Manuscript accepted on :23-04-2024
Published online on: 23-05-2024
Plagiarism Check: Yes
Reviewed by: Dr. Ameer Ali Shakr Hadi
Second Review by: Dr. Ahmed Salah
Final Approval by: Dr. Ian James Martin
Lirim Mustafa1 , Hilmi Islami2
, Hilmi Islami2 , Fitim Alidema3
, Fitim Alidema3 , Pellumb Islami4, Arta Dauti4
, Pellumb Islami4, Arta Dauti4 , Fellenza Abazi1 and Mirlinda Havolli1*
, Fellenza Abazi1 and Mirlinda Havolli1*
1Iliria College, Department of Health Management, Prishtina, Republic of Kosova
2Department of Pharmacology, Faculty of Medicine, University of Prishtina, Prishtina, Republic of Kosova
3Medical Sciences Faculty, UBT Higher Education Institution, Prishtina, Republic of Kosova
4University Clinical Centre of Kosovo-Prishtina, Republic of Kosova
Corresponding Author E-mail: mirlinda.havolli.pr@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2923
Abstract
Background: The interaction between adenosine receptor blockers and anticholinergic substances in the treatment of bronchial asthma is an area of interest. The efficacy of such combinations in managing bronchial asthma and bronchial hypersensitivity needs to be explored further. Understanding lung function parameters such as airway resistance and intrathoracic gas volume is crucial for evaluating the effects of these medications. Objective: This study aimed to investigate the effect of combining the adenosine receptor blocker, bamifylline, with the anticholinergic substance, ipratropium bromide spray, in patients with bronchial asthma. Specifically, the study sought to assess changes in lung function parameters, including airway resistance and intrathoracic gas volume, after administering ipratropium bromide alone and in combination with bamifylline. Methods: Sixteen patients with bronchial asthma were enrolled in the study. Lung function was evaluated using body plethysmography, with measurements of airway resistance (Raw), intrathoracic gas volume (ITGV), airway specific resistance (SRaw), and airway specific conductance (SGaw). Patients initially received ipratropium bromide inhalation (2 inhalations x 20µg), followed by Raw and ITGV measurements at intervals (5, 30, 60, and 120 minutes). Subsequently, patients received bamifylline (2 x 600 mg) daily for seven days at home. On the eighth day, they were administered ipratropium bromide spray (2 inhalations x 20µg), and lung function parameters were assessed similarly. Results: Administration of ipratropium bromide alone led to a significant reduction in airway resistance (p<0.05). However, the combination of ipratropium bromide with bamifylline did not significantly enhance the effects of adenosine receptor blockade (p<0.05). Specifically, there were no significant changes in Raw, ITGV, SRaw, or SGaw after combining ipratropium bromide with bamifylline. Conclusion: The study findings suggest that the addition of anticholinergic substances did not potentiate the action of adenosine receptor blockers in patients with bronchial asthma. Therefore, the anti-inflammatory effects of xanthines, such as bamifylline, were not augmented by anticholinergic substances in this study. These results highlight the need for further research to explore alternative therapeutic approaches in the management of bronchial asthma.
Keywords
Atrovent (ipratropium bromide); Bronchial asthma; Bamifix (bamifylline)
Download this article as:| Copy the following to cite this article: Mustafa L, Islami H, Alidem F, Islami P, Dauti A, Abazi F, Havolli M. Effect of Cholinergic Receptor Antagonists on the Potentiation of the Effect of Adenosine Receptor Blockers in People with Bronchial Asthma. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Mustafa L, Islami H, Alidem F, Islami P, Dauti A, Abazi F, Havolli M. Effect of Cholinergic Receptor Antagonists on the Potentiation of the Effect of Adenosine Receptor Blockers in People with Bronchial Asthma. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4dTFH1c |
Introduction
Asthma, including COPD (chronic obstructive pulmonary disease) as inflammatory airway chronic disorders, which attack millions of people and result in a significant economic burden on the health care system. Although these disorders have unique and distinctive features, they manifest continuous inflammation of the airways and remodeling of the wall of the airways that can lead to progressive loss of lung function 1.
