Awaneet Kaur 1,2 ,, Md. Aftab Alam2, Tarique Mahmood1*
,, Md. Aftab Alam2, Tarique Mahmood1* and Farogh Ahsan1
and Farogh Ahsan1
1Department of Pharmacy, Integral University, Kursi Road, Dasauli, Lucknow- 226026, Uttar Pradesh, India
2Department of Pharmacy, School of Medical and Allied Science, Galgotias University, Greater Noida-203201, Uttar Pradesh, India.
Corresponding Author E-mail: tmahmood@iul.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2931
Abstract
It is good knowledge that the nutrient-dense kiwi fruit promotes better overall health. The antioxidant properties of kiwifruit have attracted attention as a possible reason for the fruit's health-enhancing effects. In comparison to chemical antioxidant assays, this study of the antioxidant capacity of kiwifruit discusses biologically relevant in vitro assays for predicting antioxidant activity in a biological location. These assays can be performed in a laboratory setting. The topic of discussion pertains to the potential of kiwifruit to serve as a cytoprotective agent against hydrogen peroxide-induced oxidative stress, hence mitigating cell death. In the final part of this article, we explore how the antioxidant and naturally occurring defence characteristics of kiwifruit may influence the health and welfare of humans. Compounds 1 and 2 have been subjected to additional research using scopolamine (SCOP)-induced memory impairment in a mice model of Alzheimer's disease (AD). The remarkable ability of these chemicals to enhance cognitive function has been shown to be highly impacted by their antioxidant and anticholinesterase capabilities.
Keywords
Alzheimer's disease (AD); Memory; Morris Water Maze; Kiwi Fruit
Download this article as:| Copy the following to cite this article: Kaur A, Alam M. A, Mahmood T, Ahsan F. Probing the Memory-Enhancing Potential of Kiwi Fruit Against Scopolamine-Induced Memory Impairment in Experimental Rats. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Kaur A, Alam M. A, Mahmood T, Ahsan F. Probing the Memory-Enhancing Potential of Kiwi Fruit Against Scopolamine-Induced Memory Impairment in Experimental Rats. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3vKkRjP |
Introduction
Dementia is characterised by cognitive decline and behavioural changes, commonly affecting individuals aged 65 and older. Alzheimer’s disease is a neurological condition affecting 47 million people worldwide, with a projected 62% increase by 2030. By 2050, the number of people diagnosed with AD is expected to quadruple.1
Age is the main risk factor for Alzheimer’s disease. The disorder affects 3% of people aged 65-74, 17% of people aged 75-84, and 32% of people aged 85 and older. By 2050, a new case of Alzheimer’s disease is expected every 33 seconds in the United States, with a total of 16 million cases due to the aging population.
Alzheimer’s disease (AD), which is the most common reason for dementia in people of advanced age, is ranked as the sixth leading cause of death in the United States by the Centres for Disease Control and Prevention (CDC).2 On the other hand, it is possible that it will rank third, behind heart disease and cancer, as the most significant reason for death in those who are older.
