S M. Biradar1* , B. Kohima1
, B. Kohima1 , M S. Mulimani2*, Vishwanath Nayak1
, M S. Mulimani2*, Vishwanath Nayak1 , Vijayakumar Warad2
, Vijayakumar Warad2 , Avinash Jugati2, B S. Hunasagi 1
, Avinash Jugati2, B S. Hunasagi 1 , Indu Pathi1, Chetankumar M1
, Indu Pathi1, Chetankumar M1 , Santhosh R. Awasthi1
, Santhosh R. Awasthi1 , Basavaraj V1
, Basavaraj V1 and Jyoti Hawaldar1
and Jyoti Hawaldar1
1Department of PharmD (Doctor of Pharmacy), SSM College of Pharmacy and Research Centre, Vijaypura, Karnataka, India
2Department of General Medicine, Shri B M. Patil Medical College Hospital and Research Centre, Vijaypura Karnataka, India
Corresponding Author E-mail:smbiradar@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/2889
Abstract
Corona virus disease 2019 (Covid-19) is an acute respiratory illness caused by the Corona virus. Corona virus 2 (SARS-CoV-2) is a continuing global health crisis that has harmed the physiological and psychological health of people in over 200 countries worldwide. The current study performed a narrative review of its origin, epidemiology, transmission, clinical parameters, effects on mental health, management, vaccines and their trails, and future perspectives. The ease with which humans may transmit information to one another is unnoticeable in the early stages, making COVID-19 both terrifying and remarkable, but as time progresses, it can be managed successfully with the available therapies to some extent. Based on the condition and co-morbidities, therapeutic management is used in the pandemic situation, which includes drugs like antivirals, chloroquine and hydroxychloroquine, convalescent plasma therapy, Azithromycin, Corticosteroids, Cytokine’s, and oxygen therapy. Strategies such as self-quarantine, social isolation, and 70-day nationwide and state-by-state lockdowns in India aided in slowing the virus's spread, which may have been catastrophic otherwise. However, there are no clinically approved safer vaccines until the end of 2020. Several businesses have taken the lead in producing COVID-19 vaccines and came up with vaccines like Pfizer, Moderna from the US and UK, Covaxin, and Covishield from India, and vaccination programs began in India for targeting more than 300 million people.
Keywords
Covid-19; Social and Mental Health; Symptomatic therapy; Vaccinization
Download this article as:| Copy the following to cite this article: Biradar S. M, Kohima B, Mulimani S. M, Nayak V, Warad V, Jugati A, Hunasagi B. S, Pathi I, Chetankumar M, Awasthi S. R, Basavaraj V, Hawaldar J. Prognosis of Covid-19 on its 1st Anniversary: Global V/s Indian Scenario. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Biradar S. M, Kohima B, Mulimani S. M, Nayak V, Warad V, Jugati A, Hunasagi B. S, Pathi I, Chetankumar M, Awasthi S. R, Basavaraj V, Hawaldar J. Prognosis of Covid-19 on its 1st Anniversary: Global V/s Indian Scenario. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3yE9HOz |
Introduction
The Corona virus (Covid-19) is the greatest threat to world health now, and the greatest issue facing the cosmos is pandemic1. Corona viruses, which cause sickness in animals and humans, are members of the family Coronaviridae of the Nidovirales family. Corona viruses are named after the crown-like spikes on their outer surface. They are a form of enclosed virus with a non-segmented, single-stranded, positive-sense RNA genome. Corona virus subgroups include alpha, beta, gamma, and delta coronaviruses 2, 3. Seven coronaviruses can infect people all across the world, but the four most prevalent are 29E, NL63 (alpha coronavirus), OC43, and HKU1 (beta coronavirus).
MERS-CoV, the virus responsible for Middle East respiratory disease (MERS), and SARS-CoV, the virus responsible for severe acute respiratory disease (SARS), are rarer variants that induce more severe consequences. A severe new strain of SARS-CoV-2 began circulating in 2019, generating the disease COVID-192. Prior to the 2002 outbreak of SARS in Guangdong, China, caused by SARS-CoV, these viruses were thought to solely affect animals 3, 4.
