Youssry Fathy 1, Ahmed Sadek 2 , Wafaa Wafy 3, Mahmoud Elansary 2
, Wafaa Wafy 3, Mahmoud Elansary 2 , Khaled Ragab 2
, Khaled Ragab 2 , Ahmed Ali 1
, Ahmed Ali 1 , Elwy Kamal 1, Amira Mohamed Abdel Gawad 4
, Elwy Kamal 1, Amira Mohamed Abdel Gawad 4 , Hend M. Ahmed 4
, Hend M. Ahmed 4 , Mohamed A. Shahba 4
, Mohamed A. Shahba 4  and Eman R. Youness 4*
and Eman R. Youness 4*
1Department of Internal Medicine, Faculty of Medicine, Minia University, Egypt.
2Department of Hepatology and Gastroenterology, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
3Public Health Department, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
4Department of Medical Biochemistry, Medical Research and Clinical Studies Institute, National Research Centre Cairo, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2933
Abstract
The study aimed to understand the magnitude of submucosal lesions as part of the referral to the endoscopic ultrasound (EUS) unit in one year and know the percent of the different types and sites of submucosal lesions of GIT in Theodore Bilharz Research Institute as tertiary referral center draining Egyptian community. Within one year, all patients referred to the EUS unit at Theodore Bilharz Research Institute (TBRI) for assessment to assess the percent of submucosal lesions cases as part of the total referral and know the different types seen and their common sites as compared to the international literature. Patients diagnosed to have submucosal lesion will be subjected to; full clinical history, thorough physical examination, laboratory investigations, BUS for more characterization (site, size, location, echo pattern, etc.) and BUS-guided fine-needle aspiration (FNA) for histopathological examination. The work comprised 36 patients; 16 females and 20 males. Their ages ranged from 21 to 75 years. All patients had preliminary upper endoscopy or colonoscopy. According to the indication of upper preliminary endoscopy or colonoscopy, 12 (33.3%) were complaining of melena, 5 (13.8%) hematemesis, 1 (2.7%) bleeding per rectum, 7 (19.4%) upper abdominal pain, 2 (5.5%) dysphagia, finally, 8 (22.2%) vomiting. According to the site of the submucosal lesion, 24 (66.6%) were gastric, 6 (16.6%) esophageal, 4 (11.1%) duodenal, 1 gastro-esophageal (2.8%), and 1 (2.8%) rectal. 34 cases (94%) were covered by normal overlying mucosa while 2 cases (6%) had superficial ulcerations. It was concluded that EUS criteria, can be used without FNA and histopathologic examination to reduce the cost of differentiation between malignant and benign lesions. All homogenous lesions were benign. Lesion size of 4.5 cm is a cut off; > 4.5 cm were malignant whereas < 4.5 cm were benign. All submucosal lesions without areas of breakdown were benign. Those infiltrating all layers are malignant. EUS guided fine needle aspiration (FNA) and histopathological examination should be done for some submucosal masses to put a definite diagnosis. EUS with colored Doppler is necessary in differentiating cystic from vascular lesions.
Keywords
Endoscopy; FNA; GIT; Submucosal lesions; US
Download this article as:| Copy the following to cite this article: Fathy Y, Sadek A, Wafy W, Elansary M, Ragab K, Ali A, Kamal E, Gawad A. M. A, Ahmed H. M, Shahba M. A, Youness A. R. Role of Endoscopic Ultrasound in Diagnosis of Submucosal Lesions of Gastrointestinal Tract. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Fathy Y, Sadek A, Wafy W, Elansary M, Ragab K, Ali A, Kamal E, Gawad A. M. A, Ahmed H. M, Shahba M. A, Youness A. R. Role of Endoscopic Ultrasound in Diagnosis of Submucosal Lesions of Gastrointestinal Tract. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/45pIjA7 |
Introduction
Submucosal lesions are Lesions that appear as protuberance in GI tract with normal overlying mucosa they are a frequent source of Referral EUS evaluation1. Those lesions are most often found incidentally during endoscopy and colonoscopy. The majority are asymptomatic. Others, may found with hemorrhage obstruction, or dysphagia. Women and men were equally affected, and most patients were more than 50 years old at the time of diagnosis2. Some of these lesions can be benign, premalignant or malignant, they most commonly occur within the stomach, but are also regularly noted in the duodenum and esophagus. Furthermore, during colonoscopy, submucosal lesions were often detected in the cecum and rectum, but familiar lesions such as lipomas may be seen in any part of the colon3.
They might arise from any layer of the gastrointestinal tract wall (intramural) or outside of the wall (extramural). Intramural lesions originate from the submucosal layer are usually lipomas, carcinoid tumors, granular cell tumors pancreatic rests, fibromas and duplication cysts. Lesions arising from the muscularis propria usually represent GI stromal tumors (G I S T), leiomyomas4,5.
There are conventional methods of diagnosis of submucosal lesions as computed tomography C T Barium studies, Endoscopic studies with biopsies and MRI6.
EUS is now considered to be superior to conventional studies as it provides an understanding of whether the lesion arises from the bowel wall (intramural) or from a structure outside the bowel wall (extramural) It also determine the layer of origin of intramural lesions e.g.; stromal cell tumors, can be seen as evolving from the muscularis mucosa, whereas lipomas evolve from the submucosal7.
Furthermore, EUS can determine the echogenicity, size of the lesion, margins, vascularity and absence or presence of adjacent lymph nodes. Lastly, it is helpful in confirming diagnosis by EUS -guided fine needle aspiration (FNA) and sometimes in appropriate management of the diagnosed lesions8.
