Ruchi Jakhmola-Mani1 , Vikash Sharma1
, Vikash Sharma1 , Sohini Singh1
, Sohini Singh1 , Tanu Allen1
, Tanu Allen1 , Nitu Dogra2
, Nitu Dogra2 and Deepshikha Pande Katare1*
and Deepshikha Pande Katare1*
1Proteomics and Translational Research Lab, Centre for Medical Biotechnology, Amity Institute of Biotechnology, Amity University, Noida. India.
2Analytics Research Division, Research Boulevard Technologies, Sector 27, Greater Noida, Uttar Pradesh, India.
Corresponding Author E-mail: dpkatare@amity.edu
DOI : https://dx.doi.org/10.13005/bpj/2907
Abstract
Breast Cancer (BC) is a complex disease with high incidence in developed countries. According to the World Health Organization (WHO), it is accounted for 11.7% of all new cancer cases worldwide in 2020, with an estimated 2.3 million new diagnosis every year. A 2.5% annual reduction in the disease mortality could prevent 2.5 million deaths worldwide between 2020 and 2040. In the current work systematic review was conducted for drugs under clinical trials or approved for treatment of BC. It was observed that many drugs were repurposed for BC treatment over the course of time even though they were originally developed for some other disease. This is called as Drug Repurposing. It is an approach that has gained significant attention in recent years as a promising alternative to traditional drug discovery, which is often costly, time-consuming, and has a high failure rate. Thirteen drugs were observed to be repurposed for BC treatment and we dig deep into their molecular background and reasons for their efficacies in BC treatment. Molecular targets of these drugs in the human system were predicted and protein interaction networks were analysed to work out the genes responsible for their repurposed events. Few genes seen in the disease progression, were BRCA1, BRCA2, PALB-2, ATM, TP53, PTEN, and HER2/neu participate in various biological pathways, such as the PI3K/Akt/mTOR and ER pathways, and biological processes such as the tumor microenvironment, epithelial-mesenchymal transition, and DNA damage response pathways. Mutations or alterations in these genes or pathways can lead to the development and progression, and understanding their roles that can help in the development of new diagnostic and therapeutic strategies. This study offers an in-silico perspective and a powerful tool to find potentially effective drugs by analysing the molecular mechanisms and signalling pathways involved in the disease progression.
Keywords
Breast Cancer; Drug Repurposing; Drug Discovery; Precision Medicine; Therapeutic Potential
Download this article as:| Copy the following to cite this article: Mani R. J, Sharma V, Singh S, Allen T, Dogra N, Katare D. P, Drug Repurposing and Molecular Insights in the Fight Against Breast Cancer. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Mani R. J, Sharma V, Singh S, Allen T, Dogra N, Katare D. P, Drug Repurposing and Molecular Insights in the Fight Against Breast Cancer. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4aPOezw |
Introduction
Breast Cancer (BC) stands as a prominent malignancy originating from breast lobules or the glandular milk duct epithelial cells, and it’s a leading cause of global mortality 1,2. The disease presents a significant unmet medical need and poses a substantial global health challenge. While its higher incidence in underdeveloped regions contributes to the extensive global burden, implementing effective prevention and treatment strategies can significantly improve outcomes and survival rates for those affected. Interestingly, the prevalence of BC is noticeably higher in regions boasting elevated levels of human development2.
Reflecting its impact, the World Health Organization (WHO) noted that breast cancer accounted for 11.7% of all new cancer cases globally in 2020, with approximately 2.3 million new diagnoses. A mere 2.5% annual reduction in breast cancer mortality could potentially prevent 2.5 million deaths worldwide between 2020 and 2040. In the United States, it remains the most diagnosed cancer among women, with an estimated 284,200 new cases of invasive cancer and 49,290 non-invasive cases projected for diagnosis in 2021. In India, there’s been a recent upsurge in its incidence, with approximately 160,000 new cases diagnosed in 2020. The age-standardized rate of the disease in India is around 25 per 100,000 women, somewhat lower than the global average. However, the disease tends to affect women at a younger age in India compared to Western countries. Generally, the risk of breast cancer escalates with age, with most cases diagnosed in women over 50. A fraction, roughly 5-10% of cases, is presumed to be hereditary, attributed to genetic mutations passed down in families. In 2020, the disease accounted for approximately 2,261,419 new diagnoses and 684,996 deaths2,3. Routine mammogram screening is advised for women at an average risk, typically starting around age 40 or 50, based on country-specific guidelines.