Origin and Transmission
The first known human coronavirus outbreak, known as HCoV-229E, occurred in 1965. Two subsequent outbreaks of comparable size, known as SARS-CoV and MERS-CoV, occurred in 2003 and 2012, respectively 5. CoVs have been found to be human-susceptible viruses, with CoVs HCoV-229E and HCoV-NL63 and CoVs HCoV-HKU1 and HCoV-OC43 both causing moderate respiratory symptoms comparable to the flu. The other two known CoVs, SARS-CoV and MERS-CoV, cause serious and potentially fatal respiratory tract diseases 6. SARS-CoV-2’s genomic sequence shared 79.5% similarity with SARS-CoV while sharing 96.2% identity with a bat CoV, RaTG13. According to viral genome sequencing investigations and evolutionary studies, the bat is the virus’s natural host, and SARSCoV-2 may be transferred from bats to humans via unidentified intermediary animals, as seen in figure-13. SARS-CoV-2 may infect people by exploiting the angiotensin-converting enzyme 2 (ACE2) receptor, which is the same as SARS-CoV 7. According to WHO reports for 2020, almost 30% of the nations were unready and had no plans for the spread of COVID-19. In healthcare institutions, a complete infection prevention and control programme, as well as water, sanitation, and hygiene requirements, are only found in select countries. In the absence of a COVID-19 vaccine, preserving social and physical distance seeks to limit the spread of this infectious illness 8.
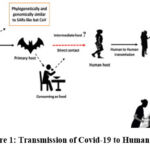 |
Figure 1: Transmission of Covid-19 to Human Host. |
Epidemiology
The first case of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, which causes coronavirus illness (COVID-19), was discovered in December 2019 in Wuhan, China, and quickly spread to many other regions. The World Health Organisation (WHO) has recorded 69,592,545 positive COVID-19 cases as of December 10th, 2020, and the death toll from COVID-19 had reached 1,581,854 globally. As of January 24, 2020, at least 830 instances had been documented in nine countries, including China, Thailand, Japan, South Korea, Singapore, Vietnam, Taiwan, Nepal, and the United States. The WHO classified COVID-19 as a public health emergency of international concern on January 30, 2020. The most impacted nations on the globe are the United States, India, Brazil, Russia, and the and the United Kingdom, and a high fatality rate is seen in countries like the United States (441K), Brazil (225K), Mexico (159K), India (154K), and the United Kingdom (106K). On January 30th, 2020, the state of Kerala reported the first SARS-CoV-2 positive case in India 9, 10. Following that, just two further instances were recorded in February. As of March 26th, the Indian Council of Medical Research and the Ministry of Family Welfare had confirmed a total of 649 cases, 42 recoveries, 1 migration, and 13 fatalities, In India, the COVID-19 infection rate is 1.7, which is far lower than in the worst-affected countries 11. The first death in India was on March 12th, 2020, in Kalaburagi, Karnataka, of a 76-year-old man with comorbidities of asthma, hypertension, diabetes, and a history of international travel. More instances occurred in March, and the number of cases has increased significantly since the second part of April 2020. According to the Ministry of Health and Family Welfare (MoHFW), as of June 9, 2020, there had been a total of 2,66,598 confirmed COVID-19 cases reported by 32 states and union territories 12. The most affected states in India are Maharashtra, Karnataka, Kerala, Andhra Pradesh, Tamil Nadu, and Delhi. High fatality rates are seen in states like Maharashtra (51k), Karnataka (12.2k), Tamil Nadu (12.3k), Delhi (10.8k), and West Bengal (10.1k). On March 22nd, 2020, the Janta Curfew was declared by the Government of India for quarantine and to prevent the spread of disease. Along with its positive effects, the government’s harsh public health policy also has negative effects on the populace, which can result in social, psychological, and economic stress, which can have long-term detrimental health effects 5.
