Ali Ata Alsarhan1* , Ashraf O. Khashroum2
, Ashraf O. Khashroum2 , Jumanah D. Al-Shawabkeh1
, Jumanah D. Al-Shawabkeh1 , Suha Khayri Ababnheh1
, Suha Khayri Ababnheh1 , Alia Khwaldeh3
, Alia Khwaldeh3  , Nidal M.F Abu Laban1
, Nidal M.F Abu Laban1  and Ahmad Bani-Khaled4
and Ahmad Bani-Khaled4
1Department of Allied Medical Sciences, Zarqa University College, Al-Balqa Applied University, Jordan
2Department of Plant Production and Protection, Faculty of Agriculture, Jerash University, Jordan.
3Faculty of Allied Medical Sciences, Department of Medical Laboratory Sciences, Jadara University, P.O.Box 733, Irbid, Jordan
4Department of Biological Sciences, Al al-Bayt University, P.O.BOX 130040, Al-Mafraq, Jordan.
Corresponding Author E-mail: asarhan@bau.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2917
Abstract
This work was carried out to evaluate levels of expression of the Heat Shock Protein 70 (Hsp70) and inducible nitric oxide synthase (iNOS) biomarkers in extracts of Artemisia sieberi (A. herba-alba) and their impacts on the activity of hypothalamic-pituitary-thyroid (HPT) axis in diabetic rats. 50 rats were separated into five experimental groups: a normal control group, a positive control group treated with dilute A. herba alba (AHE) oil extract, a diabetic non-treated group, a diabetic group treated with AHA extract, and a diabetic group treated with Metformin. Results: Orally administered 8.1 mg/kg body weight (BW) of dilute AHA oil and 14.2 mg/kg BW of Metformin were administered for 6 weeks. Serum triiodothyronine (T3) levels decreased significantly in diabetic rats and increased significantly in the rats treated with the dilute AHA oil. Furthermore, no significant differences were observed in thyroid gland Hsp70 expression between the diabetic and non-diabetic rats. Metformin and dilute AHA oil treatments significantly increased the expression of Hsp70 in the thyroid gland. The results also demonstrated that diabetes significantly increased the rate of iNOS expression in the thyroid gland. Treatment with Metformin and dilute AHA oil significantly reduced the expression of iNOS in the thyroid gland. These results suggest that dilute AHA oil plays a role in the peripheral regulation of thyroid function and provide empirical evidence that it contributes to the stimulation or improvement of thyroid function.
Keywords
Artemisia Sieberi (A. herba-alba); Hypothalamic-Pituitary-Thyroid; Hsp70; iNOS; Diabetic Rats
Download this article as:| Copy the following to cite this article: Alsarhan A. A, Khashroum A. O, Al-Shawabkeh J. D, Ababnheh S. K, Khwaldeh A, Abu-Laban N. M. F, Khaled A. B. Levels of Expression of Hsp70 and iNOS and Effect of Artemisia Sieberi (A. herba-alba) on Activity of Hypothalamic-Pituitary-Thyroid (HPT) Axis in Diabetic Rats. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Alsarhan A. A, Khashroum A. O, Al-Shawabkeh J. D, Ababnheh S. K, Khwaldeh A, Abu-Laban N. M. F, Khaled A. B. Levels of Expression of Hsp70 and iNOS and Effect of Artemisia Sieberi (A. herba-alba) on Activity of Hypothalamic-Pituitary-Thyroid (HPT) Axis in Diabetic Rats. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3RsEJ2x |
Introduction
Artemisia sieberi (A. herba-alba) belongs to the Asteraceae (Compositae) family and includes annual, perennial, and biennial plants1. Artemisia herba-alba is a short shrub usually found in Northern Africa and the Middle East. The parts that grow above the ground are used as medicine2. The herb is used traditionally to treat wounds, parasites (especially helminths), diabetes, respiratory and intestinal disorders, and injuries2. Moreover, several Artemisia herbs have historically been used to treat seizures and their efficacy has been proven by in vivo animal trials3,4,5. The Artemisia species have been studied in vitro and in vivo, as well as in clinical trials, for their anticancer, antimalarial, antibacterial, antiviral, and antidiabetic properties6. Vegetables, culinary herbs, and medicinal plants are commonly used to control diabetes in Jordan, where traditional medicine is an essential component of primary health care7.
