24. Gotu kola, scientifically known as Centella asiatica L. Urban (CA), is a promising plant source to alleviate stress-induced behavioral traits such as anxiety25, 26, depression and mood in healthy volunteers27, and mild cognitive decline in elderly individuals 28. C. asiatica is a major medicinal herb used to alleviate anxiety symptoms in traditional medicine, such as Ayurveda and Chinese medicines29. Previous studies have demonstrated the neuroprotective properties of standardized C. asiatica extracts against neurotoxin-induced oxidative stress and mitochondrial dysfunction in the brain30 which may be responsible for anti-aging potential 31, 32
Triterpenoids, such as asiaticoside (AS) and madecassoside (MA), are major bioactive components of C. asiatica leaves with stress and anxiety-reducing 33 and anti-aging properties 34. A triterpenoid-based extract from C. asiatica leaves (INDCA) has shown antidepressant effects in rats with chronic depression induced by olfactory bulbectomy35. The beneficial effects of C. asiatica triterpenoids are reportedly achieved by improving the function of the HPA axis and enhancing the levels of monoamine neurotransmitters31, 36.
In rodents, social isolation induces various behavioral changes, including anxiety, impulsivity, aggression, and suicidal traits 37. Socially isolated rats are valuable animal models for exploring options against stress-induced behavioral traits, including suicidal ideation38, 39. Existing clinical and preclinical scientific evidence indicates the potential of INDCA as an antidepressant and anxiolytic agent with stress-relieving properties36. However, a detailed exploration of INDCA to manage suicidal behavior has not yet been performed. Therefore, the present study aimed at the characterization and pharmacological assessment of oral INDCA supplementation on aggression, impulsivity, irritability, and learned helplessness induced by social-isolation stress (SIS) in laboratory rats.
Materials and Methods
Chemicals
Fluoxetine and cortisol kits (EIA-1887) were obtained from Cadila Pharma (Ahmedabad, India) and DRG Diagnostics (Marburg, Germany), respectively. Indus Biotech Limited (Pune, India) provided the test compound, INDCA, a standardized C. asiatica leaves extract, with a total triterpenoid content of 75.5 % (35.88 % MA and 39.62 % AS) by high-performance liquid chromatography as previously reported40. Fresh solutions of fluoxetine and INDCA were prepared daily immediately before oral administration to the rats.
Animals
Sprague-Dawley rats (male, weighing 200–250 g of 2-3 months of age) were procured and maintained in polypropylene cages in rooms with ambient temperature, humidity, and light: dark cycle following ethical standards and regulations for animal experiments in India41. All animal experiments were compiled the norms of “Committee for the Purpose of Control and Supervision of Experimental Animals” (CPCSEA) with protocol approval (No: SIOP/IAEC/2012/70) by the “Institutional Animal Ethics Committee” of Sinhgad Institute of Pharmacy (Narhe, Pune, India)
Induction of SIS and treatments
Rats were acclimatized to the testing room by moving them from the animal house an hour before testing. The rats were divided into six groups of six rats each. To prevent the influence of diurnal rhythms, experiments were conducted at 8:00 AM and 1:00 PM. To avoid personal bias, a person unaware of the treatments recorded the observations.
One group of rats, G1, called the normal, did not undergo SIS42 and received vehicle (1 ml/kg). The remaining groups of rats (G2–G6) were housed individually throughout the experiment received SIS, and 14-days of oral treatment as follows:
G2: Stress control – Received vehicle at dose 1 ml/kg;
G3: Fluoxetine (20) – Received positive control, Fluoxetine at dose 20 mg/kg;
G4: INDCA (3) – Received INDCA at dose 3 mg/kg;
G5: INDCA (10) – Received INDCA at dose 10 mg/kg;
G6: INDCA (30) – Received INDCA at dose 30 mg/kg.
Aggression and impulsivity in SIS-induced rats
The resident-intruder paradigm measures aggression-related parameters 43, 44. First, one rat was confined to the cage for 5 min, after which the intruder rats were brought into the same cage. A video tracking apparatus (ASTMD2467-SCH80, VJ Instruments, Akola, India) was used to record aggressive behavior for 10 min. Aggressive behavior (attack bites, tail rattling, wrestling, and chasing) was noted on days 1 (D1), Day-7 (D7), and Day-14 (D14) of isolation. The sum of all scores was considered the total aggression score. The time the intruder mouse took to initiate an attack (attack latency) was documented and used to indicate impulsiveness42.
Irritability in SIS-induced rats
The irritability of individual rats was recorded on D1, D7, and D14 immediately after the measurement of aggression scores using a reported method45. Each rat received an individual puff of uncomfortable air blown through a straw on its neck. The enhanced reaction to stimuli by rats is noted as “irritable” behavior and scored as irritability score (intensity of the response) using a six-category scale (0 to 6)45. Adding individual irritability scores represented that rat’s “total irritability score”.