Aim of the work
The study aimed to understand the magnitude of submucosal lesions as part of the referral to the EUS unit in one year and know the percent of the different types and sites of submucosal lesions of GIT in TBRI as tertiary referral center draining Egyptian community.
Subjects and Methods
Research design
This cross-sectional hospital-based study, was conducted through one year on all cases referred to the EUS unit at TBRI for assessment, whom were analyzed to assess the percent of cases of submucosal lesions as part of the total referral and to know the different types seen and their common sites as compared to the international literature.
Cases were subjected to; thorough history talking, physical examination, laboratory investigations (CBC, liver function tests, renal function tests), finally, EUS was done using a linear echo-endoscope (PENTAX EG- 3870 UTK) and processor (HITACHI-HI Vision Avius)
A preliminary upper endoscopy was performed to identify the lesions and characterize the overlying mucosa. After intubation of the esophagus, stomach, duodenum, rectum and colon the linear echo-endoscope was advanced under direct endoscopic guidance. Then, the ultrasound transducer was placed against the lesion under direct visualization whenever possible.
Lesions requiring more characterization were subjected to EUS–guided fine–needle aspiration (FNA) using needles of different calibers (25, 22 or 19) (ECHO 3-22 Cook Echotip ultra, ECHO – HD- 22-c Cook Echotip procore, ECHO 19 Cook Echotip ultra and Boston 22ga (0.72 mm)
Statistical analysis
Collected data were subjected to statistical analysis by SPSS. Ver. 22. Descriptive statistics and analytical statistics were treated according to data type.
Aspirated specimens were candidate for histopathological examination, after being stained using the two complementary types of slide-preparation techniques; air-dried and alcohol-fixed slides, the two preparations were complementary and were used to demonstrate different cytological features of lesions. Cases of GISTs and leiomyomas, further differentiation is needed using immune-histochemical staining by CD 117 and desmin to show spindle cells.
Results
The present study included 36 patients; 16 females and 20 males. As shown in Table (1); ranging in ages from 21 to 75 years. All patients had preliminary upper endoscopy or colonoscopy and according to the indication of upper preliminary endoscopy or colonoscopy, 12 (33.3%) complained of melena, 5 (13.8%) hematemesis,1(2.7%) bleeding per rectum, 7 (19.4%) upper abdominal pain, 2(5.5%) dysphagia, 8 (22.2%) vomiting and 1(2.7%) hepatic focal lesion on normal liver, upper endoscopy was done screening for the primary lesions.
Table 1: Demographic data and indication of upper preliminary endoscopy or colonoscopy.
|
Variable |
Data |
|
Age -Range -Mean +SD |
21-75 52.4+12.8 |
|
Sex: N (%) -Males -Females |
20(55.6%) 16(44.4%) |
|
Indication of upper preliminary endoscopy or olonoscopy N (%): -Melena -Hematemesis -Bleeding per-rectum -Upper abdominal pain -Dysphagia -Vomiting -Hepatic focal lesion on normal liver (screening for the primary lesion) |
12(33.3%) 5(13.8%) 1(2.7%) 7(19.4%) 2(5.5%) 8(22.2%) 1(2.7%) |
As shown in table (2), the laboratory findings of submucosal lesions were evaluated revealing Haemoglobin level (11.7±1.5), platelet count (243.9±78.2), INR (1.1±0.2), ALT and AST respectively (28.3±14.5) and (36.8±14.4), TLC (6500±154.2), urea (38.3±11.6) and creatinine (0.9±0.13).
Table 2: Laboratory data of submucosal lesions.
|
|
Range |
Mean+SD |
|
Haemoglobin Level |
10-14 |
11.7±1.5 |
|
Platelets count 10 |
60-420 |
243.9±78.2 |
|
INR |
1-2 |
1.1±0.2 |
|
ALT (U/L) |
5-65 |
28.3±14.5 |
|
AST(U/L) |
14-75 |
36.8±14.4 |
|
TLC |
4800-9900 |
6500±154.2 |
|
Urea (mg %) |
13-58 |
38.3±11.6 |
|
Creatinine (mg %) |
0.5-1.3 |
0.9±0.13 |
As shown by Table (3), the site of the submucosal lesion varied, 24 (66.67%) were gastric, 6 (16.67%) esophageal, 4 (11.1%) duodenal, one gastro- esophageal (2.8%), and one (2.8%) rectal.
Table 3: Site of submucosal lesion
|
|
Number |
% |
|
Submucosal oesophageal mass |
6 |
16.67 |
|
Submucosal Gastro oesophageal mass |
1 |
2.8 |
|
Submucosal gastric mass |
24 |
66.67 |
|
Submucosal duodenal mass |
4 |
11.1 |
|
Submucosal rectal mass |
1 |
2.8 |
|
Total |
36 |
100% |
As shown in Table (4), Gastric lesions were the commonest diagnosed submucosal lesions (66.67%), taking different locations; ten were fundal (41.67%), 6 at the greater curvature (25%), 4 at the antrum (16.67%), one at the lessor curvature (4.16%), one subcardial (4.16%), one at the junction between gastric body and antrum (4.16%), and one at the body of the stomach (4.16%).