The synthesis of nitric oxide (NO) involves enzymes that convert the amino acid L-arginine into NO8. The inducible nitric oxide synthase (iNOS) is a key physiological signaling molecule that regulates insulin production, angiogenesis, nociception, inflammation, and pain8,9. Enzymatic synthesis of NO is controlled by isoenzymes, including endothelial, neuronal, and inducible isoforms8. The concentration of the resultant NO depends on numerous parameters, which mainly include the isoform involved in NO production and duration of the production process8,9. The iNOS is more likely to generate NO than other isoforms 10. Diabetic rats have high levels of iNOS8,9,10.
The Heat Shock Protein 70 is one of the central components of thecellular network of molecular chaperones. It is naturally generated in lymphocytes, macrophages, epithelial cells, dendrites, muscles, and hepatocytes. Having a molecular mass of 70 kDA, this protein molecule has a role in regulating homeostasis of other proteins and is dependent in its action on adenosine triphosphate (ATP)11. Some Hsp70 chaperones are characterized by their genes and cell sites. Both Hsp70 and the heat shock 70 protein (Hsc70) are found in the nucleus and cytoplasm; the binding immunoglobulin protein (BiP) Grp78 is placed in the endoplasmic reticulum (ER) and mitochondrial Hsp70 (mtHsp70) Grp75 is located in the mitochondria11, 12. Changes in Hsp70 expression have been seen in people with obesity and metabolic disorders, including type 2 diabetes as evidenced by results of several studies. According to some studies, increasing insulin sensitivity through Hsp70 modulation might affect blood glucose levels12,13,14. The hypothalamus-pituitary-periphery (HPT) feedback loop regulates the production, storing, and secretion of thyroid hormones by the thyroid gland. The presence of two crucial trace elements—selenium and iodin—is necessary for these processes1. The thyroid-stimulating hormone (TSH), which is secreted by the anterior pituitary gland, is the primary stimulator of thyroid hormone synthesis. It enhances all the processes required for thyroid hormone biosynthesis, including protein biosynthesis, intra-thyroidal iodide organification, and stimulation of thyroglobulin (Tg) release15. Diabetes mellitus (DM) and thyroid problems are associated with endocrine system dysfunction. Research16 has shown that diabetes and thyroid diseases frequently co-exist in individuals. Considering their roles in cellular metabolism, insulin and thyroid hormone excesses or deficiencies can lead to functional abnormalities in the other hormones16. Bearing this in mind, this study was carried out to evaluate the levels of expressions of the Hsp70 and iNOS biomarkers in dilute oil extract of A. herba-alba (AHA) and their effects on the action of the hypothalamic-pituitary-thyroid (HPT) axis in rats suffering from diabetes.
Experimental Part
Preparation of oil
Artemisia sieberi (A. herba-alba) aerial parts were used to extract the essential oil using the extraction method described in references17,18. Dry parts of this plant were cut into 0.5 mm pieces and allowed to air dry in the dark at room temperature for five days. Afterwards, a mass corresponding to 200g of the dried material was hydrodistilled for 3 h using Clevenger apparatus. The oil yield of the dried tops (leaves, stems, and flowers) was 1.1% (v/w).
Diabetes induction in rats
For this study, adult rats weighing 150–200 g were bought from the Jordan University of Science and Technology (JUST) Faculty of Medicine’s central animal house. The animals were maintained on a 12-hour light-dark cycle. Water and food were available ad libitum. A stock diet meal consisting of 50% wheat, 21% maize, 20% soybean, 8% concentrated protein, and 1% of a combination of salts, vitamins, and dicalcium phosphate was supplied to the rats, who were kept in normal metal cages with ten rats per cage. Struggle and restraint were minimized to avoid the influence of stress on the test results as highlighted in18. The rats were administered intraperitoneal injections of alloxan monohydrate (BOH Chemicals Ltd, England) dissolved in fresh normal saline at a dose of 150 ml per kg body weight (BW) after fasting for 18 h. Rats in this study were classified as diabetics if their blood glucose concentrations ranged between 200 and 450 mg/100 ml. 17, 18.
Experimental design
50 rats were randomly divided into five experimental groups of 10 each. Normal rats have been split into two groups: 1 and 2. Group 1 rats received dimethyl sulfoxide (DMSO) at a 0.5 mL/kg dosage and were used as the negative control group. A. sieberi essential oil extract was administered by gavage to group 2 at 80 mg/kg. Rats with alloxan-induced diabetes were divided into three groups: 3, 4, and 5. Group 3 rats were only given DMSO at a 0.5 mL/kg dosage. Meanwhile, Group 4 rats received treatment with dilute AHA oil extract (80 mg/kg), whereas Group 5 rats received Metformin (14.2 mg/kg). The Metformin was obtained from Bristol-Myers Squibb Company in the United Kingdom. For six weeks, the essential oil extract and Metformin were given daily via an intragastric tube. Rats in each group received the recommended treatment for 6 weeks, with unlimited access to water and food during the trial. Blood samples were obtained from the rats at the end of the study period after the heart puncture procedure, and tissues specimens were collected from the thyroid gland to look at histological alterations. The mice were killed by cervical dislocation after the study procedure while under light ether anesthesia. The experiment was conducted in compliance with the guidelines of the animal ethics committee of JUST, which were expressed in accordance with international standard principles for laboratory animal use and care (Number 27045128/17).