Asthma is characterized by progressive and irreversible obstruction of the airways, mucus hypersecretion, and infiltration of neutrophils and macrophages in the lung 2. The reaction that regulated the type of these chronic illness are unspecified. Adenosine as signaling nucleoside is produced in hypoxic condition in lungs which are inflamed, suggesting that they may play a regulatory role in chronic lung illness 3. Adenosine is a purine nucleoside base, commonly known asmolecule of adenosine triphosphate, or ATP. The use of adenosine as a pharmacological medicine function through receptors called adenosine purinergic receptors 4. Adenosine receptors have four subtypes: A1AR, A2AAR, A2BAR, and A3AR. These subtypes are targets for the creation of novel asthma medications 5.
There is a range of documentation to maintenance the idea that adenosine has effect in asthma. Adenosine when it is inhaled produce bronchoconstriction in patients with asthma; however not in non-asthmatics6. This reaction emerges to be mediated by activation of mast cell, as it can have obstructed by antihistamines and mast cell activation suppressors. Aspirated adenosine causes discharge in bronchoalveolar liquid of mast cell mediators, along with histamine, tryptase and prostaglandin D2 7. Adenosine aspirated and adenosine 5-monophosphate and triphosphate are noted to produce bronchospasm in patients with asthma, very likely through the release of mast cell mediators and because the effect has not been determined with adenosine administrated intravenously, this indicate that bronchospastic impact is related with the way of the administration. Bronchospasm, which take place with adenosine monophosphate and triphosphate (inhaled) is also related with dyspnea progress 8.
The muscarinic receptor M3,the subclass of the cholinergic receptor is accountable for contracting bronchial smooth muscle. Despite the fact ipratropium and other similar substances stops all 5 subtypes of muscarinic receptors with same affinity, antagonism of the M3 receptor alone may have dilated effects. Bronchodilatation caused by ipratopium develops gradualy and is normally slighter intense than that caused by adrenergic agonists. Asthmatic patients in some causes may exhibit beneficial response that may take up to six hours 9. An acceptable response to ipratropium can be noticed in the patient with asthma who experience deterioration of psychogenic nature 10
This study aims to analyse the impact of cholinergic receptor antagonists (ipratropium bromide) in potentiating the effect of adenosine receptor blockers Bamifix (bamifylline) in patients diagnosed with bronchial asthma and COPD.
Material and Methods
Study design
The experimental procedures in this study received approval from the Clinical Ethics Committee of the University Clinical Center of Prishtina, with Protocol Number 502, dated March 22, 2019. The purpose of the examination was explained beforehand to each patient. Retrospective study, performed in 16 patients with bronchial asthma moderate. Average of disease duration was 10 ± 6 years (from 4-20 years). Average of their age was 40 ± 7 years (from 29 – 45 years), whilst average weight was 74 ± 7% (from 64 – 72%). The patients involved in the study were not admitted to hospitals; however, the tests were conducted in medical settings such as University Clinical Center. The anamnestic data and lung clinical and functional research were used to choose the individuals. There have been examinations, as shown in tables 1, 2, and diagram 1.
Study procedures
Using a minimum of 48 hours of prior research of bronchial reaction, patients have not received bronchodilator substance. The examinees were familiar with the way of functional analysis of the lungs. Patients have suffered from asthma with or without concomitant bronchitis. Each patient has previously been explained the purpose of the examination. Defined was lung activity at rest, which is composed of measurement of the airway resistance (Raw) and the volume of intrathoracic gas (ITGV). From obtained data, was calculated specific resistance and specific airway conductance:
SRaw = Raw x ITGV
SGaw = 1/SRaw
The bronchial response research in various substances was conducted by measuring Raw, and ITGV and SGaw and SRaw are calculated and more of very sensitive indicators of the airways; Medical Research Council 11.