Learned helplessness in SIS-induced rats
The effects on stress-induced learned helplessness were assessed immediately after irritability measurements on D14 of the experiment. The investigations were performed active avoidance paradigm46 using an active avoidance chamber (Model-DS163, INCO, Ambala India) with 15 attempts (33 s) for each rat. During the first 3 seconds, no shock was applied. In the absence of escape, an electric current (0.8 mA for 15 s) was administered at an interval of 45 s between each stimulus. In the event of the jump, the process was terminated and recorded as an “escape,” and the latency was recorded. Failure to jump the pole (helplessness) occurred, and the attempt was registered as an “escape failure,” and the number was recorded. Attempts to recover (i.e., when the rat learned to escape and showed escape instead of escape failure) were recorded individually.
Plasma cortisol levels in SIS-induced rats
Cortisol levels were assessed on D14 in plasma isolated from blood samples of rats (collected under anesthesia) using an ELISA kit (DRG Diagnostics, Germany), following the instructions of the manufacturer and supplier.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). The total scores for aggression, impulsivity, and irritability were analyzed using two-way repeated-measures analysis of variance (ANOVA) and Bonferroni multiple comparisons. Kruskal-Wallis ANOVA and Dunn’s multiple comparison tests were used to analyze data obtained on D14 from the learned helplessness test (escape latency, failure numbers, and number of recovery attempts). Data of plasma cortisol measurements were analyzed using one-way ANOVA followed by Dunnett’s test. Prism (v. 6.0, GraphPad Inc., San Diego, USA) was used for data analysis. Differences were considered statistically significant at P < 0.05.
Results
Effects on total aggression scores
The data analysis of the total aggression scores during the resident-intruder paradigm is organized in Table 1. No significant change was observed between any treatment groups on D1 and D7. On D14, stress control showed a significant (P < 0.001) increase in aggression scores compared to normal rats, whereas fluoxetine and INDCA (all tested doses) treated groups showed a decline in total aggression scores (vs. stress control).
Effects on impulsivity
Table 1 shows the attack latency (impulsivity scores) data collected during the resident-intruder paradigm. The Stress control rates significantly increased (vs. Normal) in attack latency on D14 but not D1 or D7. However, no significant changes (vs. Stress control) were present in fluoxetine- or INDCA-treated rats on any day at any tested doses of study.
Table 1: Effect on total aggression scores and impulsivity (attack latency) in social-isolation stress induced rats
|
Treatment |
Parameter score (Mean ± SEM) |
|||||
|
Total Aggression score |
Impulsivity – Attack Latency (Sec) |
|||||
|
|
D1 |
D7 |
D14 |
D1 |
D7 |
D14 |
|
Normal |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
0.00 ± 0.00 |
|
Stress |
2.33 ± 1.67 |
12.33 ± 2.76 |
49.17 ± 6.82### |
0.67 ± 0.67 |
6.50 ± 1.52 |
1.33 ± 0.21# |
|
Fluoxetine (30) |
5.33 ± 2.72 |
18.00 ± 1.75 |
30.83 ± 7.30** |
2.83 ± 2.44 |
7.33 ± 1.52 |
13.33 ± 4.07 |
|
INDCA (3) + |
5.33 ± 2.78 |
12.33 ± 2.38 |
19.83 ± 1.35*** |
1.33 ± 0.71 |
2.50 ± 0.72 |
2.17 ± 0.65 |
|
INDCA (10) + |
13.50 ± 5.86 |
23.50 ± 2.60 |
13.17 ± 2.69*** |
3.33 ± 1.31 |
2.33 ± 0.49 |
5.00 ± 1.46 |
|
INDCA (30) + |
12.67 ± 4.33 |
17.00 ± 2.97 |
16.00 ± 3.73*** |
3.50 ± 0.89 |
4.33 ± 1.15 |
13.67 ± 3.92 |
n=6, Total aggression score is calculated as the sum of scores from attack bites, tail rattling, wrestling and chasing behavior. #P < 0.05, ### P < 0.001 (vs. Normal), ** P < 0.01, *** P < 0.001 (vs. stress control) at respective day (two-way repeated measures ANOVA and Bonferroni multiple comparisons test)
Effects on irritability
The total irritability scores of the rats in the resident-intruder test are presented in Table 2. On D7 and D14, total irritability score of stress control showed a significant increase in the (vs. normal). No significant change was observed in the total irritability scores of fluoxetine-treated rats on any day. Treatment with INDCA (3) and INDCA (10) significantly reduced the total irritability score compared with the stress control on D14 but not on D1 or D7. However, INDCA (30) treatment significantly reduced the total irritability score (vs. stress control) on D7 and D14.
