Gaurav Sharma and Kiranjeet Kaur*
Chitkara School of Health Sciences, Chitkara University, Punjab, India.
Corresponding Author E-mail: kiranjeet.kaur@chitkara.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2895
Abstract
Hospital-acquired infections (HAIs) are one of the most tangled difficulties in advanced clinical practices. These infections lead to financial implications and have a significant impact on morbidity and mortality. It is very difficult to eradicate the HAIs however both disinfection and sterilization account for the best measure to control HAIs. Of all the disinfectants in use, one of the chemical disinfectants which seem to be user-friendly, non-corrosive, and used extensively are Quaternary ammonium compounds (QACs) based disinfectants. To improve the efficacy of these disinfectants and tackle the challenge of antimicrobial resistance (concerning QACs), from time to time newer QACs disinfectants were introduced which are termed as first, second, third, fourth, and fifth-generation QACs disinfectants. Manufacturers of these newer generations QACs disinfectants claim these compounds as high-level, broad-spectrum disinfectants while leading healthcare agencies like the Centre for Disease Control & Prevention (CDC) mention QACs as mild disinfectants and not sporicidal. Sadly, the antimicrobial efficacy of QACs has been largely assessed using old methods like phenol coefficient methods & suspension methods, and not using an internationally standardized method. These loopholes raise a lot of queries about the true efficacies of the QACs and thus, increase the chances of the development of resistant HAIs. Therefore, there is an urgent need for better and standardized methods to study the efficacy of different generation QACs. The present review discusses the status of currently available methods and gaps in the literature that would be useful to highlight the potential use of QACs for infection control and prevention in better ways.
Keywords
Disinfectants; Hospital-acquired infections; Infection Control; Efficacy; QACs; Quaternary ammonium compounds;
Download this article as:| Copy the following to cite this article: Sharma G, Kaur K. Quaternary Ammonium Disinfectants: Current Practices and Future Perspective in Infection Control: Review Article. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Sharma G, Kaur K. Quaternary Ammonium Disinfectants: Current Practices and Future Perspective in Infection Control: Review Article. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4bsKp4g |
Introduction
Microbial diseases have compromised human well-being for a long time and will continue to do so, due to constant developmental changes such as mutations.1 These microbes become even more deadly when they are obtained from clinics or medical institutions. The infections caused by such microbes are termed Hospital Acquired infections (HAIs), which a patient or his associate gets during his tenure in a hospital or any other healthcare facility & that were absent at the time of admission.2,3 HAIs become fatal because of excessive invasive devices, immunosuppressive drugs, and the frequent use of inappropriate antimicrobial therapy.4 Several factors are reported to contribute to HAIs like healthcare-associated factors, patient-related factors, and environment-related factors (Figure 1.0). Patients with acute illness particularly in high-risk areas like Intensive care units, burn units, transplant operation theatres, etc. are highly prone to get these infections due to contaminated hospital environments or the fomites which come in contact with the patient directly or indirectly.4
To prevent HAIs, cleaning, disinfection, and sterilization are the most important control measures. Cleaning is a process that involves washing (soap and water) and scrubbing to remove the filth, bioburden, and soil by physically removing organic matter, although it may not always kill the germs. Disinfection on the other hand is a process of making an object microbe free except spores with the use of chemicals known as disinfectants. Sterilization destroys or eliminates all forms of microorganisms including bacterial spores. It employs both physical and chemical methods.5 The leading infection control and prevention guidelines also highlight the role of cleaning & disinfection in the control of nosocomial infections.6,7
However, research studies have reported that cleaning and disinfection are not sufficient to eliminate microbial loads.8-10 Contaminated surfaces and patients shed different microorganisms, and these organisms survive for longer duration on hospital surfaces.11,12 The concentration of these pathogens is adequate for transmission for an expanded duration. It is well-reported that environmental surfaces with microbial contamination and related factors contribute significantly to the endemic and pandemic exchange of potential microbes.13 Removal of microbial loads from the hospital environment is the major tool in controlling nosocomial infections.14 However inefficient, irrational use of disinfectants and further challenges in the measurement of efficacy standards compromise the actual efficacy in practice and contribute to the increase in the HAIs.