Dementia is a broad category of neurodegenerative pathologies, whose main symptom is a decline in cognitive ability severe enough to interfere with activities of daily living. Among them, Alzheimer Disease (AD) is the most common type.3
The neuropathological hallmarks of AD include oxidative stress and disturbances in neurotransmission, particularly cholinergic transmission. Neurofibrillary tangles (NFTs) and senile plaques (SPs) made of aberrant tau protein accumulate inside cells, and extracellular amyloid (A) deposits are also present.4
Morphological changes in ageing differ from those in Alzheimer’s disease (AD). Alzheimer’s disease is characterised by Aβ accumulation and tau hyperphosphorylation in the brain. Several new hypotheses have emerged to explain the development of Alzheimer’s disease (AD). The hypotheses are cellular senescence, infection-triggered AD, and neuroimmunomodulation. There is interest in the link between immune system dysfunction and AD progression. Aβ peptide forms oligomers that hinder long-term potentiation and facilitate Aβ toxicity.5 Disease-modifying treatment strategies for Alzheimer’s disease (AD) are currently the subject of extensive research. Currently, the available treatments for this disease are limited to addressing the symptoms and attempting to restore balance to the disrupted neurotransmitters.6 The Food and Drug Administration (FDA) has only granted authorization for a limited number of medications to be used in its treatment. The medications Rivastigmine, Galantamine, Donepezil, Memantine, and the combination of Memantine with Donepezil are commonly prescribed for various medical conditions.3 In order to impede the advancement of the disease, therapeutic agents are designed to disrupt the pathogenic processes that give rise to clinical symptoms. These processes typically involve the accumulation of extracellular amyloid β plaques and the formation of intracellular neurofibrillary tangles. Neuroprotective, anti-inflammatory, growth factor promotive, metabolic efficacious agents, and stem cell therapies are employed to target various underlying mechanisms.6
These medications are effective in patients with mild to moderate AD. Tacrine, a cholinesterase inhibitor, was once used at a high dose (160 mg/d) for the treatment of AD. However, its use is limited due to adverse effects such as hepatotoxicity. For this reason, herbal supplements as safe cognitive agents have gained approval as an alternative to these medications7. It is time to explore plant-based herbal treatments, which offer a promising option for managing this complex disease. Traditional medicinal herbs have seen a resurgence in recent decades due to increased awareness and their relatively low side effects compared to allopathic medicines8.
Kiwifruit (Actinidia deliciosa), is often referred to as the “king of fruits” due to its exceptional qualities. The primary origin of bioactive compounds, specifically vitamin C and phenolic compounds (including tannins, flavanols, and phenolic acids) and rich in antioxidants such as lutein, oxy carotenoid, omega-3 fatty acids, and alpha-linolenic acid, which contribute to its robust anti-inflammatory properties. Additionally, it exhibits valuable medicinal properties, including antibacterial, anti-asthmatic, anti-platelet, anti-nociceptive, anti-tumor, and anti-hypertensive effects9. Moreover, this fruit belongs to the Actinidiaceae family and is primarily cultivated in the tropics and subtropics, including New Zealand, France, and Japan. Kiwi fruits are consumed stewed, in jams, and as juice, and they possess high medicinal value. is rich in antioxidants such as lutein, oxy carotenoid, omega-3 fatty acids, and alpha-linolenic acid, which contribute to its robust anti-inflammatory properties. Additionally, it exhibits valuable medicinal properties, including antibacterial, anti-asthmatic, anti-platelet, anti-nociceptive, anti-tumor, and anti-hypertensive effects9.
Based on the aforementioned research findings, an evaluation was conducted to assess the antimicrobial, antitumor, and antioxidant properties of kiwi extracts using ethanol, chloroform, and n-hexane solvents.10 This study aims to explore the potential of Kiwi fruit in enhancing memory function, marking the first investigation of its kind. The ethanolic extracts underwent additional analysis to assess their potential as nootropic agents using various in-vivo pharmacological models and in-vitro biochemical estimation techniques. Memory impairment was induced in a mouse model of Alzheimer’s disease (AD) through the administration of scopolamine, an antagonist that blocks the activity of Muscarinic receptors.11 This study is justified by the utilization of Piracetam as a standard drug. Piracetam has been observed to possess the ability to decrease the adhesion of erythrocytes to vascular endothelium, inhibit vasospasm, and enhance microcirculation.12
Materials and Methods
All solvents utilized in the experiment were of high purity and met the standards for the analytical grade. Thin-layer chromatography (TLC) was performed using aluminum plates that were pre-coated with silica gel. The visualization of TLC plates was conducted by exposing them to a UV lamp.
Identification, Collection, and Extraction of Plant Material
The Kiwi was collected from the local market in Noida, Uttar Pradesh, India. The plant material, Kiwi fruit, was identified and authenticated by a Scientist from the Plant Diversity, Raw Material & Herbarium and Museum, Delhi. The plant specimen was authenticated in the herbarium and assigned the reference number NIScPR/RHMD/Consult/2022/4038-39-1. The collected plant material was dried in the shade. Subsequently, the Kiwi fruits were crushed and powdered using a manual homogenizer and mixer.