Although BC often presents a poor overall survival rate, early diagnosis and proper therapy during the primary stage offer promising prospects for recovery 4. Risk factors for disease progression typically fall into two categories: causal and non-causal. Non-causal factors include age, obesity, alcohol consumption, unhealthy diet, hormonal imbalances, irregular periods, and specific genetic or epigenetic factors, whereas causal factors are thought to stem from the presence of mutant genes 5. Recent years have witnessed the emergence of computational analysis as a potent tool in drug repurposing research. By parsing extensive data, including genomic and proteomic information, computational analysis can unearth new drug targets and potential candidates for a myriad of diseases, including BC. This approach shows promise in saving substantial time and resources by identifying viable drug candidates that can be further evaluated in preclinical and clinical trials. The review aims to delve into drug repurposing and its potential benefits, highlighting reduced costs and shorter development timelines. It also navigates the challenges associated with conventional drug development methods and how system biology approaches can expedite the process. The study will encompass diverse approaches employed in drug repurposing, spanning from network pharmacology to systems biology modeling.
Background
Breast cancer (BC) is a complex disease that originates in the cells forming the milk-producing parts of the breast. Initially, cancer cells confine themselves within the duct or lobule without causing noticeable symptoms or spreading easily. However, with time, these cells might proliferate and intrude into nearby breast tissue, lymph nodes, or other parts of the body. It stands as the most prevalent type of cancer globally, representing the leading cause of cancer-related fatalities among women. While it can occur at any age post-puberty, the likelihood increases with age. A multitude of factors contribute to the risk of developing BC, encompassing being overweight, excessive alcohol consumption, a family history of BC, radiation exposure, certain reproductive patterns, smoking, and the use of hormone therapy post-menopause. Additionally, specific gene mutations might predispose some individuals to a higher likelihood of BC. Typically, the appearance of a painless lump or thickening in the breast serves as an initial indicator, accompanied by other signs like alterations in breast size, shape, or appearance, skin changes like dimpling or redness, abnormal nipple discharge, and more. Seeking medical attention upon noticing these changes is crucial, often requiring tests like breast imaging and tissue biopsy to determine the nature of the lump.
The repositioning of drugs, or drug repurposing, is a dynamic approach recognized for its efficiency and safety in drug research. Notably, the US NCCN reports that over half of disease-specific medications are used for purposes diverging from their original intended uses. Entities like the NCATS, part of the NIH, have invested substantial funds—$575 million—to drive efforts in exploring novel applications for existing drugs. Drug repurposing, defined by Ashburn and Thor (2004) as finding new uses outside the original medical indication for existing drugs, represents a significantly faster, cost-effective, and safer strategy compared to developing entirely new medications 6. Past successes in this domain, such as Aspirin transitioning from an anti-inflammatory drug in 1897 to an anti-clotting agent in 1956, and Zidovudine transforming from an anti-cancer drug in 1964 to an anti-HIV medication in 1987, underscore the potential within drug repositioning. A plethora of resources, from the Drug Repositioning Portal to scientific publications, serve as invaluable sources for information on repositioned drugs. Nevertheless, rigorous searches and thorough evaluation of data remain crucial to affirm a drug’s potential for a new application suggested by a particular article. For instance, although Ceftriaxone was initially considered a potential treatment for Amyotrophic Lateral Sclerosis, subsequent studies didn’t validate its efficacy. Hence, comprehensive analysis of information available through official platforms like the US FDA website and clinical trials database is imperative to appraise the outcomes from drug repositioning initiatives.
