Nyamdemberel Tsagaanbaatar1* , Uranzaya Dashzeveg1
, Uranzaya Dashzeveg1 , Nomindari Nyamdemberel2
, Nomindari Nyamdemberel2 , Myadagbadam Urtnasan1
, Myadagbadam Urtnasan1 and Chimedragchaa Chimedtseren1
and Chimedragchaa Chimedtseren1
1Institute of Traditional Medicine and Technology, Ulaanbaatar, Mongolia
2Mongolia National University of Medical Sciences, Ulaanbaatar, Mongolia.
Corresponding Author E-mail: nyamaa071427@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2941
Abstract
This study examines ARUR-10, the herbal prescription commonly utilized in Traditional Mongolian Medicine practices for the treatment of kidney disease and the improvement of physical strength. This investigation aims to establish a profile of the biologically active compounds, antioxidant activity, and the anti-inflammatory impact on the kidneys in the traditional ARUR-10 prescription. For this analysis, we used Thin-Layer Chromatography (TLC) to identify phenolic compounds in the traditional prescription. Quantification of these bioactive substances was determined by UV/Vis spectrophotometry. The traditional ARUR-10 prescription, or traditional drug, had a higher polyphenolic content of 12.9±0.361% (gallic acid equivalent) and a sum flavonoid content of 0.33±0.015% (Rutin equivalent). The antioxidant activity was measured using the inhibiting free radical’s DPPH method and evaluated using the IC50 value. The 100 μg/ml concentration inhibited free radicals by 68.47%, whereas 200 μg/ml inhibited them by 83.29%, with an IC50 of 92.4 μg/ml. This study concluded that the traditional ARUR-10 prescription contains many polyphenolic compounds and flavonoids and has antioxidant properties. ARUR-10 has a moderate level of toxicity and no signs of chronic toxicity have been detected. Urine excretion was 1.52 ± 1.23 times higher than in the distilled water group (0.97 ± 0.71). ARUR-10 at doses of 240 mg/kg (1.62±1.14) had a similar effectiveness to Hydrochlorothiazide (1.52±1.23). In the gentamicin-induced nephritis model, ARUR-10 reduced serum creatinine by 42.8%, uric acid by 14.9%, and urea by 32% at doses of 240 mg/kg, compared to the control group that produced the pathological model. It had a protective effect on the kidneys by preserving the tube structure; cell death and cell necrosis were not observed.
Keywords
2.2-diphenyl-1-picrylhydrazyl; flavonoid; nephroprotective activity; phenolic compounds; traditional ARUR-10 prescription
Download this article as:| Copy the following to cite this article: Tsagaanbaatar N, Dashzeveg U, Nyamdemberel N, Urtnasan M, Chimedtseren C. Results of Chemical and Pharmacological Studies of the Mongolian Traditional Prescription "Arur-10". Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Tsagaanbaatar N, Dashzeveg U, Nyamdemberel N, Urtnasan M, Chimedtseren C. Results of Chemical and Pharmacological Studies of the Mongolian Traditional Prescription "Arur-10". Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3RsHxN7 |
Introduction
Nearly 700 million people globally suffer from chronic kidney disease, with chronic kidney failure being one of the leading causes of both morbidity and mortality. In 2017, the World Health Organization (WHO) reported patients with chronic kidney disease reached 697.5 million and that the global prevalence of kidney disease reached 9.1%. Therefore, this disease has become a public health problem 1. According to the health statistics of Mongolia, diseases of the genitourinary system are among the five leading causes of diseases in the population and 932 cases were registered in 2018, 1120 in 2019, 1084 in 2020, and 836 in 2022 (per 10,000 population)2. It depends on many factors such as Mongolia’s extreme climate, social and economic conditions, and the lifestyle of its citizens including food safety, etc.
The worldwide prevalence of diseases of the kidney is increasing every year and extensive research into medications and medicinal plants that can treat and lessen this sort of disease is underway.
According to researchers, Traditional Mongolian Medicine emerged about 5000 years ago. Mongolians have long created many recipes to treat any disease. The main ingredients of prescriptions are natural drugs such as plants, animals and minerals used to treat disease, but not all traditional prescriptions were studied and validated scientifically. One of them, as written in traditional medical source books, is the traditional ARUR-10 prescription or ARUR-10 traditional drug which has been widely used to treat kidney diseases including kidney stones3. However, a thorough investigation of the safety and bioavailability of its main components is required. ARUR-10 is orange in color, slightly aromatic, has a bitter taste, and it is composed of 10 medicinal herbs. These 10 herbs are Terminalia chebula Retz., Carthamus tinctorius L., Amomum kravanh pire ex Garnep., Canavalia gladita D.C., Phytolacca acinosa L., Rubia cordifolia L., Gentian barbata L., Pyrola rotundifolia L., Trogopterus xanthipes Milne., Juniperus sabine L.4. There have been no chemical or pharmacological studies on ARUR-10. In general, when providing a theoretical evaluation and conclusion on the therapeutic mechanisms of traditional prescriptions, it is best to consider the primary biologically active ingredients that they include. Therefore, our research focuses on the bioactive compound contained in ARUR-10 with accurate methods commonly used in bioorganic chemistry and pharmacology. The investigation aim is to establish the biological activity compounds, antioxidant activity and anti-inflammatory impact of the kidneys in ARUR-10. On the other hand, to scientifically prove the knowledge about the traditional use of ARUR-10 for kidney disease, pharmacological studies will be conducted to determine the effect of treating kidney inflammation.