Table 2: Effect of on Irritability score in social-isolation stress induced rats
|
Treatment |
Total irritability score – (Mean ± SEM) |
||
|
D1 |
D7 |
D14 |
|
|
Normal |
1.33 ± 0.49 |
1.00 ± 0.37 |
0.67 ± 0.33 |
|
Stress control |
0.83 ± 0.40 |
3.67 ± 0.49# |
5.17 ± 0.54### |
|
Fluoxetine (30) + Stress |
1.17 ± 0.54 |
3.50 ± 1.20 |
5.00 ± 0.63 |
|
INDCA (3) + Stress |
0.50 ± 0.22 |
1.17 ± 0.31 |
2.50 ± 1.15* |
|
INDCA (10) + Stress |
1.50 ± 0.81 |
2.33 ± 1.12 |
1.50 ± 0.50** |
|
INDCA (30) + Stress |
1.33 ± 0.84 |
0.67 ± 0.67* |
0.33 ± 0.21*** |
n=6, Total Irritability score is calculated as the sum of scores obtained from startle, snout, biting, and resistance and vocalization responses. # P < 0.05, ### P < 0.001 vs. Normal, * P < 0.05, ** P < 0.01, *** P < 0.001 (vs. stress control) on the respective day (Two-way repeated measures ANOVA and Bonferroni multiple comparisons test)
Effects on learned helplessness
The learned helplessness data for D14 during the active-avoidance paradigm are shown in Table 3. On D14, Stress control showed a significant (P < 0.001) rise in escape latency compared with the normal group, whereas fluoxetine or INDCA (3) did not affect escape latency (vs. Stress control). However, a significant reduction in escape latency was shown by INDCA at 10 and 30 mg/kg (significant at P < 0.01 and P < 0.001, respectively).
On D14, number of escape failure attempts in Stress control (vs. normal) showed a significant (P < 0.001) increase. Fluoxetine treatment did not affect the number of escape failure attempts compared with the stress control. INDCA treatment significantly reduced the number of attempts to escape failure in a dose-dependent manner. However, rats treated with the highest tested dose of INDCA (30 mg/kg) showed a significant decrease in escape failure attempts (vs. stress control).
A significantly (P < 0.001) higher number of attempts to recover was found in the stress control group on D14 (vs. normal). Treatment with fluoxetine did not alter the number of recovery attempts compared to the stress control group. Treatment showed a dose-dependent reduction in the number of recovery attempts. Furthermore, a significant (P < 0.05, P < 0.001) reduction in the number of attempts to recover was observed in the INDCA (10) and INDCA (30) groups but not in the INDCA (3) group.
Table 3: Effects on learned helplessness during active avoidance test in social-isolation stress induced rats
|
Treatment |
Parameter Value on D14 (Mean ± SEM) |
||
|
Escape latency (sec) |
failure attempts (number) |
Attempts to recover (number) |
|
|
Normal |
64.20 ±7.83 |
0.00 ±0.00 |
5.80 ±0.20 |
|
Stress control |
183.20 ±9.59### |
5.60 ±0.24### |
16.00 ±0.00### |
|
Fluoxetine (30) + Stress |
174.00 ±6.04 |
2.00 ±0.37 |
13.00 ±0.37 |
|
INDCA (3) + Stress |
178.70 ±18.96 |
1.67 ±0.21 |
12.50 ±0.56 |
|
INDCA (10) + Stress |
102.50 ±19.33** |
1.50 ±0.22 |
9.33 ±0.88* |
|
INDCA (30) + Stress |
65.17 ±10.24*** |
0.00 ±0.00*** |
7.00 ±0.68*** |
n=6, The score in 15 attempts for each trait is recorded. ### P < 0.001 (vs. Normal), * P < 0.5, ** P < 0.01, *** P < 0.001 (vs. stress control) of respective parameters (Kruskal-Wallis ANOVA and Dunn’s multiple comparison test)
Effects on plasma cortisol levels
The data obtained from plasma cortisol measurements are shown in Fig. 1. The mean plasma cortisol concentration in the normal group rats was 14.76 ng/ml. In the stress control rats, mean plasma cortisol concentration significantly (P < 0.001) increased to 176.3 ng/ml (vs. Normal). Fluoxetine or INDCA treatment group showed the mean plasma cortisol levels to 271.6 ng/ml, 82.49 ng/ml, 69.14 ng/ml, and 14.35 ng/ml, respectively, which are significantly (P < 0.001 vs. stress control).