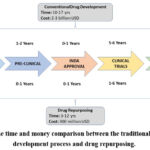 |
Figure 1: The time and money comparison between the traditional medication development process and drug repurposing. |
Current therapies in breast cancer treatment
According to research, cancer cure chances are greater when the disease is detected and treated early, and the adverse effects of various treatment options are understood. The death rate has undoubtedly dropped because of the numerous therapeutic approaches that have been created and refined with the passage of time and technology, and these treatments have made it easier for most cancer patients to recover 7. Conventional treatments, however, have several limitations, and the fundamental problem with these strategies is that they are general 7. Breast cancer treatment encompasses a range of drugs, each designed to target the disease through distinct mechanisms. Chemotherapy, a cornerstone in cancer treatment, employs various classes of drugs. Alkylating agents like Cyclophosphamide and Thiotepa damage DNA, impeding cell growth and gene expression. However, their dual response poses complexities—Cyclophosphamide, acting as an immunomodulator at lower doses and an alkylating agent at higher doses, necessitates careful dosage considerations. Anthracyclines, such as Doxorubicin and Epirubicin, intercalate with DNA, inhibiting its production, but their potential side effects require careful administration within chemotherapy regimens. Antimetabolites like 5-Fluorouracil and Methotrexate disrupt cell growth by mimicking biological molecules, hindering DNA and RNA synthesis, but their usage demands cautious management due to adverse reactions. Mitotic inhibitors, including Docetaxel and Vinblastine, halt cell division, playing vital roles in breast cancer treatment protocols.
Targeted therapies have revolutionized breast cancer treatment, providing more precise and potentially less invasive options. Palbociclib, a CDK4/6 inhibitor, impedes cells from transitioning from G1 to the S phase, significantly affecting the progression of hormone-sensitive breast cancers. Hormone therapies, designed for estrogen and progesterone receptor-positive breast cancers, include Selective Estrogen Receptor Modulators (SERMs) like Tamoxifen, Toremifene, and Raloxifene, as well as Aromatase Inhibitors such as Anastrozole, Exemestane, and Letrozole. These drugs work by either blocking the effects of estrogen or reducing its production in postmenopausal women, thereby impeding the growth of hormone-sensitive cancer cells. Selective Estrogen Receptor Degraders (SERDs) like Fulvestrant target and destroy estrogen receptors without inducing estrogen-like effects, showing promise in certain types of breast cancer. Luteinizing Hormone-Releasing Hormone (LHRH) analogs like Goserelin, initially designed for other conditions, have been repurposed for hormone-sensitive breast cancer treatment.
Another significant targeted therapy includes the mTOR Kinase Inhibitor, Everolimus, which obstructs the PI3K/AKT/mTOR pathway, crucial for cancer cell growth and survival. Its approval in advanced breast cancer cases, particularly when unresponsive to other therapies, reflects its importance in advanced disease management.
These drugs operate in diverse ways, aiming to halt cancer progression, but they also present a spectrum of potential side effects, efficacy variations among individuals, and the development of resistance over time. Treatment decisions are tailored to the patient’s cancer type, stage, and health profile, striving to balance therapeutic benefits with possible adverse effects. Individualized treatment plans and ongoing research in drug development continue to shape the landscape of breast cancer management, emphasizing the need for precision medicine and evolving treatment strategies to navigate the complexities inherent in these diverse drug treatments.
Current pharmacological options (repurposed drugs) for the treatment of Breast Cancer
The clinically authorized medications that were first prescribed for conditions other than breast cancer are covered in the section that follows. However, these medications are currently being investigated for treatment.
Alkylating Agents
These are agents that harm DNA by alkylating the guanine base, which prevents them from attaching to the complementary strand. As a result, Malignant cell development as well as gene expression is inhibited. The cell cycle is affected by the alkylating chemicals at every stage.