Materials and Methods
Materials
The traditional ARUR-10 prescription or traditional drug (registration number: L 20161122TO05802, serial number: 700622) produced by the Institute’s Traditional Medicine’s Factory was used in the study.
Laboratory Animals
The Institute’s Experimental Animal Center provided thirty adult Wistar rat males weighing 220-280 grams. The vivar meets the following conditions including: temperature 20±1°C, humidity 50-60%, light/dark cycle 12 hours and air circulation 8-15 times per hour. Rats were fed with clean water and a special animal feed.
Chemical Reagents
Gallic acid (≥98%), tannic acid (≥98%), chebulic acid (≥98%), rutin (≥92.6%), quercetin (≥98.1%), kaempferol (≥98%), apigenin (≥99.6%) were supplied by Shanghai (China). Luteolin (≥99%) and DPPH reagent (≥97%) from Sigma Aldrich (Germany). The Folin-Ciocalteu phenol reagent (≥98%) from Sangon Biotech (China). Analytical grades were included for all other reagents.
Gentamicin injections of 80 mg were supplied by Ninhua Group Co, LTD. Branch (China), and Cromatest Liner Chemicals, S.L.U. (Spain) supplied creatinine (1123010), uric acid (1161010), and urea (1158010).
Chemical analysis
A qualitative analysis method (TLC)
Preparation of the test solution
With 0.5 g of the test sample in a flask, 10 ml of 70% ethanol was added, kept at room temperature for 24 hours, filtered and the filtrate was used as the test solution.
Preparation of the standard solution
A 70% ethanol of standard substances with a 1 mg/ml concentration was used as a reference.
Identification of total phenolic compounds
TLC was carried out using a silica gel F254 (China) plate in a mixed solvent system of benzene: ethyl acetate: formic acid: acetone (5:5:1:1). The chromatogram was sprayed with 5% FeCL3 in an ethanol solution and heated at 100°C for 1-2 minutes5.
Identification of flavonoids
TLC was carried out using a silufol UV 254 (China) plate in a mixed solvent system of toluene-ethyl acetate- formic acid-methanol (6:6:1.6:0.4). The chromatogram was sprayed with 3% ALCL3 in an ethanol solution and heated at 100°C for 3 minutes5, 6.
Quantitative analysis method (UV/Vis spectrophotometer)
Sample preparation
Weigh 0.5 g of the sample and place it in a 50 ml flask with 25 ml of 70% ethanol. These were extracted in Ultrasonication at 70-80°C for 20-30 minutes. Filter the extract into a 25 ml volumetric flask and dilute up to the nominated volume with 70% ethanol (Solution A).
Determination of total phenolic compounds content
Transfer 0.5 ml of Solution A to a 25 ml volumetric flask, add 10 ml of distilled water and 1 ml of Folin-Ciocalteu’s color reagent (diluted 1:10 in water) and dilute up to the nominated volume with 10.75% Na2CO3. Then, keep it at room temperature for 30 minutes and measure the absorbance of the solution at a wavelength of 760 nm with a UV spectrophotometer (UV-M 51, China). The Gallic Acid Equivalent (GAE) was used to represent the content of polyphenolic compounds in the traditional ARUR-10 prescription5,7,8,9.
Determination of total flavonoid content
The study involved 3 ml Solution A into a 25 ml volumetric flask, adding 1 ml 5% NaNO2, 1 ml 10% AL(NO)3 and 10 ml 4% NaOH solutions to diluting with water and measuring at a wavelength of 500 nm absorbance using a UV spectrophotometer. Rutin equivalent (RE) was used to express the total flavonoid content of the Arur-10 prescription5,8,9,10.
Antioxidant activity determination method (DPPH, in vitro)
The sample extracts in methanol were prepared at five distinct concentrations (12.5-200 μg/ml). The first was a measure of 1.5 ml of the extract of the sample added 1.5 ml of 3×10-4 M DPPH (2,2-diphenyl-1-picrylhydrazyl) and shaken (Sample). The second concentration contained 1.5 ml extract of the sample added 1.5 ml of methanol and mixed well (Blank). The third was a measure 1.5 ml of DPPH solution added to 1.5 ml of methanol and mix well (Control). The sample, blank and control solutions were each kept at room temperature in a dark area for 30 minutes. The light absorbance value was measured at 517 nm by a UV-spectrophotometer and covered into the percentage antioxidant activity using the following formula.
AA%= 100- [(Absorbance Sample – Absorbance Blank) × 100 / Absorbance Control];
The value of the IC50 of the sample shows its capacity as an antioxidant11.
Effects of the ARUR-10 traditional prescription nephritis in rats
Ethics statement
The ethical review committee of Mongolia’s Ministry of Health accepted the experimental protocol (Protocol #23/033).