Effect on Mental Health
Social determinants of health include race, gender, ethnicity, sexual orientation, disability status, and resources such as a job, money, food, shelter, and social supports 13. Health care workers (HCWs) have been observed to experience the psychological effects the most. Long working hours, strict rules and regulations, the constant need for attention and attentiveness, limited social contact, and completing tasks for which one may not have been prepared are all instances of extremely high work-related stressors. The COVID-19 pandemic-related emotional discomfort that healthcare workers experienced has been strongly linked to depression, stress, and anxiety 14. The pandemic of COVID-19 has had a profound psychological impact, and the social influence on the population may have an effect on both people’s health, safety, and well-being (due to insecurity, confusion, emotional isolation, and stigma) as well as communities’ (due to economic loss, closure of workplaces and educational institutions, insufficient funding for medical responses, and improper distribution of necessities) safety and well-being. These consequences can manifest as a wide range of emotional reactions (such as distress or psychiatric conditions), unhealthy behaviours (such as excessive substance abuse), and noncompliance with public health regulations (such as home confinement and vaccination) in both those who contract the disease and the general population 15,16,17. COVID-19’s mental health implications are being referred to as the “fourth wave” of the pandemic and are expected to be responsible for the greatest and most long-lasting health footprint illustrated in figure 2.
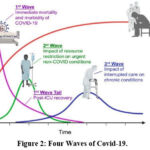 |
Figure 2: Four Waves of Covid-19. |
Risk factors effecting COVID 19
Risk factors for the Corona virus include older age (geriatric population), pre-existing cardiovascular or cerebrovascular diseases, Diabetes, Obesity, Chronic Kidney Disease (CKD), Chronic Liver Disease, Chronic Lung Illness (Chronic Obstructive Pulmonary Disease (COPD), Asthma, Interstitial Lung Disease (ILD), Idiopathic Pulmonary Fibrosis (IPF), or Bronchiectasis), Carcinoma, Dyslipidemia, Hypertension, Immune-compromised state (weakened immune system) 18,19. COVID-19 can be spread by direct contact with infected animals or through human-to-human transmission and touching contaminated surfaces, which are the main forms of transmission. Along with risk factors, some clinical findings associated with COVID-19 are shown in Table 1.
Table 1: Clinical Findings Associated with Covid-19.
|
|
Mild |
Moderate |
Severe |
|
SPO2 |
>94% in room air |
90-94% in room air |
<90% in room air |
|
RR |
<24/minute |
24-30 |
>30 |
|
|
No pneumonia |
Pneumonia + |
Pneumonia++ |
|
CT Chest Criteria |
|||
|
|
Normal or <25% |
25%-75% |
75%-100% |
|
|
Grade I |
GradeII/III |
Grade IV |
|
Laboratory Findings |
|||
|
NLR |
<3.2 |
>3.2 |
>5.5 |
|
CRP |
<40 |
40-125 |
>125 |
|
Ferritin |
<500 |
>500 |
>800 |
|
D Dimer |
<0.5 |
>0.5 |
>1.0 |
|
LDH |
<300 |
300-400 |
>400 |
|
IL6 |
<4.8 |
5-50 |
>80 |
|
LFT |
Normal |
Slight derangement |
Moderate derangement |
Treatment
COVID-19 does not have a specific antiviral therapy, and no safer vaccines were available until the end of 2020. The treatment was symptomatic, with oxygen therapy serving as the main treatment intervention for patients with severe infections. Antibiotics were also used to maintain body fluid balance and avoid subsequent bacterial infections. In situations of respiratory failure that is resistant to oxygen treatment, mechanical ventilation may be necessary; nonetheless, hemodynamic support is crucial for treating septic shock 20. Patients with refractory hypoxemia should get extracorporeal membrane oxygenation (ECMO), according to the WHO. According to their circumstances, some serious patients receive rescue therapy using convalescent plasma and immunoglobulin G 7. The MOHFW and the Government of India have implemented a revised protocol for COVID-19 clinical care. The protocol also includes instructions for investigational medicines, including Remdesivir, Tocilizumab, Convalescent Plasma Therapy, and a prophylactic dose of low-molecular-weight heparin such as Enoxaparin. Corticosteroid dexamethasone has also been included in the treatment protocols for COVID-19 patients with moderate to severe illness. It is no longer recommended to use azithromycin and hydroxychloroquine combined to treat people with severe Corona virus infections 21.