A well-known immuno-modulator, cyclophosphamide, inhibits regulatory T-cells and boosts T – cell with effector functions in the stroma of the carcinoma. It has a dual response, meaning that with low dosages it has an immunosuppressive effect while in higher doses, it functions as an alkylating agent wherein it destroys lymphoid and tumor cells 8,9. BC patients often get 600 mg/m2 of cyclophosphamide intravenously (IV), along with other anti-cancer medications (Docetaxel, Paclitaxel, Doxorubicin). Healthy patients are recommended to have either 4 cycles of Cyclophosphamide and Adriamycin or 6 cycles of Cyclophosphamide, Methotrexate, and 5-fluorouracil 10,11.
Thiotepa, an N, N’, N-triethylenephosphoramide derivative, was discovered in 1953 as an inflammatory therapy for transplantation in hemophilia 12. The medication was then recommended for solid tumors in 1959 as well as for BC in 1963 (0.3 to 0.4 mg/kg IV, repeated every 1-4 weeks) 13,14. Patients with metastatic BC as well as those who had had a relapse after adjuvant therapy responded well to the drug combination of thiotepa, vinblastine, and Adriamycin (VATH) 15.
Anthracyclines
Anthracyclines are antibiotics that intercalate between the neighbouring DNA base pairs to create a ternary complex with DNA Topoisomerase II. In highly replicative cancer cells, the ternary complex inhibits the resealing activity of the enzyme, which prevents DNA and RNA production. Doxorubicin, daunorubicin, epirubicin, and idarubicin are a few examples of common anthracyclines. Doxorubicin was authorized for medicinal use in 1974. Additionally, it took around two decades of research for approving anthracyclines in treating cancer. After that, chemotherapy regimens that included doxorubicin (a type of anthracycline) were commonly used with different doses and schedules. Doxorubicin was given by IV injection (usual dose: 60–75 mg/m2 every 21 days) along with other drugs such as cyclophosphamide, Taxotere, and 5-fluorouracil 16. Myocet is a type of doxorubicin that is wrapped in tiny fat bubbles without PEG, a coating that helps the drug stay longer in the body. It is used with another drug called cyclophosphamide to treat BC that has spread to other parts of the body. Myocet is only approved in Europe and Canada. Doxil is another type of doxorubicin that has PEG on its fat bubbles. It is different from Myocet 17.
Antimetabolites
Antimetabolites are drugs that interfere with the metabolism of cancer cells by mimicking important biological molecules. One of these drugs is 5-Flourouracil (5-FU), which is like uracil, a building block of DNA and RNA. 5-FU blocks an enzyme called thymidylate synthetase, which normally converts uracil to thymidine, another building block of DNA. 5-FU also gets incorporated into RNA instead of uracil, which disrupts both DNA and RNA synthesis in the cell. This affects the growth of fast-dividing cells, such as cancer cells. 5-FU was first used as a treatment for keratoacanthomas (KAs), which are skin tumors that usually heal on their own, by Klein and his team in 1962 18. Later, 5-FU was used to treat several cancers, including BC 19. Every 28-day cycle, the standard medication dosage for 5-FU is 500 mg/m2 or 600 mg/m2 IV (six cycles in total). For the treatment of BC, Lokich et al. (1985) first described the administration of 5-FU together with methotrexate (M) 20. Since then, a variety of regimens of combination treatment have been used to treat metastatic BC that include 5-FU (F) together with other medications such as doxorubicin (A), cisplatin (C), cyclophosphamide (CX), and epirubicin (E) (ECF, CMF, CAF) 21. Other mixtures, such as 5-FU and paclitaxel in 1998 22 together with doxorubicin and high dosage 5-FU/paclitaxel 23 were discovered to be effective in treating BC.