Extraction
The dried and ground samples, weighing 500 grams, were measured with precision. The measured amount was then transferred into a thimble and placed inside the extraction chamber. A Soxhlet apparatus, equipped with a condenser, was positioned on top of a distillation flask containing 700 mL of petroleum ether. The extraction was carried out at the temperature ranges from 70-80ᵒc. The extraction process was carried out for a duration of 2 hours. Subsequently, the cartridge was cooled to room temperature in a desiccator, and its contents were milled before being transferred back into the thimble. This entire procedure was repeated using different solvents: 700 mL of chloroform, 700 mL of methanol, 1000 mL of ethanol, and 1000 mL of water. The samples were subjected to reflux for 2 hours, 8 hours, 8 hours, and 8 hours, respectively, for each solvent. The content of the distillation flask was then concentrated to dryness under vacuum using a rotary evaporator. The dried flask was subsequently dried in a desiccator and weighed.13
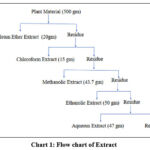 |
Chart 1: Flow chart of Extraction |
Preliminary Phytochemical Studies
The extract obtained from the fruit was analyzed using various chemical tests to identify the different active phytoconstituents present.
Experimental Animal
Animals
The Animal House at Galgotias University in Greater Noida, Uttar Pradesh, provided Swiss Albino mice that ranged in weight from 20 to 25 grams and had an age range of 8 weeks14. The mice were kept in a room with a temperature control system, and their housing consisted of polypropylene cages with steel netting. The temperature was kept at 25±5 degrees Celsius, and the relative humidity was kept at 55±5%. They were subjected to regular cycles of light and dark that lasted for a total of 12 hours each, and they were given unrestricted access to both food and water. The temperature in the room was kept at 25±4 degrees Celsius. The Central Animal House was where the feeding took place. In accordance with the recommendations made by the Institutional Animal Ethics Committee (IAEC), each and every animal was cared for in a kind and compassionate manner. Under the guidelines of Regulation GU/IAEC/2022/04/03, the Institutional Animal Ethics Committee gave their stamp of approval to the project idea.
Protocol of Pharmacological Screening
The research involved a total of thirty animals, all of which were split up into five separate groups of six animals each for the purposes of the study. Once a day, each group of mice received a dose that was proportional to their body weight as part of their treatment. The dosages were given orally at a rate of 1 ml/100 g body weight (both the saline solution and the extract), and they were given for a total of six days in a row. Before receiving the treatment dose, each group was given scopolamine intraperitoneally at a dose of 1 milligram per kilogram.15
|
S. N |
Animal |
Experimental procedure |
|
1 |
Group 1: |
Positive control group (Saline, p.o.). |
|
2 |
Group 2: |
Negative control group (Saline+ scopolamine i.p.) |
|
3 |
Group 3: |
Standard group (piracetam at a dose of 200 mg/kg, p.o +scopolamine i.p.). |
|
4 |
Group 4: |
Ethanolic extract at dose 500mg/kg body weight p.o. |
|
5 |
Group 5: |
Ethanolic extract at dose 700mg/kg body weight p.o.+ scopolamine i.p. |
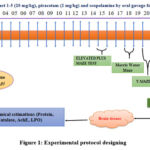 |
Figure 1: Experimental protocol designing |
Behavioral Assessment
The rationale for selecting the Morris Water Maze (MWM)16 model and the Elevated Plus Maze17 test as the behavioural assessment tests are, tests provide valuable insights into spatial learning and memory as well as anxiety and fear-related behaviours, making them powerful tools in the study of neuroscience and behaviour. The animals underwent daily training trials lasting 15–30 minutes to perform various tasks. It took 5 days to fully train the animals, during which they did not receive any drugs. Only the trained animals were selected for the study.