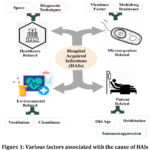 |
Figure 1: Various factors associated with the cause of HAIs |
Epidemiology of HAIs
Infections from contaminated environments have been well established. Various outbreaks have provided proof that patients get infected by the healthcare-contaminated environment and surface inside.15 As per World Health Organization (WHO) report, approximately 15% of total patients hospitalized worldwide suffer from HAIs. These infections are accountable for 4% – 56% of neonate deaths, with an incident percentage of 76% in Africa and South-East Asia.2 In the US alone, HAIs involve yearly about 2 million people and account for financial losses of approximately 4.5 billion dollars. In India and other developing nations, the issue of HAIs is significantly higher prompting well-being and money-related misfortunes.16 In 2014, CDC published a survey report on the prevalence of HAIs in the United States involving 11282 patients from 183 healthcare facilities. It was reported that approximately 4% of patients admitted to hospitals suffered from at least one type of HAIs. The major HAIs reported are Surgical site infections, Gastrointestinal infections, Pneumonia, Urinary tract infections, and primary bloodstream infections including Catheter-associated bloodstream infections with occurrence rates as 21.8%, 21.8%, 17.1%, 12.9%, and 9.9% respectively.17 In India, a research study reported 33.6% of hospital-acquired urinary tract infections in catheterized patients.18
Disinfection & QACs
Disinfection is the cycle by which an article is liberated from the pathogenic microbial entities except for spores by utilizing chemical compounds known as disinfectants. Some disinfectants, known as chemical sterilant, also destroy spores with long exposure times and are known as high-level disinfectants.6-7 Cleaning with cleaners and disinfectants plays a crucial role in the treatment of microorganisms, but the difficulties with estimating efficacy approaches have impaired the real outcomes in practice.10, 13, 19 Every disinfectant has its advantages and disadvantages and should be chosen based on its intended use or application. The normal characteristics of an ideal disinfectant incorporate i) Broad spectrum efficacy; ii) user-friendly; iii) Non-corrosive and nature friendly.7,19,20 Different chemical disinfectants are currently used for disinfection like Alcohol; Oxidizing agents; Halogens; Aldehyde; Phenolic and QACs (Table 1.0).
Table 1: List of chemical disinfectants with advantages and disadvantages
|
Chemical disinfectants groups |
Advantages /disadvantages |
|
Alcohols |
Good skin disinfectants, should not be used on surfaces as they are flammable and reacts with different surface types. Like it tends to swell or harden the rubber |
|
Phenolics |
Phenols are mild disinfectants for inanimate surfaces |
|
Halogens |
Halogens include chlorine-generating compounds and iodine-based compounds. Chlorine generating compounds are the most economical but produce health hazardous fumes. Iodine and Idophores are weak disinfectants for inanimate surfaces and are commonly used as skin disinfectants |
|
Aldehydes |
Aldehydes like formaldehyde, Glutaraldehyde, and Orthophthaldehyde (OPA) are considered to be high-level disinfectants but are toxic to users and the environment |
|
Oxidizing agents |
Hydrogen peroxide, Superoxide water, and Peracetic acid are wide spectrum and fast antimicrobial agents but are corrosive (May rust metallic material like aluminum separation and metallic instruments, etc.) |
|
Quaternary ammonium compounds (QACs) |
QACs based disinfectants are being used most in the healthcare sector, not only as good cleaners but also as good disinfectants. These disinfectants are user friendly |
Out of all these chemicals disinfectants QACs are being used extensively in the hospitals and healthcare industry due to their advantages which make them good disinfectants. The QACs are substituted ammonium compounds having a nitrogen atom in the center with a valency of five. Out of which four (R1 –R4) are the substituted radicals i.e. Heterocyclic radicals or alkyl chain and the fifth (X–) is a halide, sulfate, or similar radical (Figure 2.0).7,19,20
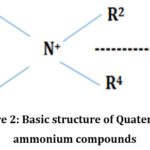 |
Figure 2: Basic structure of Quaternary ammonium compounds. |
QACs were first presented in 1916 when Walter A. Jacobs tried modifying the hexamethylenetetramine molecule synthetically with the intent to get a new molecule having bactericidal properties.21 It was demonstrated that the level of bactericidal activity was inferable from character, position, and the number of gatherings substituted in the benzene core.22 In 1935, it was reported by G Domagk that the antimicrobial properties of QACs were not limited to the Hexamethylenetetramine molecule only.23 This threshold-breaking research paved the way for the commercial application of QACs in antimicrobial use. QACs are considered broad-spectrum antimicrobials but are not sporicidal.24 These compounds are effective against gram-positive and gram-negative bacteria, fungi, and viruses.25-27 Broad-spectrum antimicrobial properties and user-friendly properties make QACs an ideal choice for disinfectants. However, there are controversies in the literature supporting the claim that QACs can be used as sporicidal agents.28 The use of QACs is not limited to disinfection but is also being used in paints, cosmetics, agriculture, and other household products. For the extensive useful properties, the QACs use has increased over the decades. The total production of QACs in the US in 1945 was about 3 million pounds, which increased to 7787 million pounds in 1993 29.