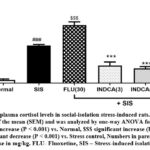 |
Figure 1: Effect on plasma cortisol levels in social-isolation stress-induced rats. n = 6, Data as mean ± standard error of the mean (SEM) and was analyzed by one-way ANOVA followed by Dunnett’s test. |
Discussion
As suicide is a complex behavior, specific animal models have not been developed42. However, four suicidal behavioral traits, namely aggression, irritability, hopelessness, helplessness, and impulsivity, are known to be closely related to suicidal ideation42. Simultaneously, social isolation in rodents induces the same behavioral changes as suicidal ideation, including impulsivity and aggression6 and mood-related behavior 47. Social isolation is a common cause of anxiety disorders in clinical settings48, 49. Due to its non-physical nature, social isolation can alleviate pain and stimulate stress responses in animals by activating the HPA axis50. Antidepressants, particularly SSRIs, induce stress-related behaviors in animal models51. The current study utilized social isolation as a stressor because of its association with clinical settings42. The most promising endpoints related to suicidal behavior in animals are cortisol levels, social stress response, and aggression/impulsivity traits involving the serotonergic system 52. Therefore, we measured the effects of oral supplementation of INDCA on aggression, impulsivity, irritability, helplessness, and plasma cortisol levels in stress-induced rats as indicators of suicidal ideation. The test compound, INDCA, was extract powder prepared from authenticated and dried leaves of Centella asiatica by hydroalcoholic extraction and purification with column chromatography based on earlier reported procedure35
Almost all animal species exhibit aggressive behavior as a natural trait53. The resident-intruder paradigm, considered one of the most reliable methods for assessing aggressive behavior-related traits, was employed in the current study44, 53. Our findings, which indicate higher total aggression scores in stress control, are consistent with a previous study42. In addition, INDCA treatment prevented stress-induced increases in total aggression scores.
The serotonergic system in the brain influences aggression by regulating the release and firing of serotonergic neurons through presynaptic 5-HT1A auto receptors54. Specific 5-HT1A/1 B receptor agonists potently reduce aggressive behavior without causing motor retardation or sedative effects55. Furthermore, fear, anxiety, and the consequent defensive behavior in aggression are regulated by 5-HT1A receptor expression in the brain 56. The 5HT1A receptor agonist property was proposed as a possible mechanism of action of C. asiatica extract as an antidepressant57 and an anti-migraine58 agent. The results of the present study provide additional support for the 5HT1A/1B receptor-mediated mechanism of INDCA against other stress-related conditions, especially aggression.
Impulsivity is an essential trait in the development of several neuropsychiatric conditions, including suicidal ideation59. The tendency towards impulsive behavior is often observed alongside other mental health conditions such as mood disorders and suicidal behavior59. Decreased attack latency (latency for first attack from intruder mouse entry) indicates the impulsivity trait of aggression42. A substantial increase in impulsivity on D14 in stress control was noted in the present study. However, Fluoxetine or INDCA failed to demonstrate any impact on impulsivity, probably because of the brief duration of the treatments and the presence of stress as reported60.
Irritability is a state of mind characterized by poor temper control and irreversible verbal or behavioral outbursts. The ability to control one’s emotional responses is often disregarded and frequently observed in individuals who display impulsive behavior61. Several studies have demonstrated a strong correlation between irritability, suicidal ideation, and suicide attempts62. Irritability measurement in rodents uses an animal’s reaction to mild touch or sound. This is complicated by the subjective nature of mental states42. The current study employed a 6-point scale to assess overall irritability in rats following exposure to unpleasant stimuli45. In the present study, SIS increased irritability in the stress control group, whereas INDCA treatment prevented stress-induced irritability in a dose-dependent manner. The absence of a substantial impact of fluoxetine on SIS-induced irritability is consistent with previous research 63.
Hopelessness, or helplessness, is one of the most notable features of suicidal ideation 64. Helplessness is defined as deep pessimism about the future65. Learned helplessness is the most validated measure and is often used as a representative trait for hopelessness assessment 66. The active avoidance paradigm, which uses animals’ natural aversion to foot shocks, has been used to investigate learned helplessness 66 . The animal will eventually display a “despair-like” behavior when the stimulus is inescapable and can no longer attempt to escape67. The 14-days SIS significantly enhanced escape latencies and failure attempts in stress-control rats, confirming the induction of learned helplessness. A significant prevention of learned helplessness in rats following oral treatment with INDCA (10 or 30 mg/kg) was observed.