On the first day is applied Atrovent-ipratropium bromide (2 inh. 20/µg) and measurements made (Raw, ITGV) after 5, 30, 60, 120 min. Afterwards, administered is Bamifix (2 times 600 mg) for 7 days at home. On the 8th day, the Raw and ITGV measurements were done again, and Atrovent (2 inh. 20/µg) was applied and again After 5, 30, 60, and 120 minutes, measurements were made using ITGV and Raw, and the airways’ Specific Resistance (SRaw) and Specific Conductance (SGaw) were computed.
According to certain theories, alterations in the respiratory system are not important, have nothing to do with the onset of bronchial asthma or other obstructive illnesses, and have nothing to do with the symptoms of allergies.
Statistical Analysis
The obtained data are grouped and examined. Utilizing statistical methods of the data involve the measurement of mean values (X), standard deviation (SD), standard error mean(SEM), as well as analyzing the significance of differences between groups of individuals receiving adenosine receptor blocker treatment and antagonist’s cholinergic receptors. The obtained results were analyzed utilizing a test (t-test). The statistical test ANOVA was applied to compare the groups.
Results and Discussion
Antagonists of cholinergic receptors (ipratropium bromide (2inh. x 20/μg), are applied on the 1stday and are examined changes in the respiratory system with body plethysmography. Registered was the important decrease of the airway’s resistance (p<0.05) and to the same patient then applied blockers of adenosine receptors (bamifylline 2 x 600 mg per os) at home for seven days in a row. On day 8, thepatient submitted for the examination of the respiratory system and again administered 1 tablet of bamifylline, and after 60 min measurements performed and again is applied ipratropium bromide 2 inh. x 20/μg, and once again are made the measurements of the respiratory system (Raw, ITGV). Based on the results obtained, there is no further decrease of the specific airway resistance (SRaw) (p<0.05) as shown in figure 1 and 2.
All constitutional data of patients are given in tables 1 and 2 and diagram 1.
Table 1: Overall characteristics of the studied patients.
|
n |
Age (years) |
Height (cm) |
Weight (kg) |
VC (L) |
FEV 1 (L) |
|
Vital capacity expressed in liters |
Enhanced expiratory volume in the first second, expressed in liters. |
||||
|
16 |
40 ± 7 |
170 ± 5.5 |
74 ± 7% |
3.75 ± 0.11 |
3.66 ± 0.23 |
Experimental group n = 16; X ± SEM.
Table 2: Characteristics of the participants’ body plethysmography in this investigation.
|
Group |
n |
Raw (kPa × s/L) |
ITGV (L) |
SRaw (kPa × s) |
SGaw (kPa × s) |
|
Volume of inthratoracic gas |
Specific airway resistance |
Specific airway conductance |
|||
|
Experimental |
16 |
1.13 ± 0.7 |
3.11 ± 0.5 |
3.5 ± 0.9 |
0.28 ± 0.11 |
Raw (kPa × s/l); Airway resistance (kilo Pascal/sec/liter)
 |
Diagram 1: Study flow chart. |
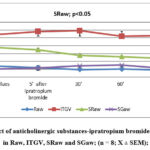 |
Figure 1: Effect of anticholinergic substances-ipratropium bromide (2inh x 20µg), in Raw, ITGV, SRaw and SGaw; (n = 8; X ± SEM); |
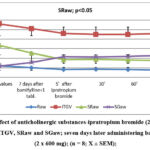 |
Figure 2: Effect of anticholinergic substances-ipratropium bromide (2inh x 20µg), in Raw, ITGV, SRaw and SGaw; |
Even though the majority of patients can effectively manage their asthma with the present treatments, many asthmatics continue to search for more potent treatments. Comprehending the variability of asthma also implies the necessity of creating novel treatments for specific forms of asthma.
This paper summarizes the current methods of treatment of asthma considering current knowledge of the pharmacology and signaling of adenosine receptor blockers in the treatment of asthma. It also addresses the debate around their use and new avenues for the development of asthma treatments. With this advancement, new treatment modalities can be employed to lessen the global rise in morbidity and death linked to chronic obstructive pulmonary disease and asthma.