Diuretic activity
The ARUR-10 prescriptions diuretic effect was determined using the Kau ST method (1984) 12, 13. In the experiment, twenty-four Wistar rats were selected and divided into four groups, n=6. 1. Control – given 5 ml/kg of water; 2. Hydrochlorothiazide – 10 mg/kg; 3. ARUR-10 – 120 mg/kg; 4. ARUR-10 – 240 mg/kg. Fast the animals for 18 hours before the experiment. The rats were separated once at a time and placed in a dedicated metabolic cage. The experimental animals were given 14 ml/kg of 0.9% NaCl solution as a fluid load. A total of 4 hours of urine will be collected and compared at intervals of 1 and 1 hour.
Nephroprotective activity
In the experiment, twenty Wistar rats weighing 240-290 grams were taken and divided equally into four groups each (n=5): healthy, control and experimental (high, medium dose). The healthy group was given normal saline (0.1 ml i.p 0.9% NaCl) and was oral for nine days. In the control group, gentamicin (80 mg/kg/day i.p.) for nine days induced a pathological model of toxic nephritis. In the experimental group, gentamicin (80 mg/kg/day i.p.) was to produce a pathological model of toxic nephritis and 120 mg/kg, 240 mg/kg of ARUR-10 traditional prescription extract was given for nine days. At the end of the study, the animal’s body weight was measured and blood serum analysis and histopathological analysis of solid organs were performed (Negatron’s method 1983)14.
Methodology for blood serum analysis
Inject ketamine hydrochloride 90 mg/kg into the animal’s abdomen, put it to sleep, puncture the heart and take blood in a tube with heparin. The collected blood is centrifuged at a speed of 3000 revolutions/min for 10 minutes plasma is separated with a semi-automatic biochemical analyzer (DR-7000D) to determine parameters such as creatinine, urea and uric acid in the blood serum.
Histopathological analysis
A kidney toxic nephritis pathological model was created. Kidney tissue microstructure analysis was carried out according to the method commonly used for animal tissue and organ samples, fixed in a 10% solution of buffered formalin for 24 hours, washed with running water, passed through ethanol, xylene and poured into paraffin. Sections were cut at 2-5 µm thickness using a (Yamato Kohki Industrial Co., Ltd) sled microtome, stained with hematoxylin-eosin, micro sections were prepared and microscopic examination was performed. Also, samples of the kidney tissue of animals will be analyzed for histopathology and the groups will be compared15.
Results of Chemical study
Qualitative analysis results
Biologically active substances were identified in the ARUR-10 traditional prescription (Table 1).
Table 1: Identification of bio-active compounds in the Traditional prescription.
|
№ |
The mobile phase of TLC |
Detection reagent and condition |
Visible color |
Diffusion coefficient of TLC (Rf value) |
|
1 |
C6H6:C4H8O2:HCOOH: CH3COCH3 (5:5:1:1) |
2% FeCl3 Heating (100-1050C) Natural light |
Deep dark blue |
0.51- Gallic acid 0.63- Tannic acid 0.48- Chebulic acid |
|
2 |
C6H5CH3:C4H8O2:HCOOH: CH₃OH (6:6:1.6:0.4) |
3% AlCI3 UV365 light |
Yellow Yellow fluorescence Yellow-green Yellow-green Orange |
0.35- Rutin 0.58 – Quercetin 0.67- Кaempferol 0.8- Apigenin 0.7- Luteolin |
Quantitative analysis results
The Folin-Ciocalteu’s reagent and aluminum chloride methods were used to establish the polyphenolic compound and total flavonoid content of the ARUR-10 traditional prescription. The content of polyphenol compounds and total flavonoids was calculated by comparing gallic acid and rutin references. Calibration curves for standard substances are shown in Graphs 1 and 2.
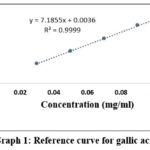 |
Graph 1: Reference curve for gallic acid |
Graph 1 shows the results of the standard gallic acid curve calibration performed using a solution containing 0.03-0.1 mg/ml concentration.
The slope of the reference curve of gallic acid was 7.1855and the linear correlation coefficient was r2 = 0.9999. The formula for the linear equation is Y= 7.1855*X + 0.0036 (r2 = 0.9999), which represents a linear relationship. The relative standard deviation (RSD) of 5 experiments is 0.21%, so not more than 2% and satisfies the criteria.
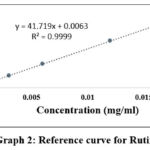 |
Graph 2: Reference curve for Rutin. |
As shown in Graph 2, the slope of the standard curve of rutin with a concentration of 0.0015-0.018 mg/ml is 41.719and the linear correlation coefficient is r2 = 0.9999. The relative standard deviation (RSD) of 5 experiments of rutin is 0.28%, so not more than 2% and satisfies the criteria. So, the calibration graphs of gallic acid and rutin showed a strong positive linear correlation (r2), close to +1. The linear equation formula of gallic acid and rutin was used to calculate the content of the polyphenolic compound and total flavonoid of traditional prescription. The ARUR-10 traditional prescription’s ethanol extract included 12.9% ± 0.361 polyphenolic compounds and 0.33% ± 0.015 total flavonoids.