By attaching to the dihydrofolate reductase (DHFR) enzyme, the folate/folic acid antagonist methotrexate prevents the creation of the DNA and RNA molecules themselves. The enzyme DHFR transforms folic acid into dihydrofolate, it is then converted to tetrahydrofolate (THDF) by the enzyme thymidine synthetase. Purine and pyrimidine production is reduced in cells because of methotrexate binding to DHFR, which prevents the cell cycle from progressing through the S-phase. Methotrexate was developed in the 1940s as a substitute for folic acid, a nutrient that was lacking in the diet and leading to more cases of acute lymphoblastic leukemia (ALL), a type of blood cancer 24,25. Jane C. Wright discovered methotrexate’s ability to treat BC in 1951 26. Methotrexate was used as a sole cytotoxic agent for advanced therapy in the 1960s and 1970s 27. Gianni and Bonadonna group, however, presented the first therapy with 12 cycles of CMF combination in 400 mastectomy patients with advanced cancer and positive lymph nodes in 1976, and they reported just 5.3% treatment failure with tolerable toxicity 28,29. In actuality, the CMF program was the first-ever treatment plan for BC. Furthermore, it was discovered that CMF was still efficacious after six cycles. Methotrexate’s immunosuppressive properties have been investigated for potential therapeutic use in autoimmune illnesses including rheumatoid arthritis 30. Cyclophosphamide (600 mg/m2), methotrexate (40 mg/m2), and fluorouracil (600 mg/m2) are typically given at 6- to 8-cycle intervals during the CMF regimen.
Another pyrimidine antimetabolite is capecitabine, a prodrug for 5-FU. Capecitabine must go through three sequential enzyme reaction cascades in the gut, liver, and tumor cells to be converted to 5-FU. The enzyme that catalyzes the final conversion step is significantly expressed in cancerous cells in contrast to normal cells. As a result, the tumor-specific conversion of capecitabine to 5-FU prevents systemic exposure to 5-FU. 31. Additionally, capecitabine is more effective than 5-FU and is safer and simpler to give. In 1998, capecitabine was initially employed to treat colon cancer 32. Capecitabine (1250 mg/m2 orally twice daily for 14 days, followed by a 7-day break in a 21-day cycle for 8 cycles) is frequently prescribed to treat aggressive or metastatic breast tumors that are resistant to docetaxel or paclitaxel. 33. It may be used alone or in combination with cabazitaxel 34, vinorelbine 35, or ixabepilone 36.
Gemcitabine is a chemotherapeutic agent that is tri-phosphorylated (dFdCTP) within the cell through a series of enzyme-catalyzed reactions. This dFdCTP incorporates into newly produced DNA under the guise of being an analog of cytidine, causing an irreversible mistake that prevents DNA replication and results in cell death 37,38. Treat enteroviruses like Coxsackievirus B3 (CVB3), Larry Hertel’s team at Eli Lilly and Company began producing gemcitabine in 1980. MERS-CoV, CVB3, EV71, human rhinoviruses (HRVs), HIV, hepatitis C virus (HCV), poliovirus, and influenza virus were also treated with the medicine 39,40. After then, it underwent pre-clinical testing for its anti-tumor property. 1995 saw the FDA’s approval of the medication for pancreatic cancer 41 and in 1998 for non-small lung cancer 42. Finally, in 2004 paclitaxel and gemcitabine, a combination therapy for metastatic BC, received approval 43,44. Some drugs are given together to treat cancer that has spread to other parts of the body when the usual drugs that contain anthracycline do not work. One of these combinations is gemcitabine and paclitaxel, which are given by IV injection on certain days of a 21-day cycle. Gemcitabine is given for 30 minutes on Days 1 and 8, and paclitaxel is given for 3 hours on Day 1 before gemcitabine. This combination is used as the first choice of treatment for this type of cancer. Other combinations that use gemcitabine with different drugs, such as vinorelbine (GemVin), cisplatin (GemCis), or capecitabine (GemCap), have also shown better results and longer survival for patients who have already received other treatments 45.