The study included 16 patients with bronchial asthma. With the exame of the variables of the respiratory system of all patients, we found increased initial ventilator values such as the specific resistance (SRaw) of the control group treated with anticholinergic substances-ipratropium bromide and the experimental group treated with adenosine receptor blocker administered 7 days at home. On day 8 is assessed the permeability of the airways and again is administered 1 tablet bamifylline; after 60 min performed the Raw and ITGV measurements and is continued by the applying antagonist of cholinergic receptors-ipratropium bromide, and performed the Raw and ITGV measurements after 5, 30, 60, 120 min. There was a significant reduction in the airways’ specific SRaw resistance in both groups—the experimental group and the control group, respectively (p<0.05). Airway resistance did not change (p<0.05) even after 7 days of administration (2×600 mg) of adenosine receptor blockers and stimulation of adenosine receptors with anticholinergic substances. This confirms the fact that blockage of adenosine receptors has no synergist action with the anticholinergic substances and as such are ineffective if administered in combination 11-14.
By preventing muscarinic acetylcholine receptors from functioning, acetylcholine is blocked by both short- and long-acting antagonists, which reduce bronchoconstriction. 15.
Recently, tiotropium, a structural analogue of ipratropium, is authorized for the management of COPD and emphysema. Tiotropium has a high affinity for all muscarinic receptor subtypes, just like ipratropium, however it releases from these receptors considerably more slowly. 16. Tiotropium specifically detaches from muscarinic M3 receptors considerably more slowly than it does from muscarinic M2 receptors, according to linkage and function tests. Tiotropium’s strong muscarinic receptor affinity and its ability to detach very slowly from them allows the administration of only one dose per day. The ability to slowly detach from the receptor offers a potential benefit by restricting the capacity of elevated levels of endogenous acetylcholine agonists to overcome receptor blockage.
Theophylline belongs to the group of methylxanthines, which causes the decrease of the release of signals that promote inflammation, such as leukotriene and TNF-alpha, and also effectively decreases the inflammation by acting as a direct antagonist of the adenosine receptors specifically causing smooth muscle relaxation and bronchiole enlargement by reducing airway respiratory obstruction 17, whereas anti-inflammatory medicines (glucocorticoids) reduce activation and infiltration of lymphocytes, eosinophils, and mast cells in bronchialmucosa 16. Each of these medicines has a unique mechanism of action 18.
Adenosine, a purine nucleoside base also recognized as adenosine triphosphate (ATP), functions pharmacologically through adenosine purinergic receptors, including four subtypes: A1AR, A2AAR, A2BAR, and A3AR, which are targeted for the development of new asthma medications.
These receptors belong to the family of G protein-linked receptors and are expressed in a variety of cells including most immune cells, further implying adenosine’s function in regulating immune cell activity 19.
The biological response to adenosine is mediated by four receptors bound to a G protein. Adenosine A1 and A3 receptors attach to Gi/0, while adenosine A2A and A2B receptors attach to Gs, resulting in the activation of phospholipase C, through Gq/11. Furthermore, adenosine A1, A3, and A2B receptors can activate both K1 and Ca12 channels, whereas cAMP-independent intracellular pathways are also described20.
The activity of receptors of adenosine A1 increases in the smooth muscles and epithelium of asthmatics’ airways. In the tissues of the human respiratory tract and HBSMC, activation of receptors A1 AR causes effects such as hyperreactivity of the respiratory tract. Activation of A1 AR leads to increased expression of the mucus hypersecretory MUC2 gene in human airway epithelial cells. Moreover, pro-inflammatory effects are produced when A1 AR is activated in a variety of human cells 21-25.
Furthermore, evidence from research and clinical settings indicates that adenosine A1 AR receptors are a key target in asthma. In Europe, bamifylline, an A1 AR antagonist, is authorized for the management of asthma. Theophylline inhibits human phosphodiesterase enzymes at a therapeutic plasma level that is less than what would cause adenosine AR receptor antagonism, which is why it has anti-asthmatic benefits in humans 26. Substantial experimental data suggest that adenosine acts as an anti-inflammatory agent, to understand the manner of activation of the various ways of adenosine receptors in specific situations of the disease, will help with the administration of agonists and specific receptor antagonists in the treatment of various inflammatory disorders.