Antioxidant activity determined the result.
The antioxidant activity of the ARUR-10 traditional prescription was determined by the DPPH method. The results were appreciated using free radical scavenging activity (%) and IC50. As a result of the study, the ARUR-10 traditional prescription has 68.47% and 83.29% free radical scavenging activity at concentrations of 100 μg/ml and 200 μg/ml. Compared to rutin (61.37-98.75%), it shows similar results and has antioxidant activity. The results are summarized in Table 2.
Table 2: The results of antioxidant activity of the ARUR-10 traditional prescription.
|
Name of samples |
Concentration, µg/ml
|
Free radical scavenging activity, % |
IC50, µg/ml
|
|
ARUR-10 traditional prescription |
200 |
83.29 |
92.4±2.08 |
|
100 |
68.47 |
||
|
50 |
32.52 |
||
|
25 |
24.97 |
||
|
12.5 |
12.69 |
||
|
Rutin |
200 |
98.75 |
78.86±1.48 |
|
100 |
61.37 |
||
|
50 |
39.08 |
||
|
25 |
27.31 |
||
|
12.5 |
20.65 |
*IC50 values are represented as mean ±SD (n=3).
In terms of IC50, the concentration required to reduce free radicals by 50% was 78.86±1.48 μg/ml for rutin, while it was 92.4±2.08μg/ml for Arur-10 traditional prescription.
Results of Pharmacological study
The result of the acute toxicity of the ARUR-10 prescription is LD50= 6.2 (7.1 – 9.4) g/kg and the acute toxicity is moderate. The pharmacological active dose is 124 (62-248) mg/kg.
Diuretic activity
Table 3: Effect of ARUR-10 prescription urine volume observed for 4 hours
|
Treatment |
Dose (mg/kg) |
Urine volume (ml/100gm/hr) |
Total |
|||
|
1 hr |
2 hr |
3 hr |
4 hr |
|||
|
Distilled water |
10 |
1.9* |
1.1 |
0.7 |
0.2 |
0.97±0.71 |
|
Hydrochlorothiazide |
10 |
1.7 |
3.2* |
0.6 |
0.6 |
1.52±1.23 |
|
ARUR-10 (120mg/kg) |
120 |
0.6 |
0.6 |
0.5 |
0.1 |
0.45±0.23 |
|
ARUR-10 (240mg/kg) |
240 |
0.1 |
2.8* |
2.1 |
1.5 |
1.62±1.14 |
*p<0.05 HCTZ vs distilled water, vs ARUR-10 240 mg/kg
Hydrochlorothiazide selected in the positive control group is a moderately diuretic that inhibits renal tubular epithelial function and reduces sodium reabsorption. The process of reabsorption keeps the balance of salt and water constant.
Table 1 shows that hydrochlorothiazide, which has a diuretic effect, doubled the level of urine output compared to the distilled water group. It is effect on urine output was much higher in the second hour. The ARUR-10 prescription 240 mg/kg dose increased urine output in the 2nd, 3rd and 4th hours with statistically significant differences compared to the distilled water group. Results show that ARUR-10 has a diuretic effect when used in high doses.
Result of nephroprotective activity
The results of the toxic nephrosis were gentamicin induced in Wistar rats administered with an extract of ARUR-10 prescription in doses of 120 mg/kg and 240 mg/kg. The creatinine level of animals in the control group of the gentamicin-induced nephritis model increased by 1.7 times compared to the healthy group and the creatinine level of the animals treated with the experimental ARUR-10 120 mg/kg dose decreased by 59.6% compared to the control group (p>0.005) (Graph 3).
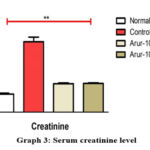 |
Graph 3: Serum creatinine level |
According to the results, the amount of urea in the blood of animals in the control group was increased by 2.8 times compared to the healthy group, while the amount of urea in the group treated with ARUR-10 prescription 240 mg/kg decreased by 67.9% compared to the control group that created a pathological model (p>0.05) (Graph 4).
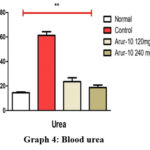 |
Graph 4: Blood urea |
The control group’s uric acid level was 1.8 times higher than the normal control group. The group treated with ARUR-10 120 mg/kg decreased uric acid by 16% compared to the positive control group, and the group treated with ARUR-10 240 mg/kg decreased it by 14.9% (Graph 5).
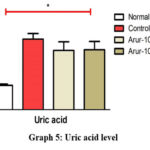 |
Graph 5: Uric acid level |
Histopathological studies
In the gentamicin model group, renal vascular endothelial cells were hyperproliferated. dystrophy, necrosis of the follicles, walls and cells, as well as an accumulation of cell decomposition products in their internal cavities, were observed. In the experimental group where ARUR-10 was an animal, a gentamicin-induced nephritis model was created in Wistar rats. The preparation effects were inflammatory cells infiltrating the kidney tissue’s tubules and vessels. According to the results, of the microstructure analysis of the kidney tissue, there was very little necrosis and no necrosis in the kidney tissue of all experimental groups only inflammatory cell infiltration occurred, while the control group rats showed necrosis.