Determination of thyroid hormone
The micro particle enzyme immunoassay (MEIA) is the basis for quantification of the Axsym thyroid hormone (triiodothyronine (T3), thyroxine (T4), and TSH). The following order is followed while pipetting the axsym-free (T3, T4, and TSH) reagents and samples. The sampling probe pipettes the sample and the assay (T3, T4, and TSH) chemicals that are needed for a single test into different reaction vessel (RV) wells. The RV is moved right away into the processing facility 18. Using the processing probe, further pipetting is performed at the processing center. An antibody-antigen combination is formed when the sample and anti-T3, anti-T4, and anti-TSH-coated micro particles are pipetted into one well of the RV. An aliquot of the reaction mixture comprising T3, T4, and TSH bound to anti-T3, anti-T4, and anti-TSH-coated micro particles was added to the matrix cell. The glass fiber matrix and the micro particles form an irreversible bond. To remove unbound components, solubilizer solution was used to wash the matrix cell. When applied to the matrix cells, T3-alkaline phosphatase interacts with the open antibody binding sites. Unbound materials are eliminated by washing the matrix cell. After adding the substrate, that is 4-methyumbelliferyl phosphate, to the matrix cell, the MEIA optical assembly measures the fluorescence18.
T3, T4, and TSH levels
AxSYM-free T3 and T4 MEIA measures free T3 quantitatively in human blood or plasma using the AxSYM analyzer17,18. The AXSYM 3rd Generation TSH Test is a useful method of concluding thyroid function, identification of thyroid conditions, and management of thyroid-related illnesses18.
Immunohistochemistry study
Schematic Overview of Immunohistochemistry:
 |
Chart 1: Schematic Overview of Immunohistochemistry |
Interpretation of the Results
The resulting number of pixels that represented the immunological reaction was divided by the total number of pixels. Following that, the number of pixels with brown color, which is the color of the marker in the present study, was determined and divided by the whole number of pixels to estimate the expression of HSP70 and iNOS based on the interpretation of output of Adobe Photoshop Version: 22.3.19.
Statistical analysis
Results are expressed as mean ±SE. One-way analysis of variance (ANOVA) was used to estimate statistical differences by using SPSS version 22. A value of p < 0.05 was considered significant18,20. Descriptive statistics for HSP70 for every group were calculated then presented as “Mean ± Standard deviation”.
Results
Effects of Artemisia and Metformin on T3, T4, and TSH levels in diabetic rats
The study found significant drops (p < 0.05) in blood T3, T4, and TSH levels in the diabetic rats below the corresponding levels in the control group (Table 1). Rats in the control group have substantially increased blood T4, T3, and TSH levels (p < 0.05) than the comparable values in the diabetic rats. Furthermore, compared to the control rats, the diabetic rats had greater blood TSH levels. These findings imply that AHA oil therapy significantly raised the control group’s blood T4 levels (p < 0.05).
Table 1: Levels of TSH, T3, and T4 in the control and diabetic rats.
|
T4 (nmol/L) |
T3 (nmol/L) |
TSH (ng/ml) |
N |
Group |
|
48.65 ± 2.45 |
1.46 ± 0.05 |
0.93 ± 0.02 |
10 |
1. Untreated Control |
|
54.82± 4.84* |
2.21± 0.13* |
1.67±0.01* |
10 |
2. Control treated with dilute AHE oil |
|
30.58± 2.27 |
0.82 ± 0.03* |
0.48 ± 0.03* |
10 |
3. Rats with alloxan-induced diabetes |
|
40.66 ± 1.28** |
1.04 ± 0.06** |
0.83 ± 0.04** |
10 |
4. Diabetic rats treated with dilute AHE oil |
|
38.54 ± 1.43** |
1.08 ± 0.07** |
0.76 ± 0.03** |
10 |
5. Diabetic rats treated with Metformin |
Values are the mean values ± standard deviation of 10 rats.
* Statistically significant when compared to the control and vehicle groups, i.e., Groups 1 and 2, at α = 0.05.