Development of new-generation QACs
The introduction and commercial application of the QACs led to the large-scale use of these disinfectants in various fields. It was found that the efficacy of the QACs was better than the other disinfectants available at that time. In an efficacy study conducted on higher-order alkyl dimethyl benzyl ammonium chloride against S. aureus and E. coli, it was reported that QACs were more effective for gram positives than gram negatives and were also better in efficacy than other commercially available disinfectants.30-32 Another study reported that QACs (Cetyl Pyridium Chloride) was microbiocidal at higher dilutions under both acidic and alkaline conditions. The nontoxic behavior of the investigated QACs compound and its germicidal properties at various conditions made QACs exceptionally useful & versatile disinfectants.33
Resistance towards QACs
Exceptionally broad-spectrum antimicrobial properties and nontoxic behavior of QACs resulted in wide-scale heavy use in the healthcare sector as disinfectants. However, the overuse of QACs led to the emergence of anti-microbial resistance towards QACs. A study in 1952 by CE Chaplin demonstrated resistance towards QACs. It was concluded that the acquired resistance was dependent on the increased lipid contents of the cell wall of the microorganism.34 Another study reported that P. aeruginosa could not only survive but even multiply in Benzalkonium chloride solutions containing ammonium acetate buffer.35 A similar study reported that at 200 μgml−1 of benzalkonium chloride, 30% of the strains were able to grow.36 To tackle the challenge of antimicrobial resistance towards QACs, manufacturers introduced newer and higher generation QACs in the next decades.37 These newer generation QACs were termed first, second, third, fourth, fifth, and so on.
Antimicrobial activity of higher generation QACs disinfectants:
Literature suggests that newer-generation QACs were more effective than the previous ones. Several studies reported higher generation QACs to be less toxic than the previous ones, being active in hard water and showing higher efficacy. A study on two higher-level QACs disinfectants showed that both disinfectants were effective against all tested microorganisms except Bacillus subtilis and the resistant strain of P. aeruginosa.38 Another study tested the antimicrobial efficacy of disinfectants used in hospitals in India against MRSA, Enterobacter aerogenes, Klebsiella pneumoniae, Multidrug-resistant Acinetobacter baumannii, and P. aeruginosa. Results demonstrated that the use of less toxic QACs in comparison to formaldehyde with mist spray agents was a good option for making the hospital environment free from microbes. For heavy contaminations, aldehyde and new QACs formulations were found to be the best disinfectants.39 Another study on third generation QACs showed that QACs disinfectants were as effective as formaldehyde and could be considered effective disinfectants and sterilization agents.40,41 Similar studies were conducted on the 5th generation QACs disinfectants and it was found that 5th generation QACs have higher efficacy than the 3rd generation. Pirsaheb et al. reported that 4th and 5th generation QACs disinfectants had higher efficacy as compared to other chemical disinfectants.42Iñiguez-Moreno et al. (2017) studied the antimicrobial activity of disinfectants commonly used in the food industry and reported that efficacy increases with the advancement of generation i.e. 5th generation QACs had higher efficacy than 3rd generation.43
The sporicidal activity of QACs
As per the guidelines issued by CDC & National Center for disease control, India (NCDC), QACs are non-sporicidal agents. A study conducted against C. difficile showed the poor sporicidal activity of QACs, validating the point mentioned in the guidelines.44 In another similar study, Deshaies et al. (2018) tested 3rd and 4th-generation QACs disinfectants against C. difficile spores at recommended and higher concentrations. None of the disinfectants showed sporicidal activity.45
Quaternary ammonium compounds and COVID 19