Table 4: Different locations of submucosal stomach lesions.
|
|
Number |
% |
|
Fundal |
10 |
41.67 |
|
At grater curvature |
6 |
25 |
|
At lesser curvature |
1 |
4.16 |
|
At the antrum |
4 |
16.67 |
|
Subcardial |
1 |
4.16 |
|
At the junction between gastric body and the antraum |
1 |
4.16 |
|
At the body of the stomach |
1 |
4.16 |
|
Total |
24 |
100 |
As shown in Table (5), it was noticed that 34 cases (94%) were covered by normal overlying mucosa while 2 cases (6%) had some superficial ulcerations.
Table 5: Overlying mucosa of submucosal lesions
|
|
Number |
% |
|
1. Normal mucosa |
34 |
94.4 |
|
2. Normal mucosa and some superficial ulceration: |
2 |
5.6 |
As shown in Table (6) and figure (1), it was found that 31 lesions (86.1%) were intramural, while 5 lesions (13.9%) were extra mural (3 of them from gall bladder and 2 from the liver).
Table 6: Intramural and extramural submucosal lesions.
|
|
Number |
% |
|
1- Intramural |
31 |
86.1 |
|
2- Extramural |
5 |
13.9 |
|
– From gall bladder – From liver |
3 2 |
60 40 |
|
Total |
36 |
100 |
As shown in Table (7) EUS examination of the outline of the intramural lesions, 93.22% were circumscribed while 6.45% were ill defined.
Regarding the echogenicity, 71% of the lesions were hypoechoic, 9.7% hyperechoic, 16.1% anechoic and 3.2% isoechoic.
Regarding the pattern, 77.4% of the lesions were homogenous, while 22.6% were heterogeneous.
The majority of the submucosal lesions were well circumscribed, hypoechoic and homogenous.
Regarding the size of the lesions, the mean size was 3.9 x 3.4 cm.
As regards the layer of origin, 32.3% of the lesions originate from the muscularis mucosa (2nd layer), 35.2% from the submucosa (3rd layer), 25.8% from muscularis propria (4th layer) and in 3.2% of lesions the layer of origin could not be defined.
As regards the regional lymph node enlargement, 16.1% of lesions had enlarged regional LN, while 83.9% had not.
Regarding areas of breakdown, 32.26% of the lesions showed areas of breakdown, while the remaining 67.74% did not.
Table 7: Outline, echogenicity, homogenicity, size, layer of origin, regional lymph node and areas of breakdown of intramural submucosal lesions diagnosed by EUS
|
|
Number |
% |
|
Out Line – Well circumscribed – Ill – defined |
29 2 |
93.55 6.45 |
|
Echogenicity – Hypoechoic – Hyperechoic – Isoechoic – Anechoic |
22 3 1 5 |
71 9.7 3.2 16.1 |
|
Homogeneity – Homogenous – Heterogonous |
24 7 |
77.4 22.6 |
|
Size (mean in Cm) |
3.9X3.4 |
|
|
Layer of origin – Muscularis mucosa (2nd layer) – Submucosa (3rd layer) – Muscularis propria (4th layer) – Infiltration all layers except serosa |
10 11 8 2 |
32.3 35.5 25.8 6.4 |
|
Regional lymph node – No – Present: |
26 5 |
83.9 16.1 |
|
Areas of breakdown – Present – Not present |
10
21 |
32.26
67.74 |
|
Total |
31 |
100 |
As shown in Table (8), figure (2), (3) and (4) twenty-five cases of submucosal lesions indicated for FNA and histopathology; 15 (60%) were GIST, 4 (16%) leiomyoma, 2 (8%) duplications cyst, 1 (4%) submucosal polyp, 1 (4%) infiltrating Adenocarcinoma and 2 (8%) non-infiltrative Adenocarcinoma.
Table 8: Submucosal lesions indicated for FNA and histopathology.
|
|
Number |
% |
|
GIST |
15 |
60 |
|
Leiomyoma |
4 |
16 |
|
Duplication cyst |
2 |
8 |
|
Submucosal polyp |
1 |
4 |
|
Adenocarcinoma Infiltrating pancreas Non infiltrating |
1 2 |
4 8 |
|
Total |
25 |
100 |
As shown in Table (9) and figure (5), six cases of submucosal lesions were not indicated for FNA, of them, 3 were anechoic lesions.
Colored Doppler EUS was used to differentiate between them, 2 lesions showed color flow therefore, were diagnosed as varices in gastric body and duodenum, while one lesion showed no evidence of flow and according to its anatomical site in biliary area, MRCP was done and aspiration of the fluid showed bile hence a diagnosis of choledocal cyst was suggested.
The other 3 lesions, because of high accuracy of EUS in diagnosing lipomas, FNA and histopathology were not required.
Table 9: Submucosal lesions not indicated for FNA (N= 6).
|
|
Number |
% |
|
Lipoma (without FNA and histology) |
3 |
50 |
|
Colored Doppler: – Body varix – Duodinal varix |
1 1 |
16.7 16.7 |
|
Choledocal cyst (MRCP, aspiration chemistry) |
1 |
16.7 |
|
Total |
6 |
100 |
As shown in Table (10), the results of histopathology, 22 lesions were benign, while 3 were malignant. The age of patients with benign lesions ranged from 21-75 years, while the ages of patients with malignant lesions range from 52 – 62 years. Benign lesions were more common in males (63.6%) while malignant lesions more common in females (66.7%)
According to upper endoscopy results, 95.5% of benign lesions were covered by normal mucosa, while 4.5% the mucosa showed some superficial ulceration. All benign lesions covered by mucosa showed some superficial ulceration proved to be GIST on histopathology. FNA and histopathology of malignant lesions showed early adenocarcinoma (non-infiltrative) covered by normal mucosa in (66.7%) and infiltrating adenocarcinoma covered by mucosa having some superficial ulceration in (33.3%).
