R. Karthikeyan 1* , G. Haema 1, V. Vijayan 1
, G. Haema 1, V. Vijayan 1 , V. Alamelu1
, V. Alamelu1 , M. Sakthiganapathy 1, S.Shanmuganathan1
, M. Sakthiganapathy 1, S.Shanmuganathan1 , Binoy Varghes Cheriyan3
, Binoy Varghes Cheriyan3 , R. Saravanan2
, R. Saravanan2  and P. Vijayarajkumar4
and P. Vijayarajkumar4
1School of Pharmacy, Sri Balaji Vidyapeeth, SBV Campus, Pillayarkuppam, Puducherry, India
2 Faculty of Pharmacy, Bharath Institute of Higher Education and Research, India
3Saveetha College of Pharmacy, Saveetha Institute of Medical and Technical Sciences, Chennai, India.
4Faculty of Pharmaceutical Sciences , UCSI University,UCSI Heights, 56000 Kuala Lumpur, Malaysia
Corresponding Author E-mail: professorrkn @gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2913
Abstract
Silver nanoparticles were combined with Ocimum Tenuiflorum L. green and purple (Krishna Tulasi) assortments using a single advance that was eco-friendly. Ultraviolet-Visible spectrophotometer, Fourier transform Infra-red, X-Ray Diffraction, Scanning Electron Microscope (SEM), and Energy Dispersive X-ray spectroscope was used to describe the particle nature of integrated AgNps (EDX). A variety of modifications confirmed the nanoparticle arrangement and UV-Visible spectroscopy was used to quantify the Surface Plasmon retention band. In the FTIR, the presence of the aromatic ring's C-H vibration is comparable to the presence of flavonoids and carbonyl gathering in the C-O stretch vibration. The inserted silver nanoparticles can clearly be seen under an SEM microscope, and the particles are estimated to be between l and 100 nm in size. The Energy Dispersive X-ray spectra demonstrate the nanomaterial’s quality. Union is traditional and viewed as proficient as far as response time as well as practicality. The combined nanoparticles were exposed to Anti-microbial and Anti proliferative investigations by in-vitro strategies and showed a portion of the subordinate movement against specific microbes and cancer cells.
Keywords
Anti-bacterial; Anti-cancer study; Characterization; Ocimum tenuiflorum; Particle size; Silver nanoparticles; Zeta potential
Download this article as:| Copy the following to cite this article: Karthikeyan R, Haema G, Vijayan V, Alamelu V, Sakthiganapathy M, Shanmuganathan S, Cheriyan B. V, Saravanan R, Vijayarajkumar P. In-vitro Anti-bacterial and Anti-cancer activity of Ocimum Tenuiflorum L. leaf Extract Induced Silver Nanoparticles – A study of Characterization Cum Evaluation. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Karthikeyan R, Haema G, Vijayan V, Alamelu V, Sakthiganapathy M, Shanmuganathan S, Cheriyan B. V, Saravanan R, Vijayarajkumar P. In-vitro Anti-bacterial and Anti-cancer activity of Ocimum Tenuiflorum L. leaf Extract Induced Silver Nanoparticles – A study of Characterization Cum Evaluation. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4aCk7wc |
Introduction
The development of nanotechnology during the 1980s and its ascent to ubiquity in the mid-2000s, with wide business applications in various areas, have been seen over the past 30 years. Bio compatible metal nanoparticles assume a significant part in biomedical applications.1,2 Silver awards have been important for the helpful field since the days of yore because of their phenomenal therapeutic capacities. Silver particles and silver-based compounds have been utilized in the treatment and the board of various afflictions because of their wide range of anti-bacterial and helpful qualities.3 In any case, innovative progressions and more prominent information on silver’s strategy for infection counteraction through antimicrobial exercises have made it ready for its use in nanomedicine. For the effective combination of silver nanoparticles, an assortment of systems and techniques has advanced, including physical, substance, and organic strategies. The utilization of plant concentrate is a crucial step for nanoparticle production. Silver nanoparticles were created by reacting fluid AgNO3 with a watery concentrate of the plant.4 Various reports are available on the biogenesis of silver nanoparticles using several plant extracts, particularly neem leaf broth (Azadirachta indica), Pelargonium graveolens, geranium leaves, Medicago sativa (Alfalfa), Aloe vera, Emblica officinalis (Amla, Indian Gooseberry) and few microorganisms.5 In the current investigation, we report the simple combination of silver nanoparticles and its utilization would be harmless to the ecosystem, methodology including the In-Situ reductions of Ag by Ocimum tenuiflorum L. leaf (figure 1) and the assessment of their Anti-microbial action and Anti-cancer activity.