Because A2A receptor activation results in anti-inflammatory actions that open the door to asthma treatment, these receptors are particularly interesting. There have been several reports of adenosine A2A AR receptors’ anti-inflammatory properties 27. Furthermore, human monocytes secrete the pro-inflammatory cytokine interleukin IL12 and block the degranulation of mast cells caused by FcɛR1 when A2A receptors are activated. Additionally, T cell effects, neutrophil adherence to endothelium, and neutrophil activation and degranulation are all suppressed by activation of the receptor A2A. 28.
Recently, regadenoson has been demonstrated to be safe for administration among patients with COPD and asthma. Another substance, apadenoson, is still in thestage of research for asthma and COPD 29.
Activation of A2BAR may cause broncho-relaxing and anti-inflammatory effects, because of a rise in cyclic AMP levels inside cells. Increases in cyclic intracellular AMP are well recognized to reduce inflammation, relax bronchial smooth muscle and bronchodilation, and stop endothelial cell alterations that would otherwise enhance endothelial permeability. Now it is reported that the use of antagonistsA2BAR can increase the permeability of the endothelium 30.
Strong antagonists of the adenosine A3 receptor have been created to treat inflammatory illnesses including asthma. Activation of A3 receptors is done by inducing phospholipase C and inhibiting adenylate cyclase. In addition to inducing inflammation, A3 agonists also stimulate phospholipase D and release histamine and other inflammatory mediators from mast cells. These factors have led to the recommendation that adenosine A3 receptor antagonists be administered clinically to treat inflammatory illnesses like asthma. 31.
The potential function of stimulation of adenosine A3 receptorsin the pathophysiology of asthma leads toward the development of a selective antagonist of the adenosine receptors A3SSR161421. SSR161421 is a nanomolar adenosine A3 antagonist receptor. SSR161421 has recently been shown to have significant in-vivo pharmacological activity against specific and allergic patterns of adenosine A3 ligand in rodents and pigs 21.
Acknowledging the part played by adenosine receptors in the development of chronic inflammatory illnesses of the respiratory system raises the possibility of inhibiting these receptors, which can be a useful therapeutic strategy for COPD and bronchial asthma. Today made intensive researches on adenosine receptors concerning the therapy of asthma and (COPD), and identified are a variety of inflammatory cell types, such as neutrophils, macrophages, lymphocytes, and eosinophils, which are crucial to the treatment of bronchial asthma 19.
The study’s limitations encompass aspects such as a small sample size, potential biases in participant selection, specific demographic characteristics of the study population, variability in individual responses to medications, the duration of follow-up, and the breadth of outcomes evaluated.
Conclusion
According to the obtained results, the following can be concluded:
Blockers of adenosine receptors – bamifylline given on a daily basis at the dosage of 2 x 600 mg tablets, oral route, results in a notable reduction in the specific airway resistance (SRaw), (p<0.05).
Anticholinergic substance Atrovent (ipratropium bromide – 2 inh x 20 μg) as a result of the effect, not emphasized the effect of bamifillyne by not causing a further decrease of the specific airway resistance (SRaw), (p<0.05).
This implies that the function of anti-inflammatory of the adenosine receptor blockers has not changed the response after administration of anticholinergic substances, reduction of transcription of pro-inflammatory genes caused with the xanthine substance, after application of the anticholinergic substance has not caused a further decrease of specific resistance (SRaw) of the airways.
Author Contributions
“Conceptualization, L.M., H.I. and F.A.; methodology, L.M..; software, M.H.; validation, L.M., H.I. and F.A.; formal analysis, L.M., A.D. and D.B.; investigation, L.M., H.I., F.A. and D.B.; resources, A.D..; data curation, L.M. and A,D,.; writing—original draft preparation, L.M., H.I. and D.B..; writing—review and editing, L.M., H.I., A.D. and D.B..; visualization, F.A. and F.A..; supervision, L.M..; project administration, L.M. and P.I.; funding acquisition, L.M.. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Sources
This research received no external funding.