EUS can differentiate between benign and malignant lesions. Regarding the lesions’ outline, all benign lesions (100%) were well circumscribed, while 33.3% of malignant lesions were well circumscribed and 66.7% of malignant lesions were ill defined.
Regarding the echogenicity of lesions, 86.4% of benign lesions were hypoechoic, 9.1% were anechoic and 4.5% were isoechoic. No benign lesions were hyperechoic.
Regarding the pattern of lesions: 81.8% of benign lesions were homogenous and 18.2% were heterogenous while all malignant lesions (100%) were heterogenous.
Regarding the size of lesions: Mean size of benign lesions was 3.7 x 3.4 cm (<4 cm.), while mean size of malignant lesions was 6.2 x 4.6 (>4.5 cm.).
Regarding the layer of origin of the lesion, it was found that any submucosal lesion infiltrating all layers is a malignant lesion.
Regarding the presence of regional lymph node 90.9% of benign lesions showed no reginal lymph node enlargement, and 9.1% of benign lesions showed benign looking regional lymph node enlargement. All malignant lesions showed malignant looking regional lymph node enlargement.
Regarding the areas of breakdown, all malignant lesions (100%) showed areas of break down, while only 31.8% of benign lesions showed areas of breakdown. The rest of benign lesions (68.2%) did not show any breakdown
It was noticed that all submucosal lesions without areas of breakdown were benign lesions, while 70% of submucosal lesions with areas of breakdown were malignant lesions while 30% of submucosal lesions with areas of breakdown were benign lesions.
Histopathological examination of benign lesions revealed that 68.2% were GIST, 18.2% were leiomyoma, 9.1% were duplication cysts and 4.2% were submucosal polyps. Histopathologic examination of malignant lesions revealed that 66.7 were early adenocarcinoma (non-infiltrative) while 33.3% were infiltrating Adenocarcinoma.
Table 10: showing benign and malignant lesions according to the results of histopathology.
|
|
Benign(n=22) |
Malignant(n=3) |
P- value |
|
Age Range Mean + SD |
28-75 55.8 12.16 |
52-62 58 5.3 |
0.432 |
|
Sex Males Females |
14(63.6%) 8(36.4%) |
1(33.3%) 2(66.7%) |
0.838 |
|
Overlying mucosa -Normal -Intact mucosa and superficial ulceration |
21(95.5%) 1(4.5%) |
2(66.7 %) 1(33.3%) |
0.451 |
|
EUS Finding |
|||
|
Outline: – Well circumscribed – Ill-defined Echogenicity: – Hypoechoic – Hyperechoic – Isoechoic – Anechoic Homogeneity: – Homogenous – Heterogenous Size (mean in Cm) Layer of origin: – Muscularis mucosa (2nd layer) with intact seraosa in some areas and loss gastric wall in others – Muscularis mucosa (2nd layer) – Submucosa (3rd layer) – Muscularis propria (4th layer) Infiltration all layer except serosa |
22(100%) 0(0%) 19(86.4%) 0)0%) 1(4.5%) 2(9.1%) 18(81.8%) 4(18.2%) 3.7×3.4 0(0%) 9(40.9%) 5(22.7%) 8(36.4%) 0(0%) |
1(33.3%) 2(66.7%) 3(100%) 0(0%) 0(0%) 0(0%) 0%) 3(100%) 6.2×4.6 1(33.3%) 0(0%) 1(33.3%) 0(0%) 1(33.3%) |
0.001
0.620
0.368 |
|
Regional lymph node – No – Present: – Benign – Malignant – Reactive Areas of break down – Present – Not present FNA – Histopathology of benign lesion: – GIST – Leiomyoma – Duplication cyst – Submucosal polyp Histopathology of Malignant lesion: – Infiltration pancreas – Non infiltration pancreas
|
7(31.8%) 15(68.2%)
15(68.2%) 4(18.2) 2(9.1%) 1(4.5%)
0(0%) 0(0%) 0(0%) |
0(0%) 3(100%) 0(0%) 1(33.3%) 2(66.7%)
3(100%) 0(0%)
0(0%) 0(0%) 0(0%) 0(0%)
3(100%) 1(33.3%) 2(66.7%)
|
0.0
0.001 |
As shown in Table (11): Immune-histochemical staining of GIST and leiomyoma, to confirm diagnosis after FNA and histopathological examination, because of difficulty in distinguishing between both lesions, being composed of spindle cells.
Spindle cells of GIST have shown positive immunostaining for CD 117 in 100% of cases and positive immunostaining for desmin in 6.7% and have shown negative immunostaining for desmin by in 93.3 of patients.
Spindle cells of leiomyoma have shown positive immunostaining for desmin in 100% of cases and negative immunostaining for CD117 in 100% of cases.
Table 11: Immune-histochemical staining of GIST and Leiomyoma.
|
|
GIST N=15 |
Leiomyoma N=4 |
P-value |
|
CD117 +ve |
15(100%) |
– |
0.002 |
|
-ve |
– |
4(100%) |
– |
|
Desmin +ve |
1(6.7%) |
4(100%) |
0.001 |
|
-ve |
14(93.3%) |
– |
|
The most common submucosal lesions in this study; GISTs, leiomyoma, duplication cyst and lipoma. The mean age of GIST is 70.8 years (old age), leiomyoma 44.5 years, duplication cyst is 39 years and lipoma is 46.6 years.