 |
Figure 1: Ocimum tenuiflorum L Purple (Krishna Tulasi). |
Materials and methods
Chemicals
Silver nitrate, M T T (3 -[4, 5-dimethylthiazol-2-yl] -2,5-diphenyl tetrazolium bromide, USA. Acridine orange (Sigma-Aldrich, Bangalore, India). Bacteriological media were bought from Hi-Media Laboratories, India, and any remaining media arrangements were done in two-fold refined Milli Q water.
Preparation of plant extract
The leaves of Ocimum Tenuiflorum (1 kg) were accumulated from the professional flowerbed of Vignan College of Pharmacy. A plant taxonomist from the Department of Botany and Microbiology, Acharya Nagarjuna University in Andhra Pradesh confirmed the case of leaf and bud. The assembled leaves were washed and dried and powdered by using a blender processor. Around 100gm of the powdered leaf was used in hot percolation extraction (Soxhlet) by including water as solvent.
Green biosynthesis of Silver Nanoparticles
In the single-step process of the green mixture, 5 ml of leaf extract was mixed with 95 ml of 1 mM liquid AgNO3, and heated at 80°C for 5 minutes, and the assortment colour change was observed. The Silver nanoparticles scheme as such gained was disinfected, and reiterated 15 minutes of centri fugation at 10000 rpm. To the obtained dried silver nanoparticles, the supernatant was transferred to an optimum dry glass beaker for more particle settlement, and repeated centrifugation was completed using a cooling microfuge. An incubation facility was used to dry the model. The particles were employed to provide more detail to the scene.6
Characterization of silver Nanoparticles
UV Spectral Study
Initially, orchestrated silver nanoparticles were described by placing a minor aliquot part of the test in an Ultraviolet-Visible spectrophotometer and obtaining assimilation spectra at 300 – 700 nm with a Spectrophotometer (Shimadzu UV-1800).
FTIR spectroscopy
Fourier-transform infrared spectroscopy Bruker model was utilized for the examination of the diminished silver. The range was kept in the mid-IR area of 400-4000cm-1 with 16 sweep speeds, utilizing the constricted complete coefficient of reflection (ATR) procedure.
SEM analysis
The examination was carried out using a Zeiss EV-18 scanning electron microscope (SEM). By employing a minor quantity of the sample on the lattice, a flimsy picture of the sample was created. The image on the SEM matrix was then given five minutes to dry under a mercury lamp.
Energy Dispersive X-ray analysis
The Energy Dispersive X-ray analysis, also known as EDX, was performed on a Zeiss EV-18 model. The elemental makeup of the sample can be determined from the peaks that are produced by EDX.
Particle size distribution
Using a particle size analyzer, we were able to determine the average particle size, as well as the size distribution and Polydispersity index (PDI), of the AgNPs that we produced.
XRD analysis
In order to determine the crystalline phase and material identification, XRD measurements of the reduced AgNPs that were done were recorded on an X-ray diffractometer (x’pert pan analytical) instrument that was running at a voltage of 40 kV and a current of 30 mA while exposed to Cu K (α) radiation. The samples were collected in airtight containers and maintained for further assessment.