** Statistically significant when compared to the rats with alloxan-induced diabetes (Group 3) at α = 0.05.
Immunohistochemistry results
Expression rates of hsp70 in control and diabetic groups
Our results demonstrate that diabetes significantly decreased Hsp70 expression in the kidneys and liver (p < 0.05). Diabetes had a molecular impact on the liver and kidneys. In addition, there was no significant difference (p > 0.05) in Hsp70 expression in the thyroid gland between diabetes and non-diabetic rats (Table 2).
Table 2: Levels of Hsp70 expression in rats of the control and diabetic groups.
|
Hsp70 Expression |
|||||
|
Liver |
Kidney |
Thyroid gland |
|||
|
Control Rats |
Diabetic Rats |
Control Rats |
Diabetic Rats |
Control Rats |
Diabetic Rats |
|
0.40 |
0.08 |
0.40 |
0.15 |
0.30 |
0.10 |
|
0.39 |
0.10 |
0.39 |
0.13 |
0.31 |
0.09 |
|
0.38 |
0.09 |
0.38 |
0.14 |
0.32 |
0.08 |
|
0.42 |
0.11 |
0.37 |
0.16 |
0.35 |
0.11 |
|
0.39 |
0.10 |
0.36 |
0.12 |
0.29 |
0.99 |
The effects of Metformin and dilute AHA oil on levels of Hsp70 expression.
The outcomes of the experiment (Table 3) revealed that both Metformin and dilute AHA oil treatments at significant (p < 0.05) raised the levels of Hsp70 expression in the livers, kidneys, and thyroid glands of the treated diabetic rats (Group 3 and Group 4) compared with the levels in non-treated diabetic rats (Group 3).
Table 3: Effects of Metformin and dilute AHA oil treatments on Hsp70 expression in diabetic rats.
|
Hsp70 Expression |
||||||||
|
Liver |
Kidney |
Thyroid |
||||||
|
Group 3 1 |
Group 4 2 |
Group 5 3 |
Group 3 1 |
Group 4 2 |
Group 5 3 |
Group 3 1 |
Group 4 2 |
Group 5 3 |
|
0.08* |
0.90** |
0.70** |
0.15* |
0.93** |
0.60** |
0.10* |
0.80** |
0.5** |
|
0.10* |
0.89** |
0.69** |
0.13* |
0.90** |
0.70** |
0.09* |
0.79** |
0.49** |
|
0.09* |
0.80** |
0.71** |
0.15* |
0.89** |
0.59** |
0.08* |
0.75** |
0.52** |
|
0.11* |
0.95** |
0.76** |
0.16* |
0.92** |
0.58** |
0.11* |
0.81** |
0.55** |
|
0.10* |
0.93** |
0.75** |
0.12* |
0.91** |
0.62** |
0.99* |
0.82** |
0.49** |
1 Rats with alloxan-induced diabetes, 2 Diabetic rats treated with Metformin. 3 Diabetic rats treated with dilute AHA oil.
* Statistically significant when compared to the control group at α = 0.05. ** Statistically significant when compared to the untreated diabetic group, i.e., Group 3, at α = 0.05.
Levels of expression of iNOS in rats of the control and diabetic groups
Diabetes resulted in a statistically significant (p < 0.05) increase in the levels of iNOS expression in the livers, kidneys, and thyroid glands of the rats (Table 4). Diabetes affects the thyroid gland, liver, and kidney at the molecular level.
Table 4: Levels of expression of iNOS in rats of the control and diabetic groups.
|
iNOS Expression |
|||||
|
Liver |
Kidney |
Thyroid gland |
|||
|
Control rats |
Diabetic rats |
Control rats |
Diabetic rats |
Control rats |
Diabetic rats |
|
0.01 |
0.75 |
0.06 |
0.62 |
0.12 |
0.62 |
|
0.02 |
0.76 |
0.04 |
0.74 |
0.13 |
0.59 |
|
0.02 |
0.77 |
0.07 |
0.83 |
0.14 |
0.70 |
|
0.03 |
0.82 |
0.07 |
0.56 |
0.09 |
0.69 |
|
0.04 |
0.79 |
0.05 |
0.70 |
0.10 |
0.63 |
Effects of Metformin and dilute AHA oil on levels of expression of iNOS
Table 5 illustrates that the rats’ thyroid glands, livers, and kidneys all had considerably lower levels of iNOS expression (p < 0.05) after receiving both the Metformin and diluted AHA oil treatments.