Also, it was found that GIST presents in males slightly more than females, leiomyoma presents in males more than females, duplication cyst presents in males and females equally and lipoma presents in females more than in males.
In this study, it was found that the commonest site for GIST is the stomach especially at the fundus and the greater curvature. Leiomyomas were present in the esophagus in 100% of cases. Duplication cysts and lipomas were more common in the stomach. oesphogus.
In this study, it was found that 46.6% of GISTs arise from muscularis mucosa (2nd layer) and 46.6% arise from muscularis propria (4th layer). Only 6.7% of GISTs came from submucosa (3rd layer).
Also, it was observed that 50% of leiomyomas arise from muscularis mucosa (2nd layer), 25% arise from submucosa (3rd layer) and 25% arise from musclaris propria (4th layer).
It was found that 100% of duplication cysts arise from submucosa (3rd layer)
As shown in Table (12), it was found that 66.7% of lipomas arise from submucosa (3rd layer) and 33.3% arise from muscularis mucosa (2nd layer).
Table 12: commonest submucosal lesions.
|
Subcardial At the junction Between gastric body and the antrum At the body of the stomach – Submucosal duodenal mass – Submucosal rectal mass |
1(7.1%) 1(6.7%) |
|
1(50%) |
|
|
Outline: – Well circumscribed – Ill-defined Echogenicity: – Hypechoic – Hyperechoic – Isoechoic – Anechoic Homgenicity: – Homogenous – Heterogenous
Size(mean in Cm) Layer of origin: – Muscularis mucosa(2nd layer) – Submucosa(3rd layer) – Muscularis propria(4th layer) |
15(100%)
15(100%)
12(80%) 3(20%)
3.65×3.3
7(46.65%)
1(6.7%)
7(46.65%) |
4(100%)
3(75%)
1(25%)
3(75%) 1(25%)
3.2×2.8
2(50%)
1(25%)
1(25%) |
2(100%)
2(100%)
2(100%)
1.9×2,1
2(100%) |
3(100%)
3(100%)
3(100%)
1.06×1.06
1(33.3%)
2(66.7%) |
As shown in table (13) the sensitivity of unaided EUS, in diagnosing of submucosal lesions, versus EUS – guided fine needle aspiration (FNA) and histopathology (gold standard) was 72%; submucosal lesions indicated for FNA and histopathology were 25 patients; 18 of them diagnosed by unaided EUS whereas the other 7 patients were diagnosed by EUS – guided fine needle aspiration (FNA) and histopathology (gold standard).
Table 13: sensitivity of unaided EUS, in diagnosing of submucosal lesions, versus EUS – guided fine needle aspiration (FNA) and histopathology (gold standard).
|
AUC |
Sensitivity |
P-value |
95% Confidence interval |
|
|
EUS |
0.860 |
75% |
0.001 |
0.733-0.942 |
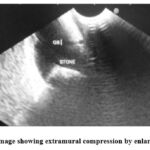 |
Figure 1: EUS image showing extramural compression by enlarged gall bladder |
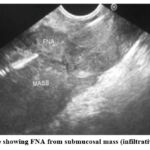 |
Figure 2: EUS image showing FNA from submucosal mass (infiltrative Adenocarcinoma) |
 |
Figure 3: EUS image showing submucosal mass (non-infiltrative Adenocarcinoma). |
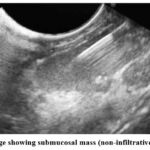 |
Figure 4: EUS image showing submucosal mass (non-infiltrative Adenocarcinoma). |
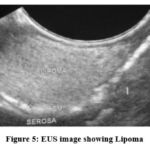 |
Figure 5: EUS image showing Lipoma |
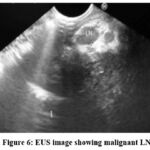 |
Figure 6: EUS image showing malignant LN. |
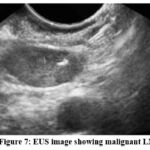 |
Figure 7: EUS image showing malignant LN. |
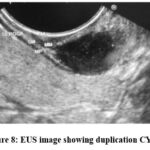 |
Figure 8: EUS image showing duplication CYST. |
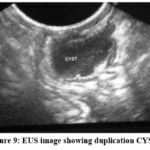 |
Figure 9: EUS image showing duplication CYST |
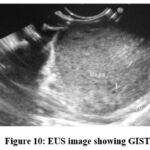 |
Figure 10: EUS image showing GIST |
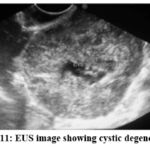 |
Figure 11: EUS image showing cystic degeneration. |
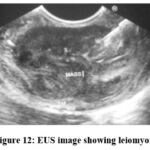 |
Figure 12: EUS image showing leiomyoma. |
Discussion
Three patients, not having FNA and histopathology were in accordance to Nakamuras who reported in 2022that biopsies or FNA were generally not needed because of high accuracy of EUS in diagnosing lipomas9.
The other 25 patients of intramural lesions underwent FNA and histopathological examination was done to reach a definite diagnosis.
Lesions were classified according to the results of histopathology as a benign and malignant.
The number of benign lesions were 22, while the malignant lesions were 3. The age of patients with benign lesions ranged from 21-75years, while the ages of patients with malignant lesions range from 52 – 62 years. Benign lesions were more common in males (63.6%) while malignant lesions were more common in females (66.7%)
According to upper endoscopy results, 95.5% of benign lesions were covered by normal mucosa, while 4.5% the mucosa showed some superficial ulceration. All benign lesions covered by mucosa showing some superficial ulceration proved to be GIST on histopathology in accordance to Souquet and Bobichon who reported in 2015 that in some cases of GIST, the overlying mucosa may be slightly ulcerated10.