Exposure to prolonged social stress and elevated corticosteroid levels can impair stress adaptation mechanisms and contribute to learned helplessness46. Furthermore, 5-HT1A activation in the dorsal hippocampus leads to enhanced stress adaptation68. The behavioral deficits of SIS resulted in escape attempts from restrained space, which are reversed by 5HT1A agonist 69. INDCA prevented learned helplessness-induced disturbances in the stress adaptation mechanism, perhaps by reducing cortisol and 5HT1A receptor activation.
SIS is recognized for its ability to cause hypothalamic-pituitary-adrenal (HPA) axis hyperresponsiveness70 and contributes to hippocampal neuronal degeneration71. Many studies have emphasized the significant role of the hippocampus under stressful conditions72. The hippocampus controls adrenocorticotropic hormone (ACTH) and cortisol secretion via the HPA axis72.
Stress responses were reliably correlated with cortisol levels73. Activation of the HPA axis increases cortisol levels and initiates physiological stress responses74. HPA axis dysfunction is associated with stress-related mental health issues 75 Cortisol levels are considered a promising approach to predict suicidal behavior in animals after social stress52. Previous studies have indicated a relationship between cortisol levels and clinical depression has been indicated by studies76. Plasma cortisol levels are commonly used as a measure of biologically active cortisol, which is influenced by social and environmental factors, such as social isolation74. The findings of this study align with previous research in stressed rats experiencing elevated cortisol levels52, 74.
INDCA showed dose-dependent prevention of plasma cortisol increase in stress control rats in the present study. However, subacute fluoxetine pretreatment failed to prevent stress-induced elevation of plasma cortisol levels. In fact, fluoxetine-administered rats showed a significant increase in plasma cortisol levels compared to the stress-control rats, a finding that is consistent with previous research 77.
The stress-relieving property of AS from C. asiatica leaves has been reported through HPA axis mediation35, 78 and normalizing cortisol levels 57. In addition, C. asiatica leaves extract exhibited neuroprotective effects against stress-induced neuronal atrophy in albino mice under stress79. Therefore, the maintenance of normal cortisol levels may largely contribute to the ability of INDCA to ameliorate stress-induced suicidal behavior.
Conclusions
This study demonstrated the ameliorative effects of oral administration of INDCA against stress-induced suicidal behavior-related traits in rats through the prevention of cortisol augmentation. The results of the present study, along with previous scientific evidence of its antidepressant efficacy, highlighted the potential of INDCA as a natural and safe therapeutic option for stress-induced disorders. However, further clinical studies are warranted.
Acknowledgment
The authors thank Dr. K. G. Bothara, former Principal of Sinhgad Institute of Pharmacy, Narhe, Pune, India, for providing the necessary infrastructure.
Conflict of Interest
The authors declare no conflicts of interest.
Funding Sources
The study was supported by Indus Biotech Limited, Pune, India (Grant Project No: IBS317).
References
- WHO. Live life: an implementation guide for suicide prevention in countries. Geneva: World Health Organizations; 2021 17 June 2021.
- Shao R, He P, Ling B, Tan L, Xu L, Hou Y, et al. Prevalence of depression and anxiety and correlations between depression, anxiety, family functioning, social support and coping styles among Chinese medical students. BMC psychiatry. 2020;8(1):1-19.
CrossRef - Good BJ, Kleinman AM. Culture and anxiety: Cross-cultural evidence for the patterning of anxiety disorders. Anxiety and the anxiety disorders: Routledge; 2019. p. 297-324.
CrossRef - Calati R, Ferrari C, Brittner M, Oasi O, Olié E, Carvalho AF, et al. Suicidal thoughts and behaviors and social isolation: A narrative review of the literature. J Affect Disord. 2019;245:653-67.
CrossRef - Choi D-H, Noh G-Y. The influence of social media use on attitude toward suicide through psychological well-being, social isolation, and social support. Inf Commun Soc. 2020;23(10):1427-43.
CrossRef - Romay-Tallon R, Pinna G. Animal Model Approaches to Understanding the Neurobiology of Suicidal Behavior. In: Kim Y-K, Amidfar M, editors. Translational Research Methods for Major Depressive Disorder. New York, NY: Springer US; 2022. p. 123-45.
CrossRef - Gutiérrez-Rojas L, Porras-Segovia A, Dunne H, Andrade-González N, Cervilla JA. Prevalence and correlates of major depressive disorder: a systematic review. Braz J Psychiatry. 2020;42(6):657-72.
CrossRef - Clevenger SS, Malhotra D, Dang J, Vanle B, IsHak WW. The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder. Ther Adv Psychopharmacol. 2018;8(1):49-58.
CrossRef - Thom RP, Alexander JL, Baron D, Garakani A, Gross L, Pine JH, et al. Selective Serotonin Reuptake Inhibitors: How Long Is Long Enough? J Psychiatr Pract. 2021;27(5):361-71.