Table 5: Results of analysis of differences in levels of iNOS expression between rats of the control group and those receiving Metformin and dilute AHA oil treatments.
|
iNOS Expression |
||||||||
|
Liver |
Kidney |
Thyroid gland |
||||||
|
Diabetic |
Metformin |
Dilute |
Diabetic |
Metformin |
Dilute |
Diabetic |
Metformin |
Dilute |
|
0.75* |
0.19** |
0.23** |
0.62* |
0.22** |
0.28** |
0.62* |
0.41** |
0.50** |
|
0.76* |
0.20** |
0.23** |
0.74* |
0.20** |
0.25** |
0.59* |
0.39** |
0.49** |
|
0.77* |
0.18** |
0.22** |
0.83* |
0.23** |
0.26** |
0.70* |
0.30** |
0.51** |
|
0.82* |
0.17** |
0.24** |
0.56* |
0.15** |
0.24** |
0.69* |
0.40** |
0.48** |
|
0.79* |
0.16** |
0.25** |
0.70* |
0.18** |
0.23** |
0.63* |
0.42** |
0.52** |
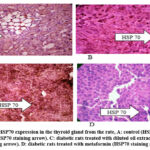 |
Figure 1: Levels of HSP70 expression in the thyroid gland from the rate, A: control (HSP70 staining arrow). B: diabetic rats (HSP70 staining arrow). |
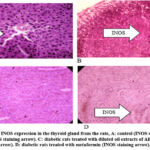 |
Figure 2: Levels of INOS expression in the thyroid gland from the rate, A: control (INOS staining arrow). B: diabetic rats (INOS staining arrow). |
Discussion
The thyroid is one of the major endocrine glands in the body. It affects how quickly the body utilizes energy, makes proteins, and influences its response to other hormones 21. With the production of thyroid hormones T4 and T3, it aids in these processes. The growth and operation of several other bodily systems are impacted by these, which also regulate metabolism. Tyrosine and iodine are used to make T3 and T422. The thyroid gland also generates calcitonin, which is a hormone that regulates calcium levels. The hypothalamus and pituitary glands regulate thyroid function23. TSH secreted by the anterior pituitary, which is produced by TRH release by hypothalamus, controls the synthesis of T4 and T3. In a negative feedback loop, the thyroid and thyrotropes produce less TSH when T4 levels are high and vice versa24.
Thyroid disorders are two to three times more common in patients with diabetes than in non-diabetic individuals. It also increases with age and is significantly impacted by autoimmune diabetes mellitus. In addition, they were substantially greater in female patients than in male patients. If a thyroid disease is linked to deterioration functions, its clinical significance, especially for diabetic individuals, increases dramatically. This is because declining functions consistently lead to several issues related to metabolic compensation of diabetes25. The two most dangerous outcomes of thyrotoxicosis are the development of potentially fatal ketoacidosis and an increased frequency of hypoglycemia in hypothyroidism. Research has demonstrated correlations between DM and several endocrine disorders, including gonadal dysfunction26. It has additionally been demonstrated that there are physiological and molecular connections between insulin and how iodothyronines and insulin affect the metabolism of lipids, proteins, and carbohydrates27. Triiodothyronine and tetraiodothynine are two thyroid hormones that serve as insulin antagonists and indirectly enhance insulin activity. T3 and T4 levels are often reduced in people and experimental animals with diabetes26,27.Diabetes causes alterations in hypothalamic thyrotropin-releasing hormone (TRH) excretion and pituitary thyrotropin (thyroid-stimulating hormone (TSH)) release and has direct effect on the thyroid gland28. Serum T3 concentrations are known to decline in diabetic patients, possibly due to inhibition of peripheral 5´-deiodinase enzyme activity, which catalyzes conversion of T4 to T3.
For thousands of years, conventional medicine has used natural therapies to maintain the robust health of the endocrine system, promoting and regulating optimal hormone production and thyroid function29. Several published clinical trials have confirmed the efficacy of various herbs in strengthening the immune system and a healthy thyroid function. Thyroid hormones are required for metabolic homeostasis, and impaired thyroid function can be a serious consequence of diabetes30. Thyroid function is mostly determined by serum T3 and T4 levels28,30. In diabetic mice, the level of T3 decreased was lower than that in the control mice. The T3/T4 ratio in the diabetic mice significantly decreased, according to these findings. Abnormal T3 levels are a sign of reduced microsomal ability to convert T4 to T3, most likely due to the emergence of oxidative conditions in many systemic non-thyroid disorders24,28. Therefore, we conclude that liver damage may be the mechanism underlying the observed decrease in T3 expression in diabetic mice. Diabetes suppresses thyroid hormones (T3 and T4) and serum insulin levels that regulate the basal metabolic rate31.