FNA and histopathology of malignant lesions showed early adenocarcinoma (non-infiltrative) covered by normal mucosa in (66.7%) and infiltrating adenocarcinoma covered by mucosa having some superficial ulceration in (33.3%). This is in accordance with Akira Dobashi who reported in 2021 in a case report that a case of early duodenal adenocarcinoma resembling a submucosal tumor cured with endoscopic resection11.
And in accordance with Nobusuke who reported in 1997 that a case of advanced gastric cancer was seen resembling submucosal tumor of the stomach12.
In this study, we report 3 cases of submucosal masses as a case report that they proved to be malignant lesions when FNA and histopathology were done, 2 of them were early Adenocarcinoma (non-infiltrative) and the other was infiltrating adenocarcinoma (Advanced cancer).
EUS can differentiate between benign and malignant lesions. Regarding the outline of lesions, all benign lesions (100%) are well circumscribed, while 33.3% of malignant lesions were well circumscribed and 66.7% of malignant lesions were ill defined in accordance to Chak and others who reported in 2016that subepithelial tumors with a smooth margin are likely to be benign, while subepithelial tumors with irregular outer borders are suggestive of malignancy13.
Regarding the echogenicity of lesions, 86.4% of benign lesions were hypoechoic, 9.1% are anechoic and 4.5% are isoechoic. No benign lesions were hyperechoic.
All malignant lesions in this study (100%) were hypoechoic, but the number of malignant lesions in this study (n=3) was insufficient and statistically insignificant.
So, we need a larger number of malignant lesions to know the different echo patterns of malignant lesions and exact role of echogenicity in diagnosing malignant lesions.
In this study 5 lesions were anechoic. Colored Doppler EUS was used to differentiate between them. Two of them showed evidence of flow in colored Doppler suggestive of varices of gastric body and duodenum. The other 3 lesions had no flow in colored Doppler, one of them proved to be a choledocal cyst according to its anatomical site, aspiration and chemistry (Bile) and confirmation by MRCP. The other 2 lesions revealed thick mucinous materials or debris by using FNA of the fluid suggestive of duplication cyst.
Regarding the pattern of lesions: 81.8% of benign lesions were homogenous and 18.2% were heterogenous while all malignant lesions (100%) were heterogenous. This means that homogenous lesions were benign in accordance with Chak and colleagues who reported in 2016 that Benign GISTs are typically hypoechoic and homogenous lesions14.
Regarding the size of lesions: Mean size of benign lesions was 3.7 x 3.4 cm (<4 cm.), while mean size of malignant lesions was 6.2 x 4.6 (>4.5 cm.). We can take the size of 4.5 cm as a cut off where > 4.5 cm were considered malignant lesions while < 4.5 cm were benign lesions. This is in accordance with Chak and others who reported in 2016 that features predictive of malignant subepithelial tumors were diameter > 4 cm and features predictive of benign subepithelial tumors were diameter < 3 cm13.
Regarding the layer of origin of the lesion, it was found that any submucosal lesion infiltrating all layers is a malignant lesion.
Regarding the presence of regional lymph node 90.9% of benign lesions showed no regional lymph node enlargement, and 9.1% of benign lesions showed benign looking regional lymph node enlargement. All malignant lesions showed malignant looking regional lymph node enlargement.
Regarding the areas of breakdown, all malignant lesions (100%) showed areas of break down, while only 31.8% of benign lesions showed areas of breakdown. The rest of benign lesions (68.2%) did not show any breakdown in accordance to Chak who reported in 2016 that EUS features of GIST including irregularity of extraluminal border, presence of cystic spaces, echogenic foci, heterogeneity and large size are associated with an invasive tumor14. It was noticed that all submucosal lesions without areas of breakdown were benign lesions, while 70% of submucosal lesions with areas of breakdown are malignant lesions while 30% of submucosal lesions with areas of breakdown were benign lesions. This means that all submucosal lesions with areas of breakdown must be biopsied for histopathologic examination to exclude malignant lesions.
Histopathological examination of benign lesions revealed that 68.2% were GIST, 18.2% were leiomyoma, 9.1% were duplication cysts and 4.2% were submucosal polyps. Histopathologic examination of malignant lesions revealed that 66.7 were early adenocarcinoma (non-infiltrative) while 33.3% were infiltrating adenocarcinoma.
After FNA and histopathological examination there was a difficulty in differentiating between GIST and leiomyoma as they are composed of spindle cells, so they needed confirmation by immunohistochemical staining.
Spindle cells of GIST have shown positive immunostaining for CD 117 in 100% of cases and positive immunostaining for desmin in 6.7% and have shown negative immunostaining for desmin by in 93.3 of patients, in accordance to Abraham who reported in 2021 that GIST is positive for kit CD117 and < 5% are positive for desmin. Spindle cells of leiomyoma have shown positive immunostaining for desmin in 100% of cases and negative immunostaining for CD117 in 100% of cases15. This is in accordance with ZHU who reported in 2019 that esophageal leiomyoma typically shows strong positivity for desmin while proves negative for CD11716.