CrossRef - Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. Treasure Island: StatPearls Publishing; 2022.
- Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, et al. Selective serotonin reuptake inhibitors and adverse effects: a narrative review. Neurol Int. 2021;13(3):387-401.
CrossRef - Pompili M. Antidepressants therapy and risk of suicide among patients with major depressive disorders. New York: Nova Biomedical Books; 2011. vi, 48 p.
- Fiksdal A, Hanlin L, Kuras Y, Gianferante D, Chen X, Thoma MV, et al. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology. 2019;102:44-52.
CrossRef - Vinkers CH, Kuzminskaite E, Lamers F, Giltay EJ, Penninx BW. An integrated approach to understand biological stress system dysregulation across depressive and anxiety disorders. J Affect Disord. 2021;283:139-46.
CrossRef - Juruena MF, Eror F, Cleare AJ, Young AH. The Role of Early Life Stress in HPA Axis and Anxiety. Adv Exp Med Biol. 2020;1191:141-53.
CrossRef - Santomauro DF, Herrera AMM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700-12.
CrossRef - Suwinyattichaiporn T, Johnson ZD. The impact of family and friends social support on Latino/a first-generation college students’ perceived stress, depression, and social isolation. J Hisp High Educ. 2022;21(3):297-314.
CrossRef - Yoon S, Cummings S. Factors protecting against suicidal ideation in south Korean community-dwelling older adults: a systematic literature review. J Gerontol Soc Work. 2019;62(3):279-305.
CrossRef - Karaca Z, Grossman A, Kelestimur F. Investigation of the Hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev Endocr Metab Disord. 2021;22(2):179-204.
CrossRef - D’Elia ATD, Juruena MF, Coimbra BM, Mello MF, Mello AF. Posttraumatic stress disorder (PTSD) and depression severity in sexually assaulted women: hypothalamic-pituitary-adrenal (HPA) axis alterations. BMC psychiatry. 2021;21(1):1-12.
CrossRef - Liu L, Liu C, Wang Y, Wang P, Li Y, Li B. Herbal Medicine for Anxiety, Depression and Insomnia. Curr Neuropharmacol. 2015;13(4):481-93.
CrossRef - Sahoo S, Brijesh S. Pharmacogenomic assessment of herbal drugs in affective disorders. Biomed Pharmacother. 2019;109:1148-62.
CrossRef - Kenda M, Kočevar Glavač N, Nagy M, Sollner Dolenc M. Medicinal plants used for anxiety, depression, or stress treatment: An update. Molecules. 2022;27(18):6021.
CrossRef - Al-Snafi AE. A complementary and alternative natural antidepressant therapy with emphasis on their mechanisms of action. Int J Pharm Biol Sci Arch. 2021;2(1):7-21.
CrossRef - Biswas D, Mandal S, Chatterjee Saha S, Tudu CK, Nandy S, Batiha GES, et al. Ethnobotany, phytochemistry, pharmacology, and toxicity of Centella asiatica (L.) Urban: A comprehensive review. Phytother Res. 2021;35(12):6624-54.
CrossRef - Bradwejn J, Zhou Y, Koszycki D, Shlik J. A double-blind, placebo-controlled study on the effects of Gotu Kola (Centella asiatica) on acoustic startle response in healthy subjects. J Clin Psychopharmacol. 2000;20(6):680-4.
CrossRef - Wattanathorn J, Mator L, Muchimapura S, Tongun T, Pasuriwong O, Piyawatkul N, et al. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol. 2008;116(2):325-32.
CrossRef - Tiwari S, Singha S, Patwardhan K, Gehlot S, Gambhira IS. Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. Dig J Nanomater Biostructures. 2008;3(4):215–20.
- Samuel K, Medikeri A, Pasha T, Ansari MF, Saudagar A. Centella asiatica: A traditional herbal medicine. World J Adv Res Rev. 2022;15(1):512-24.
CrossRef - Shinomol GK, Muralidhara. Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine. 2008;15(11):971-84.
CrossRef - Sabaragamuwa R, Perera CO, Fedrizzi B. Centella asiatica (Gotu kola) as a neuroprotectant and its potential role in healthy ageing. Trends Food Sci Technol. 2018;79:88-97.
CrossRef - Bjørklund G, Shanaida M, Lysiuk R, Butnariu M, Peana M, Sarac I, et al. Natural Compounds and Products from an Anti-Aging Perspective. Molecules. 2022;27(20):7084.
CrossRef - Wanasuntronwong A, Tantisira MH, Tantisira B, Watanabe H. Anxiolytic effects of standardized extract of Centella asiatica (ECa 233) after chronic immobilization stress in mice. J Ethnopharmacol. 2012;143(2):579-85.