Antiviral therapy
Antiviral drugs are Lopinavir/Ritonavir, Ribavirin, Oseltanavir, Penciclovir/Acyclovir, Ganciclovir, and Favipiravir. The experimental antiviral drug is Remdesivir. Antiviral medications and systemic corticosteroid therapy, such as neuraminidase inhibitors (Oseltamivir, Peramivir, Zanamivir, etc.), Ganciclovir, Acyclovir, and Ribavirin, as well as methyl prednisolone for influenza virus, are ineffective against COVID-19 and are not indicated 4,7. Remdesivir, a nucleoside analogue and broad-spectrum antiviral, significantly suppresses SARSCoV-2 infection at low micromolar dosages and has a high selectivity index. Ritonavir and Lopinavir (protease inhibitors) can bind to the endopeptidase C30 of the SARS-CoV-2 protease and have an antiviral impact by lowering SARS-CoV-2 protein synthesis. Remdesivir was claimed to have been successful in treating the first COVID case in the United States. Acute Respiratory Distress Syndrome was reduced in severe SARS or MERS patients treated with Lopinavir/Ritonavir alone or in combination with other antiviral medications, which improved the patients’ prognosis 22.
Chloroquine
Chloroquine and hydroxychloroquine are widely used anti-malarial, auto-immune disease, and broad spectrum anti-viral drugs. They function by inhibiting the cellular receptor ACE2, impairing endosome acidification, and acting against a range of pro-inflammatory cytokines (including IL-1 and IL-6) 7. One of the first medical authorities in the world to suggest the use of HCQS prophylaxis among healthcare professionals and close associates caring for COVID-19 or suspected patients was the Indian Council of Medical Research. Azithromycin and hydroxychloroquine proved to work better together, according to certain research. Before using hydroxychloroquine, the likelihood of medication toxicity (including QT interval (QTC) prolongation and retinal toxicity) should be considered, especially in people who may be more vulnerable to these effects, such as those with epilepsy, porphyria, myasthenia gravis, and retinal pathology—glucose-6-phosphate dehydrogenase (G6PD) deficiency. Remdesivir and chloroquine together have been shown to successfully suppress the recently emerging SARS-CoV-2 in vitro 20, 22. Hydroxychloroquine was exported from India to other countries like the US, Canada, UK, Mauritius, UAE, Sri Lanka, Russia, the Philippines, Brazil, Germany, Spain, Butan, Nepal, Afghanistan, Syria, etc.
Glucocorticoids
In individuals with COVID-19 pneumonia, glucocorticoids (Dexamethasone, Methyl Prednisolone) are not advised unless there are other indications (e.g., COPD). Glucocorticoids have been associated with both delayed viral clearance and an increased risk of death in individuals infected with the Middle East respiratory syndrome coronavirus (MERS-CoV) 20. According to Union Health Ministry protocol, dexamethasone is an alternative choice to methyl prednisolone for managing moderate-to-severe COVID-19.
Immunotherapy
Convalescent plasma treatment has been used for over a decade to provide passive immunity in COVID-19 patients by infusing SARS-CoV-2 convalescent plasma from recovered patients with antiviral antibodies (IgG, IgA, IgM, IgE, and IgD). Convalescent plasma therapy has previously been used to treat a variety of diseases, including influenza, poliomyelitis, influenza A (H5N1), and ebola. The Food and Drug Administration stated in the “Diagnosis and Treatment Guidelines of COVID-19 (trial 6th, 7th, and 8th)” issued by the NHC that delivery and testing of experimental CP therapy may have a therapeutic effect on COVID-19 during the public health emergency 24. Throughout India Convalescent plasma therapy, which can restrict the course of infection and reverse the inflammatory process in COVID-19 patients via numerous pathways, was first used in New Delhi. It also includes immune-modulatory cytokines and autoantibodies, which help to control the hyper-inflammatory process and cytokine storm, eventually improving respiratory function and prognosis in COVID-19 patients. Monoclonal antibodies that target the S1 domain of the SARS CoV, such as 80 R, m396, and S230.15, have been demonstrated to be successful in neutralising SARS-CoV infections by preventing their binding to ACE receptors on host cells 22.