Anti-bacterial activity of AgNPs
The Anti-microbial movement focused on microorganisms that cause infectious diseases. Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa were used as test organisms. Bacteria were grown in a supplement agar media. Next, the medium was autoclaved and transferred to Petri dishes for further testing. Different concentrations of 125 μl, 250 μl, and 500 μl of AgNPs were layered after the material was vaccinated with a newly developed test dye. After determining the zone of inhibition, anti-bacterial examination plates were brooded at 37°C for 24 hours past the hatching period.
Cell growth inhibition studies by the MTT assay
The cell lines were secured from NCCS (National Center for Cell Science, Pune) Hela cells were regularly kept within Rose Well Park Memorial Institute medium (RPMI), enhanced with 10% intensity inactivated fetal cow-like serum, penicillin/streptomycin (250U/ml), Gentamycin (100g/ml), and amphotericin B (1mg/ml) from Sigma Chemicals, USA. All cell societies were kept up at 370C in a humidified climate of 5% CO2. Cells were permitted to attain (90%) confluence north of 24 hours before use.7
Reagent Preparation
MTT Solution Preparation
Break down MTT in DPBS (Dulbecco’s Phosphate Buffered Saline, pH = 7.4) to 5 mg/ml. Channel disinfect the MTT arrangement through a 0.2 µ M channel into a clean, light safeguarded holder. Store the MTT arrangement, safeguarded from light, at 4°C for continuous use or at – 20°C for long-haul stockpiling.
Solubilization Solution
Choose a suitable safe holder in a ventilated fume hood to dissolve the following thing. Prepare 40% (vol/vol) dimethylformamide (DMF) by using 2% (vol/vol) glacial acetic acid along with 16% (wt/vol) sodium dodecyl sulfate (SDS) and adjusted to pH 4.7. Keep at room temperature to prevent the SDS from becoming precipitated. In the case that a precipitate forms, heat the mixture to 37 degrees Celsius and mix it to dissolve the SDS.
MTT Assay Protocol
Plan cells and test compounds are kept in 96-well titer plates containing 100 µl in each well and allow for reaction. Also, add 10 µl MTT Solution for every well to accomplish the last grouping of 0.45 mg/ml. Brood for 1 to 4 hours at 37°C add 100 µl of Solubilization solution for each well to disintegrate Formosan precious stones. Blend to guarantee total Solubilization. Record absorbance at 570 nm.
Statistical analysis
The analytical findings were determined in triplicate for accuracy. The results of all of the experiments are shown as the mean value accompanied by the standard error mean. The Origin programme (version 7.0383; Origin Lab Corporation, Northampton, Massachusetts 01060, United States) was utilized throughout each and every one of the statistical studies.
Results and discussion
Characterization of silver nanoparticles
Ultraviolet-Visible spectrophotometer analysis
The nanoparticles were first visualized using UV Spectroscopy, which showed to be an excellent instrument for learning nanoparticles. The color of O. tenuiflorum L. altered from red to brown as the extracts from leaves remained assorted with the fluid arrangement of the silver molecule complex. Purple (Figure 2.) shows the formation of the silver nanoparticles due to the excitement of the superficial plasma ambiances. A quartz glass cuvette with distilled water as the position was used to record the Ultraviolet-Visible Spectrum of Silver nanoparticles as a stretch component. The interaction between a 95 mL silver nitrate suspension and a 5 mL leaf removal was recorded at 90 degrees Celsius. O. tenuiflorum L. Ultraviolet-visible range retention is measured at 421 nm.