Though QACs are in use for a long time, a significant rise in the consumption of QACs was seen during the COVID-19 pandemic.46 The United States Environmental Protection Agency (USEPA) also highlighted the role of QACs disinfectants for COVID-19 prevention.47,48 A study reported the virucidal efficacy of QACs against SARS-CoV-2 on treated surfaces in the year 2021. It was found that the use of QACs led to a decrease in communicability and fomites transmission of SARS-CoV-2.29 Another study by Marteinson et al. (2022) reported that out of all disinfectants used in Canada during COVID-19, 80% of the disinfectants were QACs.The common QACs were Benzalkonium chloride (32%), alkyl dimethyl ethyl benzyl ammonium chloride (17%), and didecyl dimethyl ammonium chloride (8%).49 Despite large-scale usage of QACs disinfectants few authors believe that the effectiveness of QACs against corona viruses must be evaluated using standard protocols.29
Quaternary ammonium compounds, antimicrobial efficacy methods & gaps:
Existing literature has shown that the efficacy of most of the QACs disinfectant is performed using traditional methods like the Phenol coefficient method, Suspension method, swab method, or glass slide method. In another study, QACs were found effective when tested for germicidal efficacy against M. candidus, S. aureus, B. panis vegetative spores, gram-negative E. coli & Pseudomonas aeruginosa using the glass slide method. On similar lines, when the bactericidal efficiency of QACs was evaluated using the phenol coefficient method, these compounds were found to be bactericidal.50 In a similar study using the phenol coefficient method, the bactericidal efficiency of quaternary ammonium compounds was evaluated and these compounds were found to be bactericidal.51 Sadly, the authenticity and reliability of these methods are in question due to lack of standardization of the efficacy methods.32,52-54 Studies done by different researchers have shown contra-indicatory results concerning the efficacy of QACs. Pirsaheb et al. (2016) tested the efficacy of QACs using the traditional phenol coefficient method and the swab method. It was found that QACs have phenol coefficients <1 indicating the QACs disinfectants as weak disinfectants, while when tested in actual practice in laboratory conditions using swab methods, QACs disinfectants showed the best results.42 Contrary another study by Eyo et al. (2018) demonstrated the non-effectiveness of tested QACs using the use-dilution test that was having highest phenol coefficient.55 Research studies have emphasized that selecting the right and effective product is very difficult due to the varieties of products, various claims by the manufacturers, and contraindicatory reports from literature.56 Though there is a lack of standardized methods, looking at the need of the hour, newer QACs are being synthesized. Further, besides the discrepancies in testing methods, it has been reported that manufacturers use false claims on labels to promote and enhance sales Hence, better testing standards with strict guidelines for verifying the antimicrobial spectrum of these compounds are of utmost importance.57, 58 Literature also suggests that such data needs to be verified by independent investigations. Further, there is an urgent need to establish uniformity in the methods to evaluate the effectiveness of the QACs for their proper and effective utilization. In this context, newer standardized methods were developed by standard/ harmonized organizations like the European Committee for Standardization (CEN) and the Association of official analytical collaboration (AOAC) International.59, 60
Newer standardized international methods
The antimicrobial efficacy of any disinfectant depends on different factors, which include the Initial microbial inoculum, Experimental conditions, Neutralizer, its toxicity, and the method used.61 Newer Standardized methods like EN (European norms) standards or AOAC international methods should be utilized for disinfectant efficacy as these methods are refined and harmonized.62,63 These methods include various validation steps like validation of microbial suspension, Validation of neutralizer, Validation of method, and other experimental conditions. Validation of Microbial suspension includes verification of the required number of microbes to be used in the disinfectant efficacy. Validation of the neutralizer includes the selection of the suitable neutralizer and verification of neutralizer toxicity. A good neutralizer should be non-toxic to the microbes and able to neutralize the carry-over effect of the disinfectants. Validation of method and experimental conditions involves the verification of the method and the experimental conditions in which the test is to be conducted. Efficacy results with these methods are more realistic than the traditional methods.