Evaluation of Anti-amnesic Activity by Morris Water Maze (Mwm) Model
The water maze was a circular tank, one hundred centimeters in diameter, with walls twenty centimeters high. Just two centimeters below the surface of the water was a circular platform, its surface wrapped in white linen material for better grip. A suspension of titanium dioxide was added to the maze’s water to make it opaque, and the temperature was kept constant at around 23 degrees Celsius. Over the course of three days, each mouse participated in four daily trials of training. Six to ten minutes passed between each trial. Each day’s trial began with the mouse being placed at a different one of four predetermined starting places. When the mouse was dropped into the water, we started timing how long it took for it to swim to the platform, or the latency. The mice was placed gently on the platform for 10 seconds if it hadn’t found it within 3 minutes. The data to be collected includes the path length, escape latency, and time spent in the platform quadrant for each mice.16
Elevated Plus Maze Test
The plus-maze had an open roof and two open arms (each 50 cm in length, 10 cm in width, and 40 cm in height). The maze is currently 50 cm high. The device was tested on mice weighing 20-25 g that had been housed in pairs for 10 days. During this time, the mice were handled by the researcher every other day to lessen the stress they felt. There were 6 mice in each group. Mice were placed in the middle of the maze, facing one of the enclosed arms, 30 minutes after being given either the test medication or the standard orally. The number of times participants entered each arm, how long they stayed in each arm, and the total number of times participants entered the arms were all recorded over the course of the 5-minute test session. A TV camera operated by remote control was used to observe the procedure as it was carried out in a soundproof room.17
Homogenization of hippocampus
On the 22nd day, the animals were given ketamine (60 mg/kg) and xylazine (10 mg/kg) in order to render them unconscious prior to having their heads severed. After that, the heads were cut open in order to remove the brains. The hippocampus was dissected on a dish that was placed on ice, and the tissues were stored for a number of different biochemical testing. After that, the tissues were rinsed in an ice-cold phosphate buffer saline solution that had a pH of 7.4 and included 10% by weight of phosphate buffer. Following homogenization and centrifugation at a cold temperature (4°C) (10,000 RPM) for 15 minutes, the supernatant was collected and kept at a temperature of -20 °C.18
Protein quantification
The biuret method was applied in order to provide an accurate reading of the protein content. In order to determine the value of the protein, a standard curve based on bovine serum albumin was utilized. The value of the protein was utilized in the process of normalizing the estimated values of a number of different biochemical parameters.18
Catalase assay
A mixture that was three milliliters in volume was prepared as the catalase assay in order to test the catalase activity of the collected hippocampal tissue supernatant against the hydrogen peroxide. This was done so that the catalase activity could be evaluated. This mixture contained 50 microliters of homogenized tissue supernatant in phosphate buffer saline (1.95 milliliters of 0.05 M, pH 7.0), and 1.0 milliliters of 0.019 M hydrogen peroxide. The mixture was then mixed together. Using a LAMBDA 25 UV/Vis Spectrophotometer (made by Perkin Elmer in the United States), absorbances were measured at 240 nm for two minutes at intervals of thirty seconds each. After compiling and analyzing the data, the findings were reported in the form of moles of H2O2 decomposed every minute per milligram of protein.19
Acetylcholinesterase (AChE) assay
In order to determine the level of acetylcholinesterase activity present in homogenized tissues taken from the hippocampal area of the brain, 50 L of supernatant was dissolved in phosphate buffer saline containing 3.0 mL of 0.01 M at a pH of 8.0. After that, 100 microliters each of acetylthiocholine iodide and DTNB were added in order to conduct the AChE test. Recordings of absorbances were taken at 412 nm for two minutes at intervals of thirty seconds each. The findings were determined by doing calculations with a molar extinction coefficient of 1.36 104 M1 cm1.20
Lipid peroxidation (LPO) assay
To demonstrate the presence of oxidative stress, the lipid peroxidation product malondialdehyde, also known as MDA, is evaluated as thiobarbituric acid reactive substances, or TBARS. In order to accomplish this, one hundred microliters of the supernatant of a tissue homogenate was combined with one hundred microliters of 0.1 M Tris-HCl at a pH of 7.4. After mixing the combination with 200 L of 10% (w/v) TCA, it was allowed to incubate for two hours at a temperature of 37 degrees Celsius. The supernatant was collected (200 L) after centrifugation for 10 minutes at 1000 RPM at 4 degrees Celsius, and then it was combined with thiobarbituric acid (200 L, 0.67% w/v). After that, the samples were heated in water that was boiling for ten minutes so that the pink color could develop, and then they were cooled at room temperature. The absorbance at 532 nm was measured immediately using a UV-visible spectrophotometer in order to quantify MDA. The molar extinction coefficient value of the chromophore was taken as 1.56 105 M-1 cm-1, and the findings are shown as nano-moles of MDA per mg protein.21
Statistical Analysis
For the purpose of conducting statistical analysis during the entirety of the experiment and determining the mean, standard deviation, and standard error mean (SEM) values associated with each parameter, the GraphPad Prism 5.0 software from San Diego, California, USA was utilized. Tukey’s test and a one-way analysis of variance were used to analyze the parameters. The data are presented with a mean and a standard error of the mean, with a significance level of P<0.05.