Cytokine storm
Interleukin (IL)-1, IL-6, IL-12, interferon (IFN), and tumour necrosis factor (TNF) are cytokines that primarily target lung tissue25. A well-known clinical syndrome called cytokine storm is defined by a massive production of pro-inflammatory cytokines that triggers an out-of-control immune response that damages organs. Particularly in tissues like the colon and kidney that express high levels of ACE2, the cytokine storm will cause harm. The use of mesenchymal stem cells that have been cytokine activated during treatment can cease the inflammatory process and aid in tissue repair 26. According to a report from China, COVID-19 targeting cytokine storms is frequent in older people. Immune modulation may be required for COVID-19 at its most severe stages, in addition to antiviral and supportive therapies 27.
Interferons
Interferons are critical cytokines used in the therapy of cancer, autoimmune disorders, and hepatitis B and C. IFN is a type I IFN that inhibits viral infection by interfering with virus replication and activating immune responses, both innate and adaptive. In vitro tests revealed that IFN efficiently inhibits SARS-CoV multiplication. Clinical and observational studies of SARS-CoV patients revealed a lack of cytokines, IFN-α and IFN-β, as compared to influenza patients 28. COVID-19 patients have been found to have lower levels of IFN-α and IFN-β. IFNs were believed to be systemically efficient in restoring lung function or delaying mortality in SARS and MERS-CoV, but they generally failed to appreciably ameliorate the disease in humans 29. Potential drugs and their target sites, along with the replication mechanism of SARS-CoV2, are shown in Figure 3.
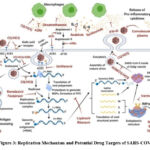 |
Figure 3: Replication Mechanism and Potential Drug Targets of SARS-COV2. |
Need for Vaccine
When combined with good testing and existing prevention efforts, a COVID-19 vaccine will be a vital instrument in bringing the pandemic under control. Although Remdesivir, an antiviral medicine, is available in developed countries, it has been demonstrated to be beneficial in treating COVID-19 illness symptoms and hastening recovery3. Several companies have agreed to develop a COVID-19 vaccine that particularly targets SARS-CoV2. A range of strategies, including live-attenuated virus, viral proteins, viral nucleic acid, virus-like particles, peptides, viral vectors (replicating and non-replicating), and recombinant protein approaches, are being employed to develop a vaccine against SARS-CoV-2 23, 27. Some of the approved vaccines by different governments worldwide are listed in Table 2.
Table 2: Approved Vaccines All Over the World.
|
Manufacturer & Vaccine |
Country |
Efficacy |
Type of vaccine |
|
Oxford university Astra Zeneca |
U.K, India |
Up to 90% |
Viral vector (Genetically modified virus) |
|
Bharat biotech ICMR- Covaxin |
India |
80.6% |
Inactivated |
|
Serum Institute of India- Covishield |
India |
70.4% |
Non replicating viral vector |
|
BioNtech- Pfizer |
Germany, America |
95% |
RNA |
|
Moderna- National Institute of allergy and infectious diseases |
Canada U.S.A |
94.50% |
RNA |
|
Gamaleya research centre |
Russia |
91.4% |
Viral vector |
|
CanSino biologics |
China |
65.7% |
Viral vector |
|
Vector institute |
Russia |
100% |
Peptide antigens |
|
Sinovac biotech |
China |
>50% |
Inactivated Virus |
|
Sinopharm |
China, U.A.E, Bahrain |
79% |
Inactivated Virus |
|
Novavax |
Russia |
96.4% |
Protein based |
Pfizer & Moderna are COVID-19 mRNA vaccines approved by the Medicines and Health Products Regulatory Agency in the U.K. This is the first emergency use authorization granted following a global phase 3 study of a pandemic vaccine 31. The US Food and Drug Administration issued the first emergency use license of vaccines for the prevention of COVID-19 for anyone over the age of 16 on December 11, 2020. Soreness at the injection site, weariness, headache, muscular soreness, chills, joint pain, and fever were described as common mild to moderate adverse effects that often lasted several days during the second dose rather than the first treatment. For the existing side effects, the developers are constantly analysing the vaccine’s safety and tolerance 32.