Conclusion
The incidence of hospital-acquired infections (HAIs) are increasing. These infections become more fatal due to the use of invasive devices, immunosuppressive drugs, and the frequent use of inappropriate antimicrobial therapy. A practical measure to lower or control the HAIs is proper cleaning and disinfection. The use of Quaternary ammonium compounds (QACs) has gained a lot of attention in this context, especially during the COVID times. However, the lack of standardized methods to evaluate the efficacies of QACs is playing a major hurdle in the appropriate use of QACs. Misuse of inefficient QACs will increase the chances of microbes adapting and developing resistance against the same. Literature reports validate the gap in efficacy testing with traditional methods and emphasize the urgent need to establish uniformity in the methods to evaluate the effectiveness of the QACs for their proper and effective utilization. In this context, the use of European (EN) standards has gained a lot of attention and is proven to be more authentic and reliable.
Acknowledgment
The authors acknowledge the Chitkara University, School of health sciences for supporting and motivating this study.
Conflict of Interest
We declare that we have no conflict of interest.
Funding Sources
We declare that there is no funding involved in this review study.
References
- Institute of Medicine (US) Committee on Emerging Microbial Threats to Health, Lederberg J, Shope RE, Oaks SC Jr., eds. Emerging Infections: Microbial Threats to Health in the United States. Washington (DC): National Academies Press (US); 1992.
- Khan HA, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pacific Journal of Tropical Biomedicine. 2017;7(5):478-482. doi:10.1016/j.apjtb.2017.01.019
CrossRef - Rutala WA, Weber DJ. Disinfection and Sterilization in Health Care Facilities: What Clinicians Need to Know. Clinical Infectious Diseases. 2004;39(5):702-709. doi:10.1086/423182
CrossRef - Al-Tawfiq JA, Tambyah PA. Healthcare Associated Infections (HAI) Perspectives. Journal of Infection and Public Health. 2014;7(4):339-344. doi:10.1016/j.jiph.2014.04.003
CrossRef - Carling PC, Von Beheren S, Kim P, Woods C. Intensive care unit environmental cleaning: an evaluation in sixteen hospitals using a novel assessment tool. Journal of Hospital Infection. 2008;68(1):39-44. doi:10.1016/j.jhin.2007.09.015
CrossRef - Ministry of Health and Family Welfare, Government of India.National Guidelines for Infection Prevention and Control in Healthcare Facilities https://www.mohfw.gov.in/pdf/ National%20Guidelines %20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
- Rutala WA, Weber DJ. Guideline for disinfection and sterilization in healthcare facilities. [Internet].2019[cited 2019 Jan 5]. Available from : https:// www .cdc.gov/infectioncontrol /pdf/guidelines/disinfection-guidelines-H.pdf
- Carling PC, Parry MF, Bruno-Murtha LA, Dick B. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission*. Critical Care Medicine. 2010;38 (4):1054-1059. doi:10.1097/ccm.0b013e3181cdf705
CrossRef - Dharan S, Mourouga P, Copin P, Bessmer G, Tschanz B, Pittet D. Routine disinfection of patients’ environmental surfaces. Myth or reality? Journal of Hospital Infection. 1999;42(2):113-117. doi:10.1053/jhin.1999.0567
CrossRef - Russotto V, Cortegiani A, Raineri SM, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. Journal of Intensive Care. 2015;3(1):54-61. doi:10.1186/s40560-015-0120-5
CrossRef - Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases. 2006;6(1). doi:10.1186/1471-2334-6-130
CrossRef - Dancer SJ. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clinical Microbiology Reviews. 2014;27(4):665-690. doi:10.1128/cmr.00020-14
CrossRef - Ayliffe GA, Collins BJ, Lowbury EJ. Cleaning and disinfection of hospital floors. BMJ. 1966;2(5511):442-445. doi:10.1136/bmj.2.5511.442
CrossRef - Weber DJ, Rutala WA. Environmental issues and nosocomial infections. Prevention and control of nosocomial infections. Baltimore: Williams and Wilkins. 1997:491-514.