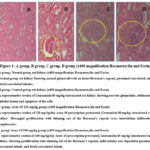 |
Figure 1: A group, B group, C group, D group (x400 magnification Haematoxylin and Eosin) |
Table 4: Histopathological studies nephroprotective activity
|
Histopathological features |
Normal group |
Control group |
Arur-10 120 mg/kg |
Arur-10 240 mg/kg |
|
Necrotic glomerular |
– |
** |
– |
– |
|
Obliterated tubular lumen |
– |
** |
– |
– |
|
Apoptosis of the cells |
– |
** |
– |
– |
|
Inflammatory cells |
– |
** |
* |
* |
(-) none, (+) mild, (++) moderate
Discussion
Mongolia’s flora contains approximately 2,860 plant species. Of these, 860 species are considered medicinal plants. From this group, 400 medicinal plants are further codified in their use in Mongolian Traditional Medicine. Mongolian scientists and researchers have conducted both basic and advanced phytochemical studies on these plants. In Mongolian Traditional Medicine’s source books, the traditional ARUR-10 prescription has been used for treating kidney disease and improving physical strength.
While Chemical and pharmacological studies of the constituent ingredients of ARUR-10 have been thoroughly studied, the formula itself has not been studied exclusively. Therefore, we are conducting initial research on the chemical pharmacology of this traditional prescription. Our research revealed the presence of gallic acid, chebulin acid, and tannic acid as a biologically active substance in the traditional formula of “ARUR-10”. Also, we have determined quercetin, rutin, kaempferol, luteolin and apigenin of the flavonoid aglycones. It is directly related to the medicinal plants in the composition of traditional prescription. Researchers found that the medicinal raw materials T.chebula., C.gladita, C.tinctorus L., A. kravanh., L.Lacca, G.decumbens L., J.sabina L., P.rotundifolia L., R.cordifolia L.and T.xanthipes contain gallic, ellagic, tannic, chebulic acids, mannitol, ascorbic acid, arbutin, anthraquinone, coumarins, flavonoids and essential oils1,4, 16, 17. For example, plants rich in phenolic compounds include T.chebula- where the gallic acid and chebulic acid found in this plant is determined by researchers to be 40-50%18, 19. Various studies conducted by researchers on T.chebula have confirmed its antioxidant, anti-diabetic, hepatoprotective, neuroprotective, cytotoxic, anti-inflammatory, anti-viral, anti-HSV-2, anti-bacterial and anti-fungal properties20,21, 22, 23, 24.
Pharmacological studies have shown that C. tinctorius has anti-inflammatory, cardioprotective, antitumor, anti-osteoporosis, lung and hepatoprotective biological activities. The phytochemistry of this plant has some bioactive substances such as flavonoids, phenylethanoid glycosides, coumarins and polysaccharides25.
In Mongolian Traditional Medicine, Juniper is widely used in inflammatory diseases such as kidney and bladder inflammation, joints rheumatism and anti-inflammatory, blood sugar lowering, oxidation inhibition, fungi and bacteria killing, memory enhancement and some anti-cancer properties have been identified17, 26. Serbian scientists Lesjak and Beara found that flavonoids rutin and quercetin have antioxidant and anti-inflammatory activity 27. Plants of the Juniper species have antioxidant and antidiabetic activity28. In addition, researchers have determined that J.sabina, which grows in Mongolia, contains mainly polyphenols, quercetin, apigenin29, coumarin and essential oils30. Phenolic compounds, flavonoid and coumarin from J.sabina, grown in China, depending on geographical location -compounds such as rhamnoside have been isolated and found to treat rheumatoid arthritis31.
The ten raw materials in ARUR-10 are rich in biologically active compounds and provide versatile medicinal services.
According to the results of our research, the content of polyphenolic compounds in the traditional prescription is 12.9%±0.361 (GAE) and the total flavonoid is 0.33%±0.015 (RE) respectively, which is directly related to the raw materials in the composition.
Natural compounds are more biologically and pharmacologically active than synthetic compounds. Researchers have isolated more than 8000 phenolic compounds and more than 6000 flavonoids from plants and determined their molecular structures. In pharmaceutical analysis, if the total phenolic compounds contained in the raw material are more than 4%, it is considered rich in phenolic compounds32. These compounds have antioxidant properties. Antioxidants fight free radicals (DPPH) through oxidation. DPPH is a stable, deep purple crystalline powder in a solid state. A deep-purple color change to pale yellow or colorless (neutralization) indicates that the DPPH free radical has scavenged 33.
According to our research, the methanolic extract of ARUR-10 at a concentration of 100 μg/ml inhibits free radicals by more than 50% or 68.47% ± 0.64.