Results and Discussion
Phytochemical Investigation of Kiwi Fruit
Table 1: Characteristics of Extract
|
Extract |
Colour of extract |
Odour |
Consistency |
Sense of touch |
Amount of extract (gm) |
%Yield (w/w) |
|
Petroleum ether |
Yellowish |
Characteristics |
Semisolid |
Sticky |
20 |
4.3 |
|
Chloroform |
Brownish green |
Characteristics |
Semisolid |
Sticky |
15 |
3.2 |
|
Methanol |
Reddish Brown |
Characteristics |
Semisolid |
Sticky |
43.7 |
9.6 |
|
Ethanol |
Reddish Brown |
Characteristics |
Semisolid |
Sticky |
50 |
10.98 |
|
Aqueous |
Yellowish brown |
Characteristics |
Semisolid |
Sticky |
47 |
10.32 |
Table 2: Phytochemical Screening Carbohydrate Test
|
Carbohydrate Test |
|||||
|
|
Pet. Ether |
Chloroform |
Methanolic |
ethanolic |
Water |
|
Benedict test |
– |
– |
+ |
++ |
+++ |
|
Barfoed’s test |
– |
– |
+ |
++ |
+++ |
|
Fehling’s test |
– |
– |
+ |
++ |
+ |
|
Molisch’s test |
– |
– |
+ |
++ |
+++ |
|
Flavonoids Test |
|||||
|
Shinoda test |
+ |
+ |
+++ |
+++ |
+++ |
|
NaoH test |
+ |
+ |
+++ |
+++ |
+++ |
|
Ferric Chloride test |
+ |
+ |
+++ |
+++ |
+++ |
|
Phenolic Test |
|||||
|
Ferric Chloride test |
+ |
+ |
+++ |
+++ |
+++ |
|
Alkaloidal Test |
|||||
|
Wagner’s test |
– |
+ |
+++ |
+++ |
+++ |
|
Dragondrof’s test |
– |
+ |
+++ |
+++ |
+++ |
|
Meyer’s test |
– |
+ |
+++ |
+++ |
+++ |
 |
Table 3: Results for Thin Layer Chromatography |
Pharmacological activity of Kiwi Extract
Anti-Alzheimer’s activity
Alzheimer’s disease (AD) is a neurological ailment that manifests itself in a peculiar way and worsens over time. In a clinical setting, this form of dementia can be recognized from overflowing dementia by a number of symptoms, including cognitive decline, progressive neurodegeneration, executive dysfunction, personality and behaviour abnormalities, and others. It is not known at this time which factors, most of which are hereditary and environmental, contribute to the aetiology of the disease. The intraperitoneal administration of scopolamine results in an increase in the generation of reactive oxygen species, which in turn leads to oxidative stress and memory loss. In addition, the breakdown of the cholinergic neurotransmitter acetylcholine is caused by the activation of the acetylcholinesterase enzyme at the postganglionic muscarinic receptor site. Oxidative stress, the death of neuronal cells, and cognitive decline are the three symptoms that come together to form Alzheimer’s disease. In order to evaluate the methanolic extract’s potential anti-Alzheimer’s action in vivo, several different animal species were tested.
Morris Water Test (MWM) Assay
The Morris Water Maze (MWM) test model was used to investigate the memory-improving effects of kiwi extracts 1 and 2 in scopolamine-treated mice. The obtained results revealed that the scopolamine group’s retention period RP was significantly longer in comparison in the normal control groups. Figure 2 depicts how the standard drug piracetam drastically decreased the retention period RP when compared to the scopolamine group. When compared to the SCOP-induced group, the treatment with kiwi fruit extract 1&2 displayed a greater level of recognition frequency in the Morris Water Maze (MWM) and also improved neuroprotective action.