- Mythri H, Kashinath K. Nosocomial infections in patients admitted in intensive care unit of a Tertiary Health Center, India. Annals of Medical and Health Sciences Research. 2014;4(5):738. doi:10.4103/2141-9248.141540
CrossRef - Magill SS, Edwards JR, Bamberg W, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. New England Journal of Medicine. 2014;370(13):1198-1208. doi:10.1056/nejmoa1306801
CrossRef - Kamat U, Fereirra A, Amonkar D, Motghare D, Kulkarni M. Epidemiology of hospital acquired urinary tract infections in a medical college hospital in Goa. Indian Journal of Urology. 2009;25(1):76. doi:10.4103/0970-1591.45542
CrossRef - Otter JA, Yezli S, French GL. The Role Played by Contaminated Surfaces in the Transmission of Nosocomial Pathogens. Infection Control & Hospital Epidemiology. 2011;32(07):687-699. doi:10.1086/660363
CrossRef - Dvorak G, Disinfection 101, Center for food security and public health, Iowa State University, Ames, IA. [Internet]. 2005 [cited 2018 Feb 8]. Available from: www.cfsph.iastate.edu
- Jeffrey DJ. Chemicals used as disinfectants: active ingredients and enhancing additives. Revue Scientifique et Technique de l’OIE. 1995;14(1):57-74. doi:10.20506/rst.14.1.828
CrossRef - Jacobs WA. The Bactericidal Properties Of The Quaternary Salts Of Hexamethylenetetramine. Journal of Experimental Medicine. 1916;23(5):563-568. doi:10.1084/jem.23.5.563
CrossRef - Jacobs WA, Heidelberger M, Amoss HL. The Bactericidal Properties Of The Quaternary Salts Of Hexamethylenetetramine. Journal of Experimental Medicine. 1916;23(5):569-576. doi:10.1084/jem.23.5.569
CrossRef - Domagk G. Eine neue Klasse von Desinfektionsmitteln. DMW – Deutsche Medizinische Wochenschrift. 1935;61(21):829-832. doi:10.1055/s-0028-1129654
CrossRef - Jiao Y, Niu LN, Ma S, Li J, Tay FR, Chen JH. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Progress in Polymer Science. 2017 Aug 1;71:53-90.
CrossRef - Haldar J, An D, Cienfuegos LÁ de, Chen J, Klibanov AM. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proceedings of the National Academy of Sciences. 2006;103(47):17667-17671. doi:10.1073/pnas.0608803103
CrossRef - Obłąk E, Piecuch A, Krasowska A, Łuczyński J. Antifungal activity of gemini quaternary ammonium salts. Microbiological Research. 2013;168(10):630-638. doi:10.1016/j.micres.2013.06.001
CrossRef - Kenawy ER, Worley SD, Broughton R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules. 2007;8(5):1359-1384. doi:10.1021/bm061150q
CrossRef - Nerandzic MM, Donskey CJ. A Quaternary Ammonium Disinfectant Containing Germinants ReducesClostridium difficileSpores on Surfaces by Inducing Susceptibility to Environmental Stressors. Open Forum Infectious Diseases. 2016;3(4):ofw196. doi:10.1093/ofid/ofw196
CrossRef - Hora PI, Pati SG, McNamara PJ, Arnold WA. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environmental Science & Technology Letters. 2020;7(9):622-631. doi:10.1021/acs.estlett.0c00437
CrossRef - Dunn CG, Meyer AE. A Mixture of High Molecular Alkyl-dimethyl-benzyl-ammonium Chlorides as an Antiseptic. Experimental Biology and Medicine. 1936;35(3):427-429. doi:10.3181/00379727-35-9005p
CrossRef - DUNN CG. Antiseptic and Germicidal properties of a mixture of high molecular Alkyl—Dimethyl—Benzyl—Ammonium Chlorides. American Journal of Epidemiology. 1937;26(1):46-53. doi:10.1093/oxfordjournals.aje.a118339
CrossRef - McCulloch EC, Hauge S, Migaki H. The Quaternary Ammonium Compounds in Sanitization. American Journal of Public Health and the Nations Health. 1948;38(4):493-503. doi:10.2105/ajph.38.4.493