CrossRef - Mando Z, Mando H, Afzan A, Shaari K, Hassan Z, Mohamad Taib MNA, et al. Biomarker triterpenoids of Centella asiatica as potential antidepressant agents: Combining in vivo and in silico studies. Behav Brain Res. 2024;466:114976.
CrossRef - Kalshetty P, Aswar U, Bodhankar S, Sinnathambi A, Mohan V, Thakurdesai P. Antidepressant effects of standardized extract of Centella asiatica L in olfactory bulbectomy model. Biomed Aging Pathol. 2012;2(2):48-53.
CrossRef - Thakurdesai PA. Centella asiatica (Gotu kola) leaves: Potential in neuropsychiatric conditions. Nutraceuticals in Brain Health and Beyond: Elsevier; 2021. p. 307-28.
CrossRef - Ieraci A, Mallei A, Popoli M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016;2016:1-13.
CrossRef - Gururajan A, Reif A, Cryan JF, Slattery DA. The future of rodent models in depression research. Nat Rev Neurosci. 2019;20(11):686-701.
CrossRef - Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomed Pharmacother 2018;105:1205-22.
CrossRef - Thakurdesai P, Nimse S, Deshpande P. Characterization and Preclinical Toxicity Assessment of Intranasal Administration of Standardized Extract of Centella asiatica (L.) Urban Leaves (INDCA-NS) in Laboratory Rats. Toxicol Int. 2023:391-407.
CrossRef - CPCSEA. CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003:257-74.
- Malkesman O, Pine DS, Tragon T, Austin DR, Henter ID, Chen G, et al. Animal models of suicide-trait-related behaviors. Trends Pharmacol Sci. 2009;30(4):165-73.
CrossRef - Takahashi A. The role of social isolation stress in escalated aggression in rodent models. Neurosci Res. 2022;In Press.
CrossRef - Wei S, Zhang H, Gao J, Xue L, Sun P, Chao Y, et al. Impact of social isolation and resident intruder stress on aggressive behavior in the male rat. Neural Regen Res. 2010;5(15):1175-9.
- Ho Y, Liu T, Tai M, Wen Z, Chow RS, Tsai Y, et al. Effects of olfactory bulbectomy on NMDA receptor density in the rat brain. Brain Res. 2001;900(2):214-8.
CrossRef - Ślifirski G, Król M, Turło J. 5-HT receptors and the development of new antidepressants. Int J Mol Sci. 2021;22(16):9015.
CrossRef - Bazovkina D, Ustinova U, Adonina S, Komleva P, Arefieva A, Kulikova E. Effect of Long-Term Social Isolation on Behavior and Brain Dopaminergic System in Mice. J Evol Biol Phyiol. 2024;60(1):397-408.
CrossRef - Teo AR, Lerrigo R, Rogers MA. The role of social isolation in social anxiety disorder: a systematic review and meta-analysis. J Anxiety Disord. 2013;27(4):353-64.
CrossRef - Mansk LM, Jaimes LF, Dias TL, Pereira GS. Social recognition memory differences between mouse strains: On the effects of social isolation, adult neurogenesis, and environmental enrichment. Brain Res. 2023;1819:148535.
CrossRef - Linge R, Pazos A, Diaz A. Social isolation differentially affects anxiety and depressive-like responses of bulbectomized mice. Behav Brain Res. 2013;245:1-6.
CrossRef - Locci A, Pinna G. Social isolation as a promising animal model of PTSD comorbid suicide: neurosteroids and cannabinoids as possible treatment options. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:243-59.
CrossRef - Preti A. Animal model and neurobiology of suicide. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):818-30.
CrossRef - Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJA. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. 2013(77):e4367.
CrossRef - Popova NK, Tsybko AS, Naumenko VS. The implication of 5-HT receptor family members in aggression, depression and suicide: similarity and difference. Int J Mol Sci. 2022;23(15):8814.
CrossRef - Olivier B, Olivier JD. Aggression and sexual behavior: overlapping or distinct roles of 5-HT1A and 5-HT1B receptors. In: Olivier B, editor. Serotonin and the CNS-New Developments in Pharmacology and Therapeutics: InTech Open; 2022.
CrossRef - Vázquez-León P, Miranda-Páez A, Valencia-Flores K, Sánchez-Castillo H. Defensive and emotional behavior modulation by serotonin in the periaqueductal gray. Cell Mol Neurobiol. 2023;43(4):1453-68.
CrossRef - Mando Z, Al Zarzour RH, Alshehade S, Afzan A, Shaari K, Hassan Z, et al. Terpenoids and Triterpenoid Saponins: Future Treatment for Depression. Curr Tradit Med. 2024;10(2):51-68.