Data Availability Statement
No new data were created for this review.
Ethics of Human and Animal Experimentation
All experimental procedures of this study were aprowed by the Clinical ethics committee of University Clinical of Prishtina. Protocol no. 502. Date 22.03.2019
References
- Chun-Xiao S, Hays W, Young J, Molina G, et al. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. The Journal of Clinical Investigation 2005; 115: 35-43. doi.org/10.1172/JCI22656.
CrossRef - Bonneau O, Wyss D, Ferretti S, et al. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. The American Journal of Physiology-Lung Cellular and Molecular Physiology. 2006; 290: L1036-43. doi: 10.1152/ajplung.00422.2005.
CrossRef - Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends in Pharmacological Sciences. 2003; 24: 66-70. doi: 10.1016/S0165-6147(02)00045-7.
CrossRef - Singh S, McKintosh R. Adenosine. In: Stat Pearls. Treasure Island (FL): .Stat Pearls Publishing; 2020. https://www.ncbi.nlm. nih.gov/books/ NBK519049/.
- Mohsenin A, Mi T, Xia Y, et al. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. The American Journal of Physiology-Lung Cellular and Molecular Physiology. 2007; 293: L753-61. doi: 10.1152/ajplung.00187.2007.
CrossRef - Jude, J.A.; Dainty, I.; Karmacharya, N.; Jester, W.; Panettieri, R. The Bronchoprotective Effects of Dual Pharmacology, Muscarinic Receptor Antagonist and β2 Adrenergic Receptor Agonist Navafenterol in Human Small Airways. Cells 2023, 12, 240. https://doi.org/10.3390/cells12020240
CrossRef - Effendi WI, Nagano T, Kobayashi K, Nishimura Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells. 2020; 9(3):785. https://doi.org/10.3390/cells9030785
CrossRef - Cox, C.A., Boudewijn, I.M., Vroegop, S.J. et al. Associations of AMP and adenosine induced dyspnea sensation to large and small airways dysfunction in asthma. BMC Pulm Med 19, 23 (2019). https://doi.org/10.1186/s12890-019-0783-0
CrossRef - Donohue, J.F., Wise, R., Busse, W.W. et al. Efficacy and safety of ipratropium bromide/albuterol compared with albuterol in patients with moderate-to-severe asthma: a randomized controlled trial. BMC Pulm Med 16, 65 (2016). https://doi.org/10.1186/s12890-016-0223-3
CrossRef - Reinoud Gosens, Nicholas Gross; The mode of action of anticholinergics in asthma European Respiratory Journal 2018 52: 1701247; DOI: 10.1183/13993003.01247-2017
CrossRef - MRC, Asthma UK. Centre in Allergic Mechanisms of Asthma. Medical Research Council (MRC). 2018; UK.
- Hyseini K, Iljazi A, Morina N, et al. Comparison of methylxanthines (doxofylline and diprophylline) effect in patients with bronchial hyperreactivity and bronchial asthma. Research Journal of Pharmaceutical, Biological and Chemical Sciences (RJPBCS). 2017; 5: 500-09.
- Shabani D, Mustafa L, Islami P, et al. Effect of glucocorticoids following application of adenosine receptor blockers in patients with chronic obstructive bronchitis and bronchial asthma. Open Access Macedonian Journal of Medical Sciences (OAMJMS). 2020; 8: 20-25.
CrossRef - Lajqi N, Ilazi A, Kastrati B et al. Comparison of glucocorticoid (budesonide) and antileukotriene (montelukast) effect in patients with bronchial asthma determined with body plethysmography. Acta Informatica Medica. 2015; 23: 347.
CrossRef - Morina N, Haliti A, Iljazi A, et al. Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity. Open Access Macedonian Journal of Medical Sciences (OAMJMS). 2018; 187: 1-5.