CrossRef - Quisno R, Foter MJ. Cetyl Pyridinium Chloride. Journal of Bacteriology. 1946;52(1):111-117. doi:10.1128/jb.52.1.111-117.1946
CrossRef - Chaplin CE. Bacterial Resistance To Quaternary Ammonium Disinfectants. Journal of Bacteriology. 1952;63(4):453-458. doi:10.1128/jb.63.4.453-458.1952
CrossRef - Adair FW, Geftic SG, Gelzer J. Resistance of Pseudomonas to Quaternary Ammonium Compounds. I. Growth in Benzalkonium Chloride Solution. Applied Microbiology. 1969;18(3):299-302. doi:10.1128/am.18.3.299-302.1969
CrossRef - Sundheim G, Langsrud S, Heir E, Holck AL. Bacterial resistance to disinfectants containing quaternary ammonium compounds. International Biodeterioration & Biodegradation. 1998;41(3-4):235-239. doi:10.1016/s0964-8305(98)00027-4
CrossRef - Schaeufele PJ. Advances in quaternary ammonium biocides. Journal of the American Oil Chemists’ Society. 1984;61(2):387-389. doi:10.1007/bf02678799
CrossRef - Hoseini SA, Shahcheraghi F, Ghaemmaghami A. Evaluation of the clinical efficacy of quaternary ammonium components (QAC) as surface disinfectant. Journal of Dentistry of Tehran University of Medical Sciences. 2006;3(4):190-4.
- Sharma R, Singh M, Taneja N, Sharma M, Gupta PK, Rana JK. Comparative efficacy evaluation of disinfectants routinely used in hospital practice: India. Indian Journal of Critical Care Medicine. 2012;16(3):123-129. doi:10.4103/0972-5229.102067
CrossRef - Mishra P, Ramalakshmi K, Verma S, Shrivastava V. Comparative efficacy of 3rd generation quaternary ammonium compounds and formaldehyde for fumigation of operation theatres. Journal of Clinical & Experimental Research. 2013;1(3):47. doi:10.5455/jcer.201332
CrossRef - Rajkumar B, Kannan I. Evaluation of third generation quaternary ammonium compounds for the sterilisation of operation theatre. International Journal of Medical Research and Review. 2015;3(3):273-277. doi:10.17511/ijmrr.2015.i3.051
CrossRef - Pirsaheb M, Hossini H, Abiri R, Sharafi K, Poorhaghighat S. Investigating The Effect Of Free Aldehyde/Phenol Antibacterial To Control The Common Hospital-Acquired Infections Including Pseudomonas Aeruginosa, Staphylococcus Aureus, Escherichia Coli, Acinetobacter, Enterococcus. Iioab Journal. 2016 Jan 1;7:572-7.
- Iñiguez-Moreno M, Avila-Novoa MG, Iñiguez-Moreno E, Guerrero-Medina PJ, Gutiérrez-Lomelí M. Antimicrobial activity of disinfectants commonly used in the food industry in Mexico. Journal of Global Antimicrobial Resistance. 2017;10:143-147. doi:10.1016/j.jgar.2017.05.013
CrossRef - Fraise A. Currently available sporicides for use in healthcare, and their limitations. Journal of Hospital Infection. 2011;77(3):210-212. doi:10.1016/j.jhin.2010.06.029
CrossRef - Deshaies F, Ahmed D, Massicotte R, Pichette G, Belhumeur P, Mafu AA. Comparison of efficacy profiles for minimum lethal concentrations (MLCs) of some commonly used commercial hospital microbicidal detergent-disinfectant products for disinfectants and sporicidal activity. International Journal of Infection Control. 2012;8(2). doi:10.3396/ijic.v8i2.013.12
CrossRef - Vereshchagin AN, Frolov NA, Egorova KS, Seitkalieva MM, Ananikov VP. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. International Journal of Molecular Sciences. 2021;22(13):6793. doi:10.3390/ijms22136793
CrossRef - Zheng G, Filippelli GM, Salamova A. Increased Indoor Exposure to Commonly Used Disinfectants during the COVID-19 Pandemic. Environmental Science & Technology Letters. 2020;7(10):760-765. doi:10.1021/acs.estlett.0c00587