The most common submucosal lesions in this study were GISTs, leiomyoma, duplication cyst and lipoma. The mean age of GIST is 70.8 years (old age), the mean age of leiomyoma is 44.5 years, the mean age of duplication cyst is 39 years and the mean age of lipoma is 46.6 years. This is in accordance with Nilsson who reported in 2012 that median age of GIST at diagnosis is 66-69 years in population-based studies, mean age of leiomyoma is 45-50 years, mean age of duplication cyst is 30-40 years, and mean age of lipoma is 40 to 45years17.
Also, it was found that GIST presents in males slightly more than females, leiomyoma presents in males more than females, duplication cyst presents in males and females equally and lipoma presents in females more than in males.
This is in accordance with Miettinen who reported in 2022 that there is a slight male predominance in adult GIST, with no difference between males and females in leiomyoma and duplication cysts, while lipoma is more common in females 18.
In this study, it was found that the most common site for GIST is the stomach especially at the fundus and the greater curvature. This is in accordance with Săftoiu who reported in 2020 that subepithelial tumors are mostly gastric GISTs. Leiomyomas were present in the esophagus in 100% of cases. Duplication cysts and lipomas were more common in the stomach19. This is in accordance with Yamashita who reported in 2015 that approximately two thirds of all GISTs occur in the stomach20. Punpale who reported in 2021 that leiomyoma is predominantly found in the esophagus21. Maderal F et al who reported in 2016 that gastric lipoma accounts for about 5% of all gastrointestinal lipomas and 75% are located in the antrum22. Wieczorek who reported in 2019 that approximately 50% of duplication cysts are found in the small intestine, with the remainder in the esophagus, stomach and colon23.
In this study, it was found that 46.6% of GISTs arise from muscularis mucosa (2nd layer) and 46.6% arise from muscularis propria (4th layer). Only 6.7% of GISTs came from submucosa (3rd layer)., This is in accordance with Ando who reported in 2012 that most GISTs arise from the 2nd or the 4th layer of gastrointestinal tract, corresponding to muscularis mucosa and the muscularis propria24.
Also, it was observed that 50% of leiomyomas arise from muscularis mucosa (2nd layer), 25% arise from submucosa (3rd layer) and 25% arise from muscularis propria (4th layer). This is in accordance with Shim who reported in 2015 that part of esophageal leiomyomas is derived from muscularis propria (4th layer) and others arise from muscularis mucosa (2nd layer)25.
It was found that 100% of duplication cysts arise from submucosa (3rd layer) in accordance with Yasuda who reported in 2017 that EUS of duplication cysts usually appears as an anechoic lesion in the third hypoechoic layer (submucosa)26.
Also, it was found that 66.7% of lipomas arise from submucosa (3rd layer) and 33.3% arise from muscularis mucosa (2nd layer) in accordance to Kim who reported in 2020 that lipoma usually originates from the third echo – Rich layer (submucosa), though sometimes from other layers as well27.
Finally, in this study, we compare the ability of EUS alone in diagnosing of submucosal lesions versus EUS – guided fine needle aspiration (FNA) and histopathology (gold standard)
It was found that number of submucosal lesions indicated for FNA and histopathology were 25 patients, 18 of them diagnosed by EUS alone and the other 7 patients not diagnosed by EUS alone and FNA and histopathology were needed for diagnosis this means that sensitivity of EUS alone in diagnosing of submucosal lesions versus EUS – guided fine needle aspiration (FNA) and histopathology (gold standard ) was 72% in accordance with Kwon who reported in 2015 in a similar study that shows the accuracy of endoscopic ultrasonographic impression compared with pathologic diagnosis in gastrointestinal submucosal tumors, in which 58 cases of gastrointestinal SMTs with both EUS findings and pathologic reports were compared retrospectively. It was found that EUS and pathologic diagnosis coincided in 46/58 with sensitivity (79.3%) of the cases.
Conclusion
EUS guided fine needle aspiration (FNA) and histopathological examination should be done for some submucosal masses to put a definite diagnosis. EUS with colored Doppler is necessary in differentiating cystic from vascular lesions.
Acknowledgment
We thanks all the participants
Funding source
None
Conflict of interest
None
References
- Joo MK, Park JJ, Lee YH, Lee BJ, Kim SM, Kim WS, Yoo AY, Chun HJ, Lee SW. Joo MK, et al, Clinical Efficacy and Safety of Endoscopic Treatment of Gastrointestinal Stromal Tumors in the Stomach. Gut Liver.2023; 15;17(2):217-225. doi: 10.5009/gnl210454.
CrossRef - Park EY, Baek DH, Hong SM, Lee BE, Lee MW, Kim GH, Song GA. Park EY, et al, Feasibility of endoscopic resection and impact of endoscopic ultrasound-based surveillance on colorectal subepithelial tumors. Surg Endosc.2023;37(9):6867-6876. doi: 10.1007/s00464-023-10195-7.
CrossRef - Vasilakis, T.; Ziogas, D.; Tziatzios, G.; Gkolfakis, P.; Koukoulioti, E.; Kapizioni, C.; Triantafyllou, K.; Facciorusso, A.; Papanikolaou, I.S., EUS-Guided Diagnosis of Gastric Subepithelial Lesions, What Is New? Diagnostics 2023; 13:2176. https://doi.org/10.3390/ diagnostics13132176
CrossRef - Kobayashi N, Yoshida H, Fujikawa N, Yoshimachi S, Kohyama A, Kawaguchi S, Kobayashi N, et al, Diagnostic utility of EUS-FNA in distinguishing hepatocellular carcinoma from gastric submucosal tumor: A case report. Int J Surg Case Rep.2023;110:108715. doi: 10.1016/j.ijscr.2023.108715.