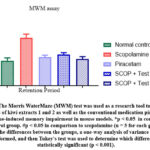 |
Figure 2: The Morris Water Maze (MWM) test was used as a research tool to investigate the effects of kiwi extracts 1 and 2 as well as the conventional medication piracetam on scopolamine-induced memory impairment in mouse models. |
Elevated Plus Maze
The Elevated Plus Maze test was carried out on mouse models in order to determine the extent of their short-term and unpleasant memories (Figure 3). Memory dysfunction can be deduced from the significantly elevated values of the parameters when they are stated in terms of escape latency and number of errors in mice that have been treated with scopolamine.
 |
Figure 3: Elevated Plus Maze test: (A) Effect of kiwi extract 1&2 and piracetam on retention time period in open arm (sec) in mice model; (B) Effect of kiwi extract 1&2 and piracetam on retention time period in close arm (sec) in mice model. |
Compared to mice treated with scopolamine, treatment with kiwi fruit extract 1&2 significantly reduced the retention time period in both the open arm and the closed arm (Figure 3). They most likely work as reversal agents on a pre-existing cholinergic disruption problem. The data shows that treatment with kiwi extracts 1&2 and piracetam significantly improved the mice’s memory. A potentially effective phytoconstituent for the therapy of cognitive impairment is kiwi extract.
Biochemical estimations
When examining biochemical indicators, it was found that the scopolamine-treated group’s hippocampus had much less catalase activity than the control group. In comparison to the scopolamine group, treatment with kiwi extracts 1&2 and piracetum significantly increased catalase activity; the effects of the standard drugs proved to be more prominent, as seen in Figure 4a.
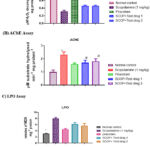 |
Figure 4: The effect of kiwi fruit extracts 1 and 2 and piracetum on catalase activity, acetylcholinesterase activity, and lipopolysaccharide oxidase activity in mice that had been treated with scopolamine. |
Discussion
Compared to the scopolamine-induced group, where higher levels of AChE were found, compound-treated groups showed a considerable reduction in AChE enzyme activity (Figure 4b). Additionally, it was noted that the test drugs 1 and 2 of kiwi fruit extract decreased AChE activity. A substantial body of research indicates lipid peroxidation caused by free radicals as a fundamental contributor to neurodegeneration in the Alzheimer’s brain. According to expectations, the parameters in the current investigation showed that the LPO levels were greater than expected in the hippocampal region of the brains of the scopolamine group animals compared to the control group. In mice given the conventional medication piracetam plus the kiwi fruit extract 1&2, the MDA levels were significantly lowered (Figure 4c). There is strong evidence that kiwi fruit extracts 1 and 2 can significantly lower levels of lipid peroxidation in the hippocampal region of the brain.
It is widely known that the naturally occurring phytoconstituent in kiwi fruit extract plays a neuroprotective role in the central nervous system. Kiwi fruit is known to be a good source of vitamin C, vitamin K, and dietary fibre, among other nutrients. There are many opportunities for its application in the prevention and treatment of neurodegenerative disorders like AD, Parkinson’s disease, etc. because it may pass the blood-brain barrier and become dispersed in different sections of the brain. Kiwi fruit extract has been reported to reduce Aβ-aggregation and may encourage hippocampus neurogenesis to enhance cognitive performance.
The effectiveness of kiwi fruit extracts 1&2 in preventing and treating scopolamine-induced memory impairment has been demonstrated in the current investigation. The inhibition of oxidative stress and the neuroinflammatory cascade in the mice’s brain appears to be the mechanism by which the kiwi extracts 1 and 2 enhance cognitive performance in SCOP-induced mice. It has been noted that the kiwi fruit extracts 1 and 2 may be able to counteract the effects of oxidative stress and peroxidation. Additionally, these substances have been found to be remarkably effective at enhancing cognitive performance via the cholinergic pathway by decreasing AChE activity. The findings back up past research on the neuroprotective properties of kiwi fruit extract.