CrossRef - Stacy GW, Hao F, Cheng Zh. G Protein–Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacology Review. 2020; 72: 1–49. doi: 10.1124/pr.118.016899.
CrossRef - Barnes PJ. Triotropium bromide. Expert Opin Investig Drugs. 2001; 70: 733-740.
CrossRef - Sharma S, Hashmi MF, Chakraborty RK. Asthma Medications. In: Stat Pearls Treasure Island (FL): Stat Pearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK531455/.
- Daiana S, Maria Gabriella M, Paola R, et al, Current and future developments in the pharmacology of asthma and COPD: ERS seminar, Naples 2022; Breathe 2023 19: 220267; DOI: 10.1183/20734735.0267-2022
CrossRef - Melani, A.S.; Croce, S.; Fabbri, G.; Messina, M.; Bargagli, E. Inhaled Corticosteroids in Subjects with Chronic Obstructive Pulmonary Disease: An Old, Unfinished History. Biomolecules 2024, 14, 195. https://doi.org/10.3390/biom14020195
CrossRef - Thian-Sze Wong, Guangzhi Li, Shiliang Li, Wei Gao, Geng Chen, Shiyi Gan, Manzhan Zhang, Honglin Li, Song Wu & Yang Du; G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders; Signal Transduction and Targeted Therapy volume 8, Article number: 177 (2023)
CrossRef - Luigino C, Domenico S, Mario C, et al. Pharmacological Characterization of Adenosine Receptors on Isolated Human Bronchi. The American Journal of Respiratory Cell and Molecular Biology. 2011; 45: 1222–1231. doi: 10.1165/rcmb.2011-0056OC on june 14, 2011.
CrossRef - Wilson CN, Nadeem A, Spina D, Brown R, et al. Adenosine receptors and asthma. The Handbook of Experimental Pharmacology. 2009; 193: 329-362. doi:10.1007/978-3-540-89615-9-11
CrossRef - Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. https://doi.org/10.3390/cells9030785
CrossRef - Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2019, 205, 9–15. [Google Scholar] [CrossRef]
CrossRef - Constance N. Wilson, Constance O. Vance, Melissa G. Lechner, George M. Matuschak, and Andrew J. Lechner; Adenosine A1 receptor antagonist, L-97-1, improves survival and protects the kidney in a rat model of cecal ligation and puncture induced sepsis; Eur J Pharmacol. 2014 Oct 5; 0: 346–352. doi: 10.1016/j.ejphar.2014.07.012
CrossRef - Wiwin I. Effendi S, Tatsuya N, et al. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells. 2020; 9: 785. doi:10.3390/cells9030785.
CrossRef - Wilson CN. Adenosine receptors and asthma in humans. British Journal of Pharmacology. 2008; 155: 475–486. doi: 10.1038/bjp.2008.364.
CrossRef - Massimo CS, Holgate T. and Riccardo P. Adenosine signalling in airways. Current Opinion in Pharmacology.2006; (4): 251-256. https://doi.org/10.1016/j.coph.2006.02.002.
CrossRef - Bhagwan Singh Patidar, Anil Meena, Manoj Kumar, Balakrishnan Menon, Vishwajeet Rohil &Surendra Kumar Bansal; Adenosine Metabolism in COPD: A Study on Adenosine Levels, 5′-Nucleotidase, Adenosine Deaminase and Its Isoenzymes Activity in Serum, Lymphocytes and Erythrocytes; COPD: Journal of Chronic Obstructive Pulmonary Disease Volume 15, 2018 – Issue 6
- Endre GM, Peter A, Kinga B, et al. Effect of novel adenosine A3 receptor antagonist SSR161421 in allergic sheep models. Critical Care/Pulmonary Journals. 2016; 1: 58-62. doi: 10.15761/PCCM.1000112.
CrossRef - Sachdeva S, and Gupta M. Adenosine and its receptors as therapeutic targets: An overview. The Saudi Pharmaceutical Journal (SPJ). 2013; 21: 245–253. doi: 10.1016/j.jsps.2012.05.011
CrossRef