Researchers consider when the concentration of 50% inhibition of free radicals is less than 200 μg/ml, it is antioxidant-active 34. As a result of our research, it was concluded that the concentration of 50% inhibition of free radicals (IC50) in the traditional prescription is less than 200 μg/ml (92.4±2.08 μg/ml), which has an antioxidant effect. The IC 50 values for rutin were 78.86±1.48 μg/ml, respectively. During the experiment, the color of the sample extract changed from brownish purple to pale yellow, similar to rutin. Phenolic compounds in the extract act as electron donors and catalyze the conversion of hydrogen peroxide to water.
Phenolic compounds form highly active biopolymers in the body and can complex compounds with single electrons when they connect with free radicals, explaining their antioxidant activity. Phenol and its traditional flavonoids show more antioxidant properties than vitamins and protect human health from cancer, diabetes, hyperglycemia, hypercholesterolemia, arteriosclerosis, stomach and liver inflammation, Parkinson’s, Alzheimer’s diseases and age 35, 36. Therefore, scientists have found that phenolic compounds slow the antioxidant effects on proteins, DNA and lipids by generating stable radicals37.
The antioxidant activity of the methanolic extract of ARUR-10 is related to the phenolic components. In other words, many plants rich in phenolic compounds and flavonoid compounds are good sources of natural antioxidants. We conclude that the ARUR-10 under study is antioxidant-active and supports the body’s prevention and treatment of disease and evidence-based traditional knowledge.
The result of phytochemistry revealed that the traditional prescription includes a high concentration of gallic acid, rutin and quercetin the effect of these substances on renal illness was investigated, yielding definite results. Gallic acid is a phytochemical belonging to the class of phenolic acid and has several pharmacological properties. Gallic acid has been shown to reduce markers of kidney function, oxidative stress, inflammation, apoptosis and electrolyte imbalance. Also, according to Hossam Ebaid et al.’s study, gallic acid reduces thioacetamide (TAA) induced liver and kidney toxicity, suppresses oxidative stress and inflammatory markers and reduces liver fibrosis or liver and kidney histological abnormalities38.
We chose a gentamicin-containing model for pharmacological studies. Gentamicin is a common antibiotic used to treat gram-negative bacterial infections and can induce severe hepatotoxicity and nephrotoxicity. When gentamicin is used in large doses, inhibition of vasodilator metabolism reduces blood supply, causes inflammation and edema and increases tissue peroxidation processes, resulting in free radicals (O2, OH, H2O2), also, by activating the synthesis of intracellular molecule-1, leukocyte molecule and monocyte chemotaxis protein-1, monocytes and macrophages migrate to the injured area and cause nephritis. Renal toxicity has been demonstrated in 25% of patients treated with gentamicin, of which 10-20% develop renal failure. Gentamicin induces acute kidney injury, tubular obstruction, decreased filtration rate and necrosis in renal tubules, vasculature and kidney39, 40.
We experimented by inducing a nephritis model with gentamicin and comparing ARUR-10 at doses of 120 mg/kg and 240 mg/kg with the control group results by laboratory and histopathological analysis. According to the experiment’s findings, creatinine and urea levels in the control group that created pathological models increased compared to the healthy group, when creatinine and urea levels in the Arur-10 traditional prescription group animals decreased statistically significantly compared to the control group. In rat kidney tissues, vascular stromal cells were hyperproliferated and queened. It was observed that there was degeneration and necrosis in the wall cells of the follicles and the accumulation of cell decomposition products in its inner cavity.
In the kidney tissue of rats in the experimental groups, there was very little necrosis, no necrotic changes and only inflammatory cell infiltration Kannappan N., Madhukar A. (2010)14 used experimental methods to investigate the effects of herbal preparations in a nephritis model. The results of the traditional formulation at a dose of 200 mg/kg reduced blood creatinine and urea levels in animals, similar to other researchers’ studies. Based on the results of chemical pharmacology research conducted on this topic, ARUR-10 has a kidney-protecting effect. It is associated with biologically active substances (gallic acid and quercetin) in the prescription because researchers found that polyphenolic compounds inhibited inflammatory macrophage cells generated during kidney injury41.
Treatment of nephritis with traditional medicine prevents the development of fibrosis. In renal fibrosis research, Chinese scientists have confirmed that many natural medicinal products can alleviate the process of renal fibrosis, protect kidney structure, and improve kidney function by regulating various cytokines. As well as the use of quercetin in renal obstruction inhibits tubulointerstitial damage and reduces the synthesis and release of inflammatory factors. Another study found that quercetin reduced structural damage to the kidneys, improved kidney function, and reduced oxidative stress42.
Also, at a dose of 240 mg/kg, ARUR-10 had a strong diuretic effect, which indicates that it is related to polyphenols and flavonoids, especially quercetin. Therefore, the traditional ARUR-10 prescription we are researching lowers renal inflammation, consistent with previous research results.
ARUR-10 has moderate toxicity, and no signs of chronic toxicity have been detected. Urine excretion was 1.52 ± 1.23 times higher than in the distilled water group (0.97 ± 0.71). Arur-10 at doses of 240 mg/kg (1.62±1.14) had a similar effectiveness to Hydrochlorothiazide (1.52±1.23). Hydrochlorothiazide is a standard drug with a diuretic effect.