CrossRef - Salah W, Faigel DO, Salah W, et al, When to puncture, when not to puncture: Submucosal tumors. Endosc Ultrasound.2014;3(2):98-108. doi: 10.4103/2303-9027.131038.
CrossRef - Li ZX, Li XD, Liu XB, Xing WQ, Sun HB, Wang ZF et al., Clinical evaluation of right recurrent laryngeal nerve nodes in thoracic esophageal squamous cell carcinoma. J Thorac Dis. 2020;12(7):3622-3630. doi: 10.21037/jtd-20-774.
CrossRef - Tsurumaru D, Nishimuta Y, Kai S, Oki E, Minoda Y, Ishigami K. Tsurumaru D, et al. Clinical significance of dual-energy dual-layer CT parameters in differentiating small-sized gastrointestinal stromal tumors from leiomyomas. Jpn J Radiol.2023;19. doi: 10.1007/s11604-023-01473-4.
CrossRef - Mehershahi S, Ghazanfar H, Shaikh DH, Baiomi A, Ihimoyan A. Mehershahi S, et al. Recent Anti-platelet Therapy Revealing Underlying Undiagnosed Gastrointestinal Stromal Tumor in Otherwise Healthy Patient. Cureus. 2020;12(3): e7450. doi:10.7759/cureus.7450.
CrossRef - Nakamura K., Takarabe S., Arahata K., Katayama T., Ojiro K., Kishikawa H., Sasaki A., Hasegawa H., Nishida J. A case of early gastric cancer resembling a subepithelial lesion diagnosed by endoscopic ultrasound-guided fine needle aspiration. Clin. J. Gastroenterol. 2022; 15:1048–1054.
CrossRef - Souquet Jc, Bobichon R. Role of endoscopic ultrasound in the management of submucosal tumors in the esophagus and stomach. Acta Endoscop 2015; 26: 307–12.
- Akira Dobashi, Shingo Ono, Akira Dobashi, Hiroto Furuhashi, Akio Koizumi, Hiroaki Matsui, Yuko Hara, and Kazuki Sumiyama. Characteristics of superficial esophageal squamous cell carcinomas undetectable with narrow-band imaging endoscopy. Gastroenterol Rep (Oxf). 2021; 9(5): 402–407.
CrossRef - Nobusuke ohara osamu tominaga, masavuki uchiyama and Haruo Nakano. A case of Advanced Gastric Cancer Resembling Submucosal Tumor of the stomach. Jpn.J clinoncol 1997: 27: 6: 423-426.
CrossRef - Chak A, Canto M, Stevens PD, Light dale CJ, Van de Mierop F, Cooper G, Pollack BJ, Sivak MV Jr. Clinical applications of a new through-the-scope ultrasound probe: prospective comparison with an ultrasound endoscope. Gastrointest Endosc.2016; 45: 291–295.
CrossRef - Chak A, Canto MI, Rosch T Dittler HJ, Hawes RH, Tio Tl. Endosonographic differentiation of benign and malignant stromal tumors. Gatrointes Endosc 2016; 45: 468-73.
CrossRef - Abraham SC, Krasinskas AM, Correa AM, Hofstetter WL, Ajani JA, Swisher SG, et al. Duplication of the muscularis mucosae in Barrett esophagus: an underrecognized feature and its implication for staging of adenocarcinoma. Am J Surg Pathol. 2021;31(11):1719–25.
CrossRef - Zhu S, Lin J, and Huang S. Successful en bloc endoscopic full-thickness resection of a giant cervical esophageal leiomyoma originating from muscularis propria. J Cardiothorac Surg. 2019; 14: 16
CrossRef - Nilsson B, Bumming P, Meis-Kindblom JM et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer 2012;103(4):821–829.
CrossRef - Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 2022; 29, 52–68.
CrossRef - Săftoiu A, Vilmann P, Ciurea T. Do we need contrast agents for EUS? Endosc Ultrasound 2020;9(6):361-368.
CrossRef - Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound. 2015; 43:89–97.
CrossRef - Punpale A, Rangole A, Bhambhani N, et al. Leiomyoma of esophagus. Ann Thorac Cardiovasc Surg. 2021;13(2):78–81.
- Maderal F, Hunter F, Fuselier G et al. Gastric lipomas—an update of clinical presentation, diagnosis, and treatment. Am J Gastroenterol 2016; 79:964–7.
- Wieczorek RL, Seidman I, Ranson JH, Ruoff M. Congenital duplication of the stomach: case report and review of the English literature. Am J Gastroenterol. 2019; 79:597–602.
- Ando N, Goto H, Niwa Y, Hirooka Y ohmiya N, Nagasaka T. The diagnosis of GI stromal tumors with EUS – guided fine needle aspiration with immunohistochemical analysis Gastrointest Endose 2012; 55: 37 – 43
CrossRef - Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2015;37:646–654.
CrossRef - . Yasuda K, Cho E, Nakajima M, Kawai K. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 2017;36:S17-S20.
CrossRef - Kim GH, Ahn JY, Gong CS, et al. Efficacy of endoscopic ultrasoundguided fine-needle biopsy in gastric subepithelial tumors located in the cardia. Dig Dis Sci 2020;65(02):583–590
CrossRef - Kwon JG, Kim EY, Kim YS, et al. Accuracy of endoscopic ultrasonographic impression compared with pathologic diagnosis in gastrointestinal submucosal tumors. Korean J Gastroenterol. 2015;45:88–96.