Kiwi extracts 1&2 in general demonstrated notable neuroprotective effects. Examining the responsible phytochemical of these substances’ biological data. It is still too early to draw any firm conclusions, and further in-depth research is needed to back up these findings. The kiwi fruit extract may yield interesting therapeutic possibilities for the treatment of Alzheimer’s disease with the right structural research.
Conclusion
The efficacy of kiwi extract in mitigating cognitive dysfunction in Alzheimer’s disease (AD) has been demonstrated through its positive impact on memory function in mice induced with scopolamine. These findings highlight the potential of kiwi extract as a promising therapeutic agent for the treatment of Alzheimer’s disease. The anti-Alzheimer effects of kiwi fruit extracts 1 and 2 are comparable to those of piracetam, which is a standard drug. The drugs exhibited positive anti-Alzheimer efficiency in vitro, as indicated by biochemical estimations that focused on the regulation of oxidative stress and LPO activity as the primary mechanisms for reversing scopolamine-induced memory impairment. The aforementioned results demonstrate that the effectiveness of the extract as a nootropic is directly linked to its ability to reduce the activity of the cholinesterase enzyme.
This provides additional evidence that an elevated level of acetylcholine in the central nervous system results in an increase in memory. Therefore, this section has the potential to be further investigated in the future for the development of more secure therapeutic approaches for cognitive disorders such as Alzheimer’s disease, dementia, and others.
Future Perspectives
This study opens up promising avenues for future research in the field of memory enhancement. Further investigations are warranted to explore the underlying mechanisms responsible for the observed memory-enhancing effects of Kiwi fruit. Elucidating the specific bioactive compounds present in Kiwi fruit that contribute to its beneficial effects on memory could provide valuable insights for the development of novel therapeutic agents for memory disorders. Additionally, conducting in-depth studies to understand the molecular pathways involved in the anti-amnesic activity of Kiwi fruit would be crucial. Furthermore, exploring the long-term effects of Kiwi fruit consumption on memory and cognitive function could provide valuable information on its potential as a preventive or therapeutic intervention for age-related memory decline and neurodegenerative disorders. It would also be worthwhile to investigate the optimal dosage, duration, and mode of administration of Kiwi fruit extract for maximum memory enhancement. Overall, further research in this area holds promise for harnessing the memory-enhancing potential of Kiwi fruit for the benefit of cognitive health and well-being.
Acknowledgment
The authors would like to thank Hon. Chancellor, Prof. Syed Waseem Akhtar, Integral University, and Vice-Chancellor, Prof. Javed Musarrat, Integral University for providing the research environment and all the equipment needed to carry out the research. The university has provided a communication number for further internal communication (IU/R&D/2023-MCN0002030).
Conflict of Interest
The authors declare that there are no possible conflicts of interest with respect to the research, authors, and/or publication of this article.
Funding Sources
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
AK has performed the experiment and written the manuscript, TM has designed the experimental protocol, AA has edited and checked the manuscript, FA has communicated the manuscript. All authors have read and approved the final manuscript.
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors so this section is not applicable.
Consent for Publication
All author have given their concern for publication of this manuscript.
Availability of Data and Material
All data have been mentioned in the manuscript, no other data are with authors.
References
- Anonymous. Alzheimer’s disease facts and figures. Alzheimers Dement., 2021; 2021;17: 327-406.
CrossRef - Scarpini E, Schelterns P, Feldman H. Treatment of Alzheimer’s disease; current status and new perspectives. The Lancet Neurology. 2003; 2(9): 539-547.
CrossRef - Chen X, Drew J, Berney W, Lei W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells. 2021; 10(6): 1309.
CrossRef - Calabrò M, Rinaldi C, Santoro G, Crisafulli C. The biological pathways of Alzheimer disease: a review. Neuroscience., 2020; 86-132.
CrossRef - Uddin M. S, Mamun A. A, Kabir M. T, Jakaria M, Mathew B, Barreto G. E, Ashraf G. M. Nootropic and Anti-Alzheimer’s Actions of Medicinal Plants: Molecular Insight into Therapeutic Potential to Alleviate Alzheimer’s Neuropathology. 2019; 56: 4925-44.