Conclusion
In the modern era of medical research, methods, substances, and formulas used as traditional medicines have grown into a worldwide research trend. There is still very little chemical and pharmacological research on ARUR-10 traditional prescription. This study concludes that the traditional prescription called ‘ARUR-10’ contains polyphenolic compounds and flavonoids and has antioxidant properties. These biologically active compounds are significant for explaining the therapeutic mechanisms and medicinal effects and resolving theoretical conclusions in Traditional Mongolian Medicine.
Therefore, pharmacological studies have shown that ARUR-10, a traditional drug (240 mg/kg), had a significant diuretic effect.
Also, the ARUR-10 has a protective effect on the kidney by reducing blood creatinine, uric acid, and urea in a model of gentamicin-induced nephritis and maintaining and restoring renal tubular structure. It is directly related to the biologically active substances (gallic acid and quercetin) contained in the ARUR-10 traditional drug. Therefore, the evidence-based lore knowledge of the ARUR-10 recipe used in the treatment of kidney disease is certifiable by modern science.
We will isolate and identify many more chemical compounds in this traditional prescription and conduct further in-depth investigations of the chemical and pharmacological research.
Acknowledgment
The authors would like to thank the staff of the Mongolian Ministry of Education and Science, Science and Technology Foundation, and the Research Center of the Institute of Traditional Medicine Technology for their help during this research.
Conflict of Interest
The authors have declared no conflicts of interest.
Funding Source
The study was funded by the Ministry of Education and Science, the Science and Technology Foundation, and the Institute of Traditional Medicine Technology of Mongolia. Grant number: 2022/137.
References
- Zijun Zhou., Yanheng Qiao., Yanru Zhao. Natural products: potential drugs for the treatment of renal fibrosis. Chin Med. 2022; 17;17(1):98.
CrossRef - Ministry of Health of Mongolia. Health indicators of Mongolia. Ulaanbaatar: 2022. p. 132-134.
- Oldokh S., Tserentsoo B., Batkhuyag P. Traditional Mongolian Medicine. Ulaanbaatar: Blue Mongolian Press; 2013. p.181-189.
- Dagvatseren B., Khishigjargal L., Narantsetseg D. Reference Book of Traditional Medicines and Herbal Materials. Ulaanbaatar: Jicom Press; 2014. p.98-98.
- Institute of Traditional Medicine and Technology of Mongolia. Pharmacopoeia of Raw Materials and Drugs Used in Traditional Mongolian Medicine. Ulaanbaatar: Jicom press; 2017.
- Hildebert W., Sabine B. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Second edition. Springer-Verlag. Berlin: 1996. p.196-197.
- Wei Zhao., Yueping Ma., Zhong Yuan. Optimization of extraction method for the polyphenols from twigs of Cinnamomum cassia. Asian Journal of Traditional Medicines. 2012;7(6): 256-258.
- Preeti Tiwari. Phenolic and Flavonoids and Antioxidant Potential of Balarishta Prepared by Traditional and Modern Methods. Asian J. Pharm.Ana. 2014; 4(1): 05-10.
- Nyamdemberel Ts., Chimedragchaa Ch. Results of quantitative and qualitative analyses of traditional prescription “Lonlunsemberu-13”. Proceedings of the Mongolian Academy of Sciences. 2020; 60(234):31-38.
CrossRef - Li G., YU S., Zhou Y-H., Chen Q-F. Spectrophotometric determination of flavonoid content in leaves of Fagopyrum cymosum complex. Asian Journal of Chemistry. 2013; 25(13):7575.
CrossRef - Altanzul J., Dumaa M., Chunsriimyatav G. Investigation of Chemical Composition and Free Radical Scavenging Activity of Lagochilus Ilicifolius Bge. Mongolian Journal of Chemistry. 2010;11(37):61-63.
- Kau ST., Keddi JR.andrews D. A method for screening diuretic agents in the rats. J. Pharmacol Meth. 1984; 11, 67, 15.
CrossRef - Abdalaa S., Martin-Herreraa D., Benjumeaa D., Gutierrez-Luisb J. Diuretic activity of some Withania aristata Ait. Fractions. J. Ethnopharmacol. 2008; 117, 496.
CrossRef - Kannappan N., Madhukar A., Mariymmal P. Evaluation of the nephroprotective activity of orthosiphon stamineus benth extract using a rat model. International Journal of PharmTech Research. 2010; 2(1): 209-215.
- MNS 6961:2021. Мал эмнэлэг. Мал, амьтны эрхтнээс эд судлалын шинжилгээ хийх арга. MASM TC. 2021; 17. 11.220.
- World Health Organization Regional Office for Western Pacific. Medicinal Plants in Mongolia. Ulaanbaatar: 2013. p. 67, 95,160.
- Ligaa U., Davaasuren B., Ninjil N. Medicinal Plants of Mongolia Used in Western and Eastern Medicine. Ulaanbaatar: Admon press; 2005. p. 65, 163, 377.
- Anil D. Mahajan., Nandini R. Pai. Development and Validation of HPLC Method for Quantification of Phytoconstituents in Haritaki Churna. International Journal of ChemTech Research. 2011; 3(1): 329-336.