CrossRef - Bobade V, Bodhankar SL, Aswar U, Mohan V, Thakurdesai PA. Prophylactic effects of asiaticoside based standardized extract of Centella asiatica (L.) Urban leaves in experimental migraine: Involvement of 5HT1A/1B receptors. Chin J Nat Med. 2015;13(4):274-82.
CrossRef - Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neurosci. 2012;215:42–58.
CrossRef - Li G, Jing P, Liu Z, Li Z, Ma H, Tu W, et al. Beneficial effect of fluoxetine treatment aganist psychological stress is mediated by increasing BDNF expression in selected brain areas. Oncotarget. 2017;8(41):69527-37.
CrossRef - da Cunha-Bang S, Knudsen GM. The modulatory role of serotonin on human impulsive aggression. Biol Psychiatry. 2021;90(7):447-57.
CrossRef - Jha MK, Minhajuddin A, Chin Fatt C, Kircanski K, Stringaris A, Leibenluft E, et al. Association between irritability and suicidal ideation in three clinical trials of adults with major depressive disorder. Neuropsychopharmacol. 2020;45(13):2147-54.
CrossRef - Liu Q, Cole DA. The association of phasic irritability (aggressive outbursts) and tonic irritability (irritable mood) to depression occurrences, symptoms, and subtypes. J Affect Disord. 2021;293:9-18.
CrossRef - McCallum SM, Batterham PJ, Christensen H, Werner-Seidler A, Nicolopoulos A, Newton N, et al. Personality factors associated with suicidal ideation, plans and attempts in adolescents. J Affect Disord. 2022;310:135-41.
CrossRef - American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed., text revision. ed. Washington, DC: American Psychiatric Association; 2000. xxxvii, 943 p. p.
- Hernandez SC, Overholser JC. A systematic review of interventions for hope/hopelessness in older adults. Clin Gerontol. 2021;44(2):97-111.
CrossRef - Banerjee R, Mondal AC, Ghosh B. Effects of chronic stress and antidepressant treatment on behavioral, physiological and neurochemical aspects in male and female rats. Al Ameen J Med Sci. 2012;5(2):165-76.
- Zhou J, Cao X, Mar AC, Ding YQ, Wang X, Li Q, et al. Activation of postsynaptic 5-HT1A receptors improve stress adaptation. Psychopharmacology. 2014;231(10):2067-75.
CrossRef - Aswar U, Shende H, Aswar M. Buspirone, a 5-HT1A agonist attenuates social isolation-induced behavior deficits in rats: a comparative study with fluoxetine. Behav Pharmacol. 2022;33(5):309-21.
CrossRef - Moradi K, Badripour A, Moradi A, Bagheri S, Soltani ZE, Moassefi M, et al. Sumatriptan attenuates fear-learning despair induced by social isolation stress in mice: Mediating role of hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinol. 2024;164:107006.
CrossRef - Du X, Pang TY. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Front Psychiatry. 2015;6:32.
CrossRef - Fa M, Xia L, Anunu R, Kehat O, Kriebel M, Volkmer H, et al. Stress modulation of hippocampal activity – Spotlight on the dentate gyrus. Neurobiol Learn Mem. 2014;112C:53–60.
CrossRef - Sun X, Li C, Zhong X, Dong D, Ming Q, Gao Y, et al. Influence of psychosocial stress on activation in human brain regions: moderation by the 5-HTTLPR genetic locus. Physiol Behav. 2020;220:112876.
CrossRef - Stafford M, Gardner M, Kumari M, Kuh D, Ben-Shlomo Y. Social isolation and diurnal cortisol patterns in an ageing cohort. Psychoneuroendocrinology. 2013;38(11):2737-45.
CrossRef - Sze Y, Brunton PJ. Sex, stress and steroids. Eur J Neurosci. 2020;52(1):2487-515.
CrossRef - Bernhard A, Mayer JS, Fann N, Freitag CM. Cortisol response to acute psychosocial stress in ADHD compared to conduct disorder and major depressive disorder: A systematic review. Neurosci Biobehav Rev. 2021;127:899-916.
CrossRef - Jin Z, Han Y, Zhang D, Li Z, Jing Y, Hu B, et al. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics. 2022;14(10):2070.
CrossRef - Chen Y, Han T, Qin L, Rui Y, Zheng H. Effect of total triterpenes from Centella asiatica on the depression behavior and concentration of amino acid in forced swimming mice. Zhong Yao Cai. 2003;26(12):870-3.
- Hemamalini A, Rao MS. Anti stress effect of Centella asiatica leaf extract on hippocampal CA3 neurons – A quantitative study. Int J Pharmacol Clin Sci. 2013;2:25–32.