The first vaccine developed in India, “Covaxin” by Bharat Biotech ICMR, is an inactivated whole virus vaccine for BBV 152 that has successfully completed Phase 1 (375 subjects) and Phase 2 (380 subjects) trials. The virus strain used in these trials was obtained from the Indian ICMR National Institute of Virology, Pune. The DNA-based second vaccination in India, “ZyCovD,” produced by Zydus Cadila, has completed Phase 1 and Phase 2 trials (1048 individuals), and following Phase 3, it may receive fast track approval from DCGI 33. A restricted emergency approval for Covaxin and Covishield (the Oxford-AstraZeneca-designed vaccine that is also produced in India) was given by the country’s medicines authorities in January 2021. On January 16, 2021, India initiated the world’s largest COVID-19 vaccination campaign, with a target population of 300 million people 34.
Conclusion
As the disease is growing at an alarming rate around the world, the COVID-19 pandemic has raised problems for almost every industry1. Even though the state-wide lockdown has slowed the spread of COVID-19, the country’s constantly rising population, extraordinarily high population density, and deplorable socioeconomic conditions are important difficulties in India’s struggle 12. Transmission of the virus can be prevented by adhering to the safety guidelines issued by the WHO and their respective governments. Specific therapy was not available, but it can be managed to some extent by the available drugs, and no safer vaccine was available until the end of 2020. Several companies taking initiative for the development of vaccines and vaccination programmes were taking place in India for the prevention of the COVID-19 pandemic.
References
- Albaraa M. Current situation of Corona virus Disease: (COVID-19) review article. Health Science Journal. 2020;1(1):1-4.
- Shrikrushna S, Shubham S, Suraj T. A Review on Corona Virus (COVID-19). World Journal of Pharmaceutical and Life Sciences. 2020;6(4):109-115.
- Muhammad A, Suliman K, Abeer K. COVID-19 infection: Origin, transmission and characteristic of human corona viruses. Journal of Advanced Research. 2020;24(1):91-98.
CrossRef - Abdul H, Shmmon A, Sameera S. A Review of COVID-19 (Corona virus Disease-2019) Diagnosis, Treatments and Prevention. Eurasian Journal of Medicine and Oncology. 2020;4(2):116-125.
- Pranab C, Nazia N, Anup A. The 2019 novel corona virus disease (COVID-19) pandemic: A review of the current scenario. Indian Journal of Medical Research. 2020;151(1):147-159.
CrossRef - Aritra G, Srijita N, Tapas M. How India is dealing with COVID-19 pandemic. Sensors International. 2020;1(1):1-9.
CrossRef - Yan-rong G, Qing-dong C, Zhong-si H. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research. 2020;7(11):1-10.
CrossRef - Nilima N, Siddharth K, Bhaskar T. Psycho-social factors associated with the national wide lockdown in India during COVID-19 pandemic. Clinical Epidemiology and Global Health. 2021;9(1):47-52.
CrossRef - Udhayakumar S, Thirumalkumar D, Prabhu C. The Rise and Impact of COVID-19 in India. Frontiers in Medicine. 2020;7(1):1-7.
CrossRef - David M, Lokeshwar P, Suraj D. COVID-19 (CORONAVIRUS): A Global Emergency Outbreak and Implications in India. International Journal of Zoology and Applied Biosciences. 2020;5(2):89-98.
- Varsha K. Novel Corona virus (COVID-19) in India: Current scenario. International Journal of Research and Review. 2020;7(3):435-447.
- Rimesh P, Urmila Y. Covid-19 pandemic in India: Present scenario and steep climb ahead. Journal of Primary Care & Community Health. 2020;11(1):1-4.