CrossRef - Wu, K. M, Zhang, Y. R, Huang, Y. Y, Dong Q, Tan L, & Yu J. T. (2021). The role of the immune system in Alzheimer’s disease. Ageing Research Reviews, 2021; 70: 101409.
CrossRef - Yiannopoulou K. G, Papageorgiou S. G. Current and future treatments in Alzheimer disease: an update. Journal of central nervous system disease. 2020; 12: 1179573520907397.
CrossRef - Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. International Journal of Nanomedicine. 2019; 14: 5541-5554.
CrossRef - Uddin M. S, Al Mamun A, Kabir M. T, Jakaria M, Mathew B, Barreto G. E, Ashraf G. M, et al. Nootropic and Anti-Alzheimer’s Actions of Medicinal Plants: Molecular Insight into Therapeutic Potential to Alleviate Alzheimer’s Neuropathology. Molecular Neurobiology. 2018; 56(7): 4925-4944.
CrossRef - Akram M, Nawaz A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017;12(4):660-670.
CrossRef - Leuner K, Kurz C, Guidetti G, Orgogozo J. M, Müller W. E. Improved mitochondrial function in brain aging and Alzheimer disease–the new mechanism of action of the old metabolic enhancer piracetam. Frontiers in neuroscience. 2010; 7: 4-44.
CrossRef - Satpal D, Kaur J, Bhadariya V, Sharma K. Actinidia deliciosa (Kiwi fruit): Acomprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. Journal of Food Processing and Preservation. 2021; 45(6): e15588.
CrossRef - Zhang J, Gao N, Shu C, Cheng S, Sun X, Liu C, Xin G, Li B, Tian J. Phenolics profile and antioxidant activity analysis of kiwi berry (Actinidia arguta) flesh and peel extracts from four regions in China. Frontiers in plant science. 2021; 1(12): 689038.
CrossRef - Carter R. J, Lione L. A, Humby T, Mangiarini L, Mahal A, Bates G. P, Dunnett S. B, Morton A. J. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. Journal of Neuroscience. 1999; 19(8): 3248-57.
CrossRef - El Azab E. F, Mostafa H. S. Phytochemical analysis and antioxidant defense of kiwifruit (Actinidia deliciosa) against pancreatic cancer and AAPH-induced RBCs hemolysis. Food Science and Technology. 2021; 11(42): e06021.
CrossRef - Sun X, Jia P, Bu T, Zhang H, Dong M, Wang J, Wang X, Zhe T, Liu Y, Wang L. Conversional fluorescent kiwi peel phenolic extracts: sensing of Hg2+ and Cu2+, imaging of HeLa cells and their antioxidant activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 202; 244: 118857.
CrossRef - Bhanumathy M, Harish M. S, Shivaprasad H. N, Sushma G. Nootropic activity of Celastrus paniculatus seed. Pharmaceutical Biology. 2010; 48(3): 324-7.
CrossRef - Sareetha A.V, YP S. P. Nootropic activity of ethanolic extract of Woodfordia fruticosa (L.) Kurz flowers on scopolamine-induced mouse model of Alzheimer’s disease. National Journal of Physiology, Pharmacy and Pharmacology. 2021; 11(5): 495-9.
- Jatav S, Pandey N, Dwivedi P, Akhtar A, Jyoti, Singh R, Bansal R, Mishra B. B. Synthesis of deoxy-Andrographolide Triazolyl Glycoconjugates for the Treatment of Alzheimer’s Disease. ACS Chemical Neuroscience. 2022; 13(23): 3271-80.
CrossRef - Kumar V, Vishalakshi M, Gangaraju M, Das P, Roy P, Banerjee A, Gupta S. D. Evaluation of antibacterial, antioxidant and nootropic activities of Tiliacora racemosa Colebr. leaves: In vitro and in vivo approach. Biomedicine & Pharmacotherapy. 2017; 86: 662-8.
CrossRef - Chaudhari K. S, Tiwari N. R, Tiwari R. R, Sharma R. S. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer’s Disease. Ann Neurosci. 2017; 24(2): 111-122.
CrossRef