Diabetic rats administered with dilute AHE oil or Metformin had substantially decreased T3, T4, and TSH levels compared to the control group (p < 0.05). Current research suggests that the diluted AHE oil has a moderating function during endocrine system disruptions. Related findings were reported in the literature study: oral treatment of A. herba-alba significantly increased thyroid blood levels of T3 and TSH in the protective group compared to the control group. On the other hand, it was revealed that AHA extract had a protective impact on serum T4 levels like that of the control. A. herba-alba includes several phytochemicals, including phenols and flavonoids, which are known to have a variety of pharmacological properties 37. A similar role can be argued for Metformin in view of the study findings. While the exact reasons of thyroid dysfunction in diabetes mellitus are yet unclear, several elements of thyroid function can be directly impacted by metabolic changes brought on by diabetes or by insulin deficiency28,31.
Our study showed that the single dose of alloxan was effective in inducing DM in the early stages of type 2 DM. Serum T3 and T4 levels dropped in short-term diabetic rats. Similar results were found in literature32. This demonstrates a shift in the variables that determine reduced thyroid hormone production during the early stages of insulinopeinia33. Short-term insulinopeinia in diabetic mice cannot be attributed to decreased TSH activation of the thyroid gland, according to researchers 33, 34. However, dilute AHA oil or Metformin treatment independently returned blood T3 levels to normal levels, which appears to be related to its antioxidant activity. It is possible that the effect of the extract on raising thyroid hormone concentrations reflects its influence on increasing insulin secretion. Insulin stimulates hepatic T4/T3 conversion and improves the synthetic capacity of thyroid cells. Hence, the present results suggest a role for dilute AHE oil or metformin. As a result, the effect of dilute AHE oil on the increase in the level of hormones may indicate the influence on insulin secretion. Our findings indicate that dilute AHE oil participates in the peripheral control of thyroid function and may stimulate or enhance thyroid roles. However, further research is needed into the processes and modes of activity.
The results of this investigation demonstrated that diabetes significantly reduced Hsp70 expression in both the rat liver and kidney (p < 0.05). This conclusion is consistent with earlier investigations that reveal a limited impact of HSP70. This conclusion is consistent with earlier investigations that reveal a limited impact of HSP7035. We believe that diabetes may mediate its adverse effects by lowering the expression of Hsp70.
Our results indicate that treatment with dilute AHA oil and Metformin substantially (p < 0.05) raised the expression of Hsp70 in the livers, kidneys, and thyroid glands of rats. This finding may explain the positive changes embodied in lowered glucose levels and other associated chemicals, such as lipid profile. We believe that both treatments could restore the normal functions associated with Hsp70 and reverse some of the passive effects of diabetes. In addition, iNOS expression was significantly increased (p < 0.05) because of induced diabetes in the liver, kidney, and thyroid gland. However, its expression was significantly decreased (p < 0.05) by both treatments.
According to a research study, NO is involved in several critical body processes, including blood pressure regulation, brain neurotransmitter function, and immune system defense against viruses and bacteria34. But NO may also be produced excessively, or mis regulated, which adds to the etiology of several illnesses, including diabetes34.
Conclusion
The outcomes of the current research indicate that the diluted AHE oil stimulates or enhances thyroid activity, and they also point to a potential role for the oil in peripheral thyroid control. From a molecular point of view, Hsp70 and iNOS seem to act in a counteracting mechanism in both disease and treatment. Therefore, these molecules are good targets for therapeutic and clinical applications36.
Data availability
All data generated or analyzed during this study are included in this article.
Acknowledgement
None
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
No funding agency has provided support for this research.
References
- Bora KS, Sharma A. The genus Artemisia: a comprehensive review. Pharmaceutical biology. 2011 Jan 1;49(1):101-9.
CrossRef - Alshehri MA, Panneerselvam C, Murugan K, Trivedi S, Mahyoub JA, Maggi F, Sut S, Dall’Acqua S, Canale A, Benelli G. The desert wormwood (Artemisia herba-alba)–From Arabian folk medicine to a source of green and effective nanoinsecticides against mosquito vectors. Journal of photochemistry and photobiology B: biology. 2018 Mar 1;180:225-34.
CrossRef - Sayyah M, Nadjafnia L, Kamalinejad M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. Journal of ethnopharmacology. 2004 Oct 1;94(2-3):283-7.
CrossRef - 4Miño JH, Moscatelli V, Acevedo C, Ferraro G. Psychopharmacological effects of Artemisia copa aqueous extract in mice. Pharmaceutical Biology. 2010 Dec 1;48(12):1392-6.