CrossRef - Sharma A, Singh N, Thomas A, Shrivastava PK, Sharma A. Quaternary Ammonium Compounds: Usage in Households during COVID-19 Pandemic, Boon, or Bane?.Asian Pac. J. Health Sci., (2022); DOI: 10.21276/apjhs.2022.9.4S.26
CrossRef - Marteinson SC, Lawrence MJ, Taranu ZE, et al. Increased use of sanitizers and disinfectants during the COVID-19 pandemic: identification of antimicrobial chemicals and considerations for aquatic environmental contamination. Environmental Reviews. Published online July 30, 2022. doi:10.1139/er-2022-0035
CrossRef - Johns CK. A Method for Assessing the Sanitizing Efficiency of Quaternary Ammonium and Hypochlorite Products. American Journal of Public Health and the Nations Health. 1947;37(10):1322-1327. doi:10.2105/ajph.37.10.1322
CrossRef - TUNCAN EU. Effect of Cold Temperature on Germicidal Efficacy of Quaternary Ammonium Compound, lodophor, and Chlorine on Listeria. Journal of Food Protection. 1993;56(12):1029-1033. doi:10.4315/0362-028x-56.12.1029
CrossRef - Tyski S, Bocian E, Laudy AE. Application of normative documents for determination of biocidal activity of disinfectants and antiseptics dedicated to the medical area: a narrative review. Journal of Hospital Infection. 2022;125:75-91. doi:10.1016/j.jhin.2022.03.016
CrossRef - Beuchat LR, Farber JM, Garrett EH, et al. Standardization of a Method To Determine the Efficacy of Sanitizers in Inactivating Human Pathogenic Microorganisms on Raw Fruits and Vegetables. Journal of Food Protection. 2001;64(7):1079-1084. doi:10.4315/0362-028x-64.7.1079
CrossRef - Van Klingeren B, Koller W, Bloomfield SF, et al. Assessment of the efficacy of disinfectants on surfaces. International Biodeterioration & Biodegradation. 1998;41(3-4):289-296. doi:10.1016/s0964-8305(98)00020-1.
CrossRef - Eyo AA, Ibeneme EO, Ogba OM, Asuquo AE. Antibacterial Efficacy of the In-Use Dilutions of Common Disinfectants against Pseudomonas aeruginosa Isolates in a Tertiary Care Hospital in Calabar, Nigeria. IOSR Journal of Pharmacy and Biological Sciences. 2018;13(3): 88-91.
- Molinari JA, Gleason MJ, Cottone JA, Barrett ED. Cleaning and disinfectant properties of dental surface disinfectants. The Journal of the American Dental Association. 1988;117(1):179-182. doi:10.14219/jada.archive.1988.0251
CrossRef - Gerba CP. Quaternary Ammonium Biocides: Efficacy in Application. Applied and Environmental Microbiology. 2015;81(2):464-469. doi:10.1128/AEM.02633-14
CrossRef - Acosta-Gío AE. Safer use of disinfectants for instrument reprocessing – a call to action. International Journal of Infection Control. 2008;4(1). doi:10.3396/ijic.v4i1.008.08
CrossRef - Tomasino SF. Development and assessment of disinfectant efficacy test methods for regulatory purposes. American Journal of Infection Control. 2013;41(5):S72-S76. doi:10.1016/j.ajic.2012.11.007
CrossRef - Gröschel DHM. Disinfectant testing in the USA. Journal of Hospital Infection. 1991;18:274-279. doi:10.1016/0195-6701(91)90033-5
CrossRef - Langsrud S, Sundheim G. Factors influencing a suspension test method for antimicrobial activity of disinfectants. Journal of Applied Microbiology. 1998;85(6):1006-1012. doi:10.1111/j.1365-2672.1998.tb05265.x
CrossRef - Reybrouck G. The testing of disinfectants. International Biodeterioration & Biodegradation. 1998;41(3-4):269-272. doi:10.1016/s0964-8305(98)00024-9
CrossRef - Reybrouck G. International standardization of disinfectant testing: is it possible? Journal of Hospital Infection. 1991;18:280-288. doi:10.1016/0195-6701(91)90034-6
CrossRef