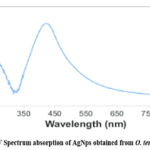 |
Figure 2: UV Spectrum absorption of AgNps obtained from O. tenuiflorum L. |
Fourier transfer Infra-red– Spectroscopy
The bands formed by together kinds are nearly undistinguishable, with minor changes in absorbed wavelengths and percentage transmittance. Figure 3 shows the FTIR range of silver nanoparticles from O. tenuiflorum L. Purple. The region of wavelengths at 3263 cm-1 is designated as the O-H extension that encompasses H-fortified alcohols and phenols. The carboxylic acid O-H stretching is assigned to the band 2928 cm-1. The region of wavelengths at 1603 cm-1 corresponds to the N-H twisting of essential amines. The groups at 1340 cm-1 are linked to the sweet-smelling ring structure’s C extending, while the top at 1369 cm-1 is linked to the sweet-smelling amine bunch’s C-N extending. Carboxylic acids are found in the 1224-1142 cm-1 range of the C extending of alcohols.
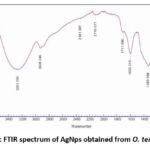 |
Figure 3: FTIR spectrum of AgNps obtained from O. tenuiflorum |
SEM Analysis
The scanning electron microscope pictures showed high-thickness silver nanoparticles mixed with the leaf extract, indicating positive silver nanostructure development. In O. tenuiflorum L. Purple, the SEM copy discloses the growth of the leaky surface with round nanoparticles and part textured round nanoparticles distinctly. They were effortlessly different, ranging from 30.56 to 82.62 nm for O. tenuiflorum L. Purple (Figure 4).
 |
Figure 4: SEM image of silver nanoparticles obtained from O. tenuiflorum L. |
Energy Dispersive X-ray Analysis
The EDX spectra reflect the sanctity of the substance and the full chemical structure of integrated silver nanoparticles. The EDX examination illustrates that 93.5 percent of the silver nanoparticle samples produced from O. tenuiflorum L. Purple (Figure 5) (Table 1) are present in the amalgamation at the time of analysis. It revealed a significant amount of silver, demonstrating the value of the included sample.
 |
Figure 5: Energy dispersive X-ray spectra of silver nanoparticles from O. tenuiflorum L. |
Table 1: Energy dispersive X-ray result of silver nanoparticles from O. tenuiflorum L
|
Metal |
Element Weight % |
Atomic % |
Error % |
|
|
AgL |
100.00 |
100.00 |
15.09 |
|
Particle size, Polydispersity, and Zeta potential analysis
The results show that the average diameter of the particles is 101 nm, and the Polydispersity index is 0.263. The generated AgNPs were found to be mono dispersed in their natural state, as shown by the average particle size and PDI. (Figure 6 and 7)
 |
Figure 6: Particle size of AgNP from the O.tenuiflorum.L. Leaves. |
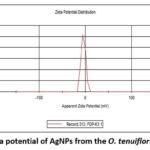 |
Figure 7: Zeta potential of AgNPs from the O. tenuiflorum L. Leaves. |
XRD analysis
The phase distribution, crystallinity, and purity of the newly synthesized AgNps were investigated with XRD analysis. The XRD patterns of AgNps extracted from O. tenuiflorum are displayed in Figure 8. It was determined, with reference to the typical XRD pattern of purified nanoparticles, that the nanoparticles were crystalline in character, had a cubical structure, and included no such contaminants.
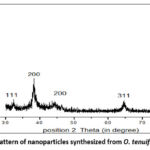 |
Figure 8: XRD pattern of nanoparticles synthesized from O. tenuiflorum L. leaves |
Antimicrobial activity of Ag NPs
In our study, the AgNPs orchestrated utilizing Tulsi extract had a huge anti-bacterial activity on the microorganisms we investigated. This is obvious by the upsides of the distance across the zone of hindrance got during the appraisal of antibacterial movement (Table 2). Figure 9 and 10 shows the zones of restraint of E. coli and B. subtilis against AgNPs, silver nitrate, Tetracycline, and DMSO as control. For both bacterial strains, no zone of restraint was noticed for the control arrangement. Bio-decreased silver nanoparticles showed significant development hindrance of two of the notable pathogenic bacterial species. Zones of 1.6 mm and 1.4 mm were noticed for E. coli and B. subtilis, individually at 500µg/ml.