CrossRef - Mahboubi M. Artemisia sieberi Besser essential oil and treatment of fungal infections. Biomedicine & pharmacotherapy. 2017 May 1; 89:1422-30.
CrossRef - Mohammed HA, Qureshi KA, Ali HM, Al-Omar MS, Khan O, Mohammed SA. Bio-evaluation of the wound healing activity of Artemisia judaica L. as part of the plant’s use in traditional medicine; phytochemical, antioxidant, anti-inflammatory, and antibiofilm properties of the plant’s essential oils. Antioxidants. 2022 Feb 8;11(2):332.
CrossRef - Afifi-Yazar FU, Kasabri V, Abu-Dahab R. Medicinal plants from Jordan in the treatment of cancer: traditional uses vs. in vitro and in vivo evaluations–Part 1. Planta medica. 2011 Jul;77(11):1203-9.
CrossRef - Al-Mazari, I. S., Alsarhan, A., Amawi, K. F., Deeb, A. S. A., Al-Emerieen, A. A., & Alkhatib, A. J. Compound expression of inducible nitric oxide synthase (inos) and p53 in prostate tissue of diabetic rats. (2021).
- Toda N, Kishioka S, Hatano Y, Toda H, Warner DS, Warner MA. Modulation of opioid actions by nitric oxide signaling. The Journal of the American Society of Anesthesiologists. 2009 Jan 1;110(1):166-81.
CrossRef - Garme Y, Moudi M, Saravani R, Galavi H. Nitric oxide synthase 2 polymorphisms (rs2779248T/C and rs1137933C/T) and the risk of Type 2 diabetes in Zahedan, Southeastern Iran. Iranian Journal of Public Health. 2018 Nov;47(11):1734.
- Mulyani WR, Sanjiwani MI, Sandra, Prabawa IP, Lestari AA, Wihandani DM, Suastika K, Saraswati MR, Bhargah A, Manuaba IB. Chaperone-based therapeutic target innovation: Heat shock protein 70 (HSP70) for Type 2 diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity. 2020 Feb 27:559-68.
CrossRef - Malyshev I, Malyshev I. HSP70 in Damaged Cells. Immunity, Tumors and Aging: The Role of HSP70. 2013:31-45.
CrossRef - Krause M, Bock PM, Takahashi HK, Homem De Bittencourt Jr PI, Newsholme P. The regulatory roles of NADPH oxidase, intra-and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clinical Science. 2015 Jun 1;128(11):789-803.
CrossRef - Shamsi MM, Mahdavi M, Quinn LS, Gharakhanlou R, Isanegad A. Effect of resistance exercise training on expression of Hsp70 and inflammatory cytokines in skeletal muscle and adipose tissue of STZ-induced diabetic rats. Cell stress and chaperones. 2016 Sep 1;21(5):783-91.
CrossRef - Hamann I, Seidlova-Wuttke D, Wuttke W, Köhrle J. Effects of isoflavonoids and other plant-derived compounds on the hypothalamus–pituitary–thyroid hormone axis. Maturitas. 2006 Nov 1;55:S14-25.
CrossRef - 16Mohamed AE, Abdel-Aziz AF, El-Sherbiny EM, Mors R. Anti-diabetic effect of Aloe vera juice and evaluation of thyroid function in female diabetic rats. Bioscience Research. 2009 Jan 1;6(1):28-34.
- Irshaid F, Mansi K, Bani-Khaled A, Aburjia T. Hepatoprotetive, cardioprotective and nephroprotective actions of essential oil extract of Artemisia sieberi in alloxan induced diabetic rats. Iranian journal of pharmaceutical research: IJPR. 2012;11(4):1227.
- A. khaled S. Bani-khaled, K. Mansi, and T. Aburjai, Effects of Oral Administration of Volatile Oil Extract of Artemisia Sieberi (A. Herba Alba) on the Activity of Hypothalamic Pituitary Thyroid (HPT) Axis in Diabetic Induced Rats. Al Al-Bayt University, 2000.
- Al-khatib A. Co-expression of iNOS and HSP70 in diabetes type 1 makes a rational hypothesis to explain the diabetic neuropathy. European scientific journal. 2013 Jan 28;9(3)..
- Alsarhan A, Mansi K, Aburjai T, Al-Khatib A, Alzweiri M, Sultana N. Hsp70 and iNOS biomarkers in evaluating the healing properties of Rubia tinctorum. European Scientific Journal. 2013 May 1;9(15):1857-7881.
- Pitt-Rivers R, Tata JR. The thyroid hormones. Elsevier; 2013 Oct 22.