- Mahdi Vaziriana., Mahnaz Khanavi., Yaghoub Amanzadeha. Quantification of gallic acid in fruits of three medicinal plants. Iranian Journal of Pharmaceutical Research. 2011; 10 (2): 233-236.
- Manisha Nigam., Abhay P Mishra., Anjana Adhikari-Devkota. Fruits of Terminalia chebula Retz.: A review of traditional uses, bioactive chemical constituents and pharmacological activities. Phytother Res. 2020; 34(10): 2518-2533.
CrossRef - Ravi Shankara Birur Eshwarappa., Y L Ramachandra, Sundara Rajan Subaramaihha. Antioxidant activities of leaf galls extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae).Acta Sci Pol Technol Aliment. 2015; 14(2): 97-105.
CrossRef - Ajay Kesharwani, Suja Kizhiyedath Polachira, Reshmi Nair. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement Altern Med. 2017; 17(1): 110.
CrossRef - Katarzyna Szewczyk., Anna Bogucka-Kocka., Natalia Vorobets. Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity. Molecules. 2020; 25(7): 1749.
CrossRef - Darzuli, Natalia1., Budniak, Liliia1., Slobodianiuk, Liudmyla. Investigation of the antibacterial and antifungal activity of the Pyrola rotundifolia L. Leaves dry extract. Pharmacology Online. 2021; 1: 395-403.
- Yanling Liu., Mengna Wang., Yangang Cao. Chemical Constituents from the Flowers of Carthamus tinctorius L. and Their Lung Protective Activity. Molecules. 2022; 27(11): 3573.
CrossRef - Javzmaa N., Atantsetseg Sh. Chemical composition of essential oil of Juniperus sibirica L. and its antimicrobial and growth-inhibitory activity against breast cancer (MCF 7) cells. Mongolian Journal of Chemistry. 2019; 7: 41-48
- Marija M. Lesjak., Ivana N. Beara., Dejan Z. Orčić. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti-inflammatory agents, Food Chemistry. 2011;124 (3): 850–856.
CrossRef - Nilüfer Orhan., Didem Deliorman Orhan., Alper Gökbulut. Comparative Analysis of Chemical Profile, Antioxidant, In-vitro and In-vivo Antidiabetic Activities of Juniperus foetidissima Willd and Juniperus sabina L. Iran J Pharm Res. 2017; 16(l): 64–74.
CrossRef - Yumchinsuren B., Nyamdemberel Ts. Some phytochemistry research results of species of Juniper native (Juniperus sabina L.) in Mongolia. Российская наука в современном мире. Сборник статей L IV международной научно-практической конференции. 2023; 30-31.
- Shatar S., Chimedragchaa Ch., Altantsetseh Sh. Chemical composition of essential oils of aromatic plants of ancient Mongolian traditional medicine. Ulaanbaatar: 2017. p. 150.
- Jun Zhao. Study on chemical constituents from Juniperus sabina L. Journal of Chinese Pharmaceutical Sciences. 2008; 43(19):1461-1463.
- Maisuthisakul P., Suttajit M., Pongsawatmanit R. Assessment of phenolic content and free radical scavenging capacity of some Thai indigenous plants. Food Chemistry. 2007; 100:1409-1418.
CrossRef - Baliyan S., Mukherjee R., Priyadarshini A. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. 2022; 27(4): 1326.
CrossRef - Selenge E., Odontuya G., Batkhuu J. Antioxidant activity of some medicinal plants from Mongolia. Proceedings of the Mongolian Academy of Sciences. 2010; 50 (195): 48-56.
- Galati EM, Tripodo MM, Trovato A, Miceli N, Monforte MT. Biological effect of Opuntia ficus indica (L.) Mill. (Cactaceae) wastematter. Note I: diuretic activity. J Ethnopharmacol. 2002; 79: 17-21.
CrossRef - Hatano et al., Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chem. Pharm. Bull. 1980; 37: 2016-21.
CrossRef - Shahidi F., Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr: 1992; 32: 67-103.
CrossRef - Hossam Ebaid., Samir A.E. Bashandy. Protective effect of gallic acid against thioacetamide-induced metabolic dysfunction of lipids in hepatic and renal toxicity. Journal of King Saud University – Science. 2023; 35(3): 3-11.
CrossRef - Maged Abdel Kader., Mubarak T Alanazi., Abdulaziz S., Bin Saeedan. Hepatoprotective and nephroprotective activities of Juniperus sabina L. aerial parts. Journal of Pharmacy & Pharmacognosy Research. 2016; 5(1): 29-39.
CrossRef - Mohana Lagshmi. S, Usha Kiran Reddy .T. A review on medicinal plants for nephroprotective activity. Academic science Asian Journal of pharmaceutical and clinical research. 2012; 5(4): 8-14.
- Hong Lu., Lianfeng Wu., Leping Liu. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization.PubMed. 2018; 154: 203-212.
CrossRef - Ding, T., Wang, S., Zhang, X. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018; 41, 45–53.
CrossRef