Table 2: Determination of zone of inhibition of AgNPs against E. Coli and B. subtilis.
|
S.No |
Bacteria |
Zone of inhibition (mm) |
||
|
125µg/ml |
250 µg/ml |
500 µg/ml |
||
|
1. |
E. coli |
0.9 |
1.2 |
1.6 |
|
2. |
Bacillus subtilis |
0.5 |
0.9 |
1.4 |
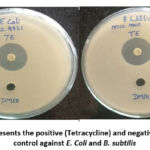 |
Figure 9: Represents the positive (Tetracycline) and negative(10%DMSO) |
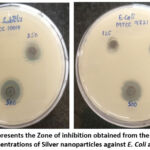 |
Figure 10: Represents the Zone of inhibition obtained from the treatment of different concentrations of Silver nanoparticles against E. Coli and B. subtilis |
Cytotoxicity study by MTT–based assay on Hela cell lines
The viability of HeLa cancer cells with O. tenuiflorum – AgNPs for 72 hrs was resolved to utilize the colorimetric MTT-based measure. The O. tenuiflorum AgNPs showed a portion subordinate action inside the fixation scope of 5 – 100 μg/ml (Fig.11). The O. tenuiflorum AgNPs showed the most extreme movement against HeLa and it was recorded as 1.22, 25.24,29.65,42.15, and 54.65 % at 100μg/ml individually.
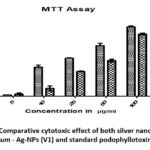 |
Figure 11: Comparative cytotoxic effect of both silver nanoparticles of O. tenuiflorum – Ag-NPs (V1) and standard podophyllotoxin AgNPs(V2). |
Discussion
Tulsi (purple) has been very much exploited, both usually and mechanically, for its microbiological potential which is an outcome of restorative oil parts. Anyway, there are relatively few examinations associated with organized silver nanoparticles. In the continuous audit, we have coordinated the leaf removal of O. tenuiflorum of silver nanoparticles. Nano silver has been genuinely used in a couple of utilization, including finding and treatment of cancer and as a drug carrier.8-11
Arranged silver nanoparticles were initially portrayed by taking a small aliquot of test into UV-Visible spectrophotometer, Fourier-transfer infrared spectroscopy (FTIR), Scanning electron microscope (SEM), Energy Dispersive X-shaft examination (EDX), and the commonplace size of the particles by atom size analyzer for the appreciation of nanoparticle which was joined AgNP from O. tenuiflorum leaves. The particles route blend of AgNP exhibited its characteristics like the size in the manometer, the Metal of silver and its perfection, and the Shape of the globe to inconsistent globe12
Depicted and cleaned AgNP were presented to antibacterial and in-vitro anticancer development. The development was clear that the nanoparticles which are procured from O. tenuiflorum leaves showed the part subordinate zone of restriction of microorganisms used in the survey. The outcomes of the continuous survey were similar to the previously reported studies.13-16
Followed with the anticancer survey was performed by the well-established procedure called MTT assessment, in which the HeLa cancer cell was used to certify the development and the audit showed the degree of cell downfall happened segment restrictively, the results of cancer cells (e.g., HeLa) are more indulgent in terrible charge than that of common cells. Thus, the ability to attract Ag+ particles by the layers of cancer cells is one of the factors that perhaps impact the practicality of nanoparticles in the disguise of cancer cells. It was moreover stated that the antibacterial and anticancer development might be of nano pore improvement and destruction of the cell divider by the zeta ability of AgNPs The eventual outcomes of the present are facilitated with another equivalent audit. Further, the survey leaves the degree of the arrangement of sensible estimations of structure and its evaluation. Furthermore, the toxicological profile of AgNPs in natural organs would be considered and optimized.