- Wróblewski M, Wróblewska J, Nuszkiewicz J, Pawłowska M, Wesołowski R, Woźniak A. The role of selected trace elements in oxidoreductive homeostasis in patients with thyroid diseases. International Journal of Molecular Sciences. 2023 Mar 2;24(5):4840.
CrossRef - Sai Kumar BA. Hormonal regulation of metabolism, water, and minerals. InTextbook of Veterinary Physiology 2023 ,Sep 1 (pp. 391-415).
CrossRef - L. H. Duntas, “Environmental Endocrinology and the Hypothalamus-Pituitary-Thyroid Axis,” in Environmental Endocrinology and Endocrine Disruptors: Endocrine and Endocrine-targeted Actions and Related Human Diseases, Springer, 2023, pp. 1–19.
CrossRef - Grubišić B, Švitek L, Ormanac K, Sabo D, Mihaljević I, Bilić-Ćurčić I, Kolarić TO. Molecular Mechanisms Responsible for Diabetogenic Effects of COVID-19 Infection—Induction of Autoimmune Dysregulation and Metabolic Disturbances. International journal of molecular sciences. 2023 Jul 18;24(14):11576.
CrossRef - Krentz AJ. Classic endocrine disorders: implications for cardiovascular disease. InCardiovascular Endocrinology and Metabolism 2023 Jan 1 (pp. 233-270).
CrossRef - La Fauci D, Bionda A, Liotta L, Crepaldi P, Chiofalo V, Lopreiato V, Fazio E. Effects of pregnancy and lactation on thyroid hormones, insulin, and metabolic blood parameters of modicana dairy cows. Large Animal Review. 2023 Feb 6;29(1):1-7.
- KUTLU Z, KAMACI AD. The Relationship Between Euthyroid, Hyperthyroid, Hypothyroid, and Type 2 Diabetes. Pharmata. 2023 Oct 9;3(4):105-11.
CrossRef - Khan KM, Hameed S, Shamim S. Natural products to cure bad breath. Pharmacological Studies in Natural Oral Care. 2023 Oct 3:217-52.
CrossRef - Macvanin MT, Gluvic Z, Zafirovic S, Gao X, Essack M, Isenovic ER. The protective role of nutritional antioxidants against oxidative stress in thyroid disorders. Frontiers in Endocrinology. 2023 Jan 4;13:1092837.
CrossRef - Le Moli R, Malandrino P, Russo M, Tumino D, Piticchio T, Naselli A, Rapicavoli V, Belfiore A, Frasca F. Levothyroxine therapy, calculated deiodinases activity and basal metabolic rate in obese or nonobese patients after total thyroidectomy for differentiated thyroid cancer, results of a retrospective observational study. Endocrinology, Diabetes & Metabolism. 2023 Mar;6(2):e406.
CrossRef - Shi H, Korejo NA, Kamboh AA, Korejo RA, Shi F. Effects of hyperthyroidism and diabetes mellitus on spermatogenesis in peri-and post-pubertal mice. Frontiers in Endocrinology. 2023 Aug 15;14 P:1191571.
CrossRef - Nascimento-Saba CC, Breitenbach MM, Rosenthal D. Pituitary-thyroid axis in short-and long-term experimental diabetes mellitus. Brazilian journal of medical and biological research. 1997;30:269-74.
CrossRef - Yuen KC, Johannsson G, Ho KK, Miller BS, Bergada I, Rogol AD. Diagnosis and testing for growth hormone deficiency across the ages: a global view of the accuracy, caveats, and cut-offs for diagnosis. Endocrine Connections. 2023 Jul 1;12(7).
CrossRef - Smolka MB, Zoppi CC, Alves AA, Silveira LR, Marangoni S, Pereira-Da-Silva L, Novello JC, Macedo DV. HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2000 Nov 1;279(5):R1539-45.
CrossRef - Barcellos LF, Ramsay PP, Caillier SJ, Sawcer S, Haines J, Schmidt S, Pericak-Vance M, Compston DA, Gabatto P, Hauser SL, Oksenberg JR. Genetic variation in nitric oxide synthase 2A (NOS2A) and risk for multiple sclerosis. Genes & Immunity. 2008 Sep;9(6):493-500.
CrossRef - El-Sawi, N.M., El-Hady, O.M., El-Aref, A.H. and Hefny Gad, M.,. Estimation of the Efficacy of Oral Administration of Egyptian Artemisia herba-alba Extract in Combating Thyroid Dysfunction in Diabetic Male Rats. Sohag Journal of Sciences, 9(3), pp.234-240, 2024
CrossRef