CrossRef - Emily J, Corey M, Saima H. A portrait of the early and differential mental health impacts of the COVID-19 pandemic in Canada: Findings from the first wave of a nationally representative cross sectional survey. Preventive Medicine. 2020;1(1):1-12.
- Javier S, Juan B, Darren M. Prevalence of anxiety in health care professionals during COVID-19 pandemic: A rapid systemic review (on published articles in Medline) with meta analysis. Progress in Neuropsychopharmacology and Biological Psychiatry. 2021;107(1):1-15.
CrossRef - Valeria S, Davide A, Vincenzo A. The Psychology and Social Impact of Covid-19: New Perspectives of Well-Being. Frontiers in Psychology. 2020;11(1):1-6.
CrossRef - Jianbo L, Simeng M, Ying W. Factors associated with mental health outcomes among health care workers exposed to corona virus disease. JAMA. 2020;3(3):1-12.
CrossRef - Betty P, Carol S. Mental Health and the COVID-19 Pandemic. The New England Journal of Medicine. 2020;6(383):510-512.
CrossRef - Tao L, Wenjia L, Haojie Z. Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerging Microbes & Infections. 2020;9(1):1-10.
CrossRef - Min cheol C, Yu-kyung P, Bong-ok K. Risk factors for disease progression in COVID-19 patients. BMC Infectious Disease. 2020;20(445):1-6.
CrossRef - Francesco D, Damiano P, Claudia M. CoronaVirus Diseases (COVID-19) Current Status and Future Perspective: A Narrative Review. International Journal of Environmental Research and Public Health. 2020;17(1):1-11.
CrossRef - Sugin S, Ananthalakshmi V. The current situation of COVID-19 in India. Brain, Behavior and Immunity- Health. 2021;11(1):1-5.
CrossRef - Heng L, Shang-ming L, Xiao-hua Y. Corona virus Disease (COVID-19): Current Status and Future Perspectives. International Journal of Antimicrobial Agents.2020;55(1):1-9.
- Harmanjit S, Prerna C, Ashishkumar K. Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead. European Journal of Pharmacology. 2021;1(1):1-11.
- Ying W, Pengfei H, Rulin D. Convalescent plasma therapy may be a possible treatment for COVID-19: A systemic review. International Immunopharmacology. 2021;91(1):1-18.
CrossRef - Rishabh H, Mohdaslam S, Chandraiah G. Targeting inflammatory cytokine storm to fight against COVID-19 associated severe complications. Life Sciences. 2020;267(1):1-14.
CrossRef - Faheem P, Tareq A, Abdallah M. Immune system response during viral infections: Immunomodulators, Cytokine storm and Immunotherapeutics in COVID-19. Saudi Pharmaceutical Journal. 2020;1(1):1-15.
CrossRef - Yatin M, Subhal D, Kapil Z. Cytokine storm in novel Corona Virus Disease (COVID-19): Expert management consideration. Indian Journal of Critical Care Medicine. 2020;24(6):429-434.
CrossRef - Bianza M, Feng H, Tiatou S.Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin and vaccine. Biomedicine and Pharmacotherapy. 2021;133(1):1-20.
CrossRef - Parastoo T, Samane E, Milad C. A review of potential suggested drugs for Corona virus Disease (COVID-19) treatment. European Journal of Pharmacology. 2021;21(1):1-87.
- Pfizer and Biotech achieve first authorization in the world for a vaccine to combat Covid-19. December 02, 2020. https://www. pfizer.com/ news/press-release/press-release-detail/p
- A side by side comparison of the Pfizer biotech and Moderna vaccines. December 12, 2020. Available from: https://www.statnews.com/ 2020/12/19/a-side-by-side-comparison-of-the-pfizer-biontech-and-moderna-vaccines/
- Shashank J. COVID-19 Immunity to Vaccination in India. JAPI. 2021;69(4):1-3.
- Bhuyan A. World report. India begins COVID-19 vaccination amid trial allegations. 2021;397:1.
CrossRef - Bagcchi S. The world’s largest COVID-19 vaccination campaign. Lancet Infect Dis. 2021 Mar;21(3):323.
CrossRef