Conclusion
The conclusion of the study on silver nanoparticles synthesized from O. tenuiflorum leaves extract indicates promising anti-cancer activity against HeLa cell lines and effective anti-microbial properties against the tested microorganisms. Overall, the results of this study provide evidence supporting the potential biomedical applications of silver nanoparticles synthesized from O. tenuiflorum leaves extract. However, it is important to note that further research, including in vivo studies and toxicity evaluations, would be necessary before considering their practical application in cancer therapy or as antimicrobial agents in real-time application.
Acknowledgement
We thank Mr.Yamarthi venkateswara rao, Assistant Professor, Vignan pharmacy college, Guntur,Andhra Pradesh for helped in doing Nanoparticles characterization work.
Conflict of Interest
There is no conflict of interest
Funding Sources
There are no funding Sources
References
- Bilal M, Rasheed T, Iqbal HMN, Hu H, Zhang X. Silver nanoparticles: Biosynthesis and antimicrobial potentialities. Int. J. Pharmacol., 2017;13 : 832–845.
CrossRef - Sharma A et al. Algae as crucial organisms in advancing nanotechnology: a systematic review. J . Appl Phycol., 2015;28 : 1759–1774.
CrossRef - Chaloupka K, Malam Y, Seifalian AM. Nano silver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol., 2010;28: 580–588.
CrossRef - Mohanpuria P, Rana NK,Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res.,2008; 10: 507–517.
CrossRef - Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, and Thangamani Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their anti-plasmodial activities. Asian Pac J Trop Biomed. 2012 Jul; 2(7): 574–580.
CrossRef - Arthi C and Hikku G S. Biological routes for the synthesis of Silver nanoparticles. Chettinad Health City Medical Journal., 2021; 10(1): 42-48.
CrossRef - Gurunathan S, Han JW, Kim ES, Park JH, Kim JH. Reduction of graphene oxide by resveratrol: A novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int.J.Nanomed., 2015:2951-2969.
CrossRef - Sapsford KE, Tyner KM, Dair BJ, Deschamps JR, Medintz IL. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem., 2011; 83:4453–4488.
CrossRef - Patil MP, Singh RD, Koli PB, Patil KT, Jagdale BS, Tipare AR, Kim GD. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb Pathog., 2018; 121:184-189.
CrossRef - Mosmann T, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods.,2017; 65: 55–63.
CrossRef - Liu JY, Wang ZY, Liu FD, Kane A.B., Hurt R.H. Chemical transformations of nanosilver in biological environments. ACS Nano., 2012;6:9887–9899.
CrossRef - Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on small medicine: The state of nanomedicine products approved for use or in clinical trials. Nanomedicine. 2013;9:1–14.
CrossRef - Ge LP, Li QT, Wang M, Yang JO, Li XJ, Xing MMQ. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014;9:2399–240.
CrossRef - Saravanan RD, Haneesha CH, Gowtham A, Hanuma S. Formulation and Evaluation of Nanoparticles Loaded with Lamivudine. Euro . Jour. Bio. Pharm. Sci, 2017; 4(9): 611-616.
- Namratha N, Monica PV. Synthesis of silver nanoparticles using Azadirachta indica (Neem) extract and usage in water purification. Asian J Pharm Tech., 2013; 3: 170–174.
- Rout Y, Behera S, Ojha AK, Nayak PL. Green synthesis of silver nanoparticles using Ocimum sanctum (Tulsi) and study of their antibacterial and anti-fungal activities. J Microbiol Antimicro., 2012; 4: 103–109.
CrossRef - Faezeh BG, Shabnam S. Evaluation of anti-bacterial (Escherichia coli and Staphylococcus aureus) and Anticancer Effects of Silver Nanoparticles Synthesized by Melissa officinalis L. Extract on Several Cancer Cells (A549, MCF-7, and HeLa). International Journal of Advanced Biological and Biomedical Research., 2022; 10(1): 57-71.
- Narayanaswamy K, Athimoolam R, and Ayyavoo J. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria ternatea and Solanum nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. Journal of Nanoscience, 2015: 1-8.
CrossRef