CDK4/6 Inhibitor
A CDK4/6 inhibitor called Palbociclib prevents cells from moving from the G1 to the S phase. Along with hormone therapy, Palbociclib (125 mg, 28-day cycle with aromatase inhibitor) is used as a targeted treatment for advanced and metastatic ER+/HER2 BC. Recently, either letrozole, an aromatase inhibitor, or Palbociclib was used in the open-label PALOMA clinical trials (PALOMA-1 or PALOMA-2 Trials) 46,47 or hormone treatments with fulvestrant (PALOMA-3 trails) 48. According to phase III clinical studies for PALOMA-3, individuals with metastatic BC are now living longer overall. Inhibiting CDK4/6 activity postpones the development of hormone treatment resistance and dramatically increases patients’ progression-free survival (PFS) 49,50.
Hormone Therapy or Endocrine Therapy
Endocrine treatment is often administered for 5 to 10 years. In patients with estrogen and progesterone BC, this treatment either directly targets the synthesis of hormones (estrogen and/or progesterone) or adversely affects the functional consequences.
SERMs are drugs that block the effects of estrogen by attaching to hormone receptors in a different way. Some of these drugs have been repositioned as BC treatments, such as tamoxifen (1977), toremifene (1997), and raloxifene (2007). The oldest SERM, tamoxifen, has been used to treat early-stage BC in both pre-and post-menopausal women for more than 30 years. Only postmenopausal women with advanced BC should use tamoxifen; toremifene and raloxifene are safe, similarly effective substitutes 51,52. Multiple Results the Raloxifene Evaluation (MORE) clinical study, which was created to treat postmenopausal women’s osteoporosis, also had the other goal of assessing how well it reduced the disease risk. Raloxifene Use for the Heart (RUTH), Tamoxifen with Raloxifene Study, and Evista Continuing Outcomes (CORE) clinical studies were developed because of MORE (STAR) 53.
Only postmenopausal women get aromatase inhibitor hormone treatment to treat early-stage or late-stage ER+ BC 54. It works by inhibiting the aromatase enzyme, which stays active in the adipose tissue of post-menopausal women or females without functioning ovaries. Thus, in post-menopausal women with BC, the aromatase inhibitor lowers the amount of oestrogen that would otherwise support the cancer cells’ development. The early uses of the aromatase inhibitors (anastrozole, exemestane, and letrozole) were to stimulate the ovaries and induce ovulation in females who were infertile or had polycystic ovarian syndrome. Aromatase inhibitors were developed as an alternative to tamoxifen in postmenopausal individuals and can be used as neoadjuvant or adjuvant treatment, either alone or in combination 55.
SERDs are drugs that stop and destroy estrogen receptors without having any estrogen-like effects. Fulvestrant is an example of a SERD that has been repositioned as a BC treatment 56. Fulvestrant is a drug that blocks and destroys estrogen receptors, which are involved in some types of BC. It was first used in 2002 as a “SERD hormone therapy” for women who had stopped having periods and had HR+ HER2 BC that had spread to other parts of the body and did not respond to regular hormone therapy 57. It is used in conjunction with anti-PI3K/AKT/mTOR pathway medications including pictilisib (FERGI) and buparlisib, as well as CDK4/6 inhibitors like Palbociclib (PALOMA-3) and ribociclib (MONALEESA-3) (BELLE-2 and BELLE-3) 58.
The signaling system that promotes the manufacture of oestrogen in the ovaries is interfered with by luteinizing hormone-releasing hormone (LHRH) analogs, resulting in temporary menopause. Goserelin was first used to treat prostate cancer and for assisted reproduction in 1987. In 1989, goserelin was then authorized for the treatment of hormone-sensitive BC in pre-menopausal women. It can be used separately or with other hormone therapy 59. A Phase II clinical trial is now being conducted on goserelin as an adjunct to the typical neoadjuvant treatment for patients with triple negative breast cancer. By 2023, the goserelin phase II study is expected to be finished 60.
mTOR Kinase Inhibitor
Everolimus is a drug that blocks a pathway called PI3K/AKT/mTOR that helps cancer cells grow and survive. It was first used for other types of cancer, such as pancreatic cancer in 2011, kidney cancer in 2009, and to prevent the rejection of kidney transplants in 2010. In 2012, the US FDA approved everolimus for HR+, HER2+ BC that had spread to other parts of the body and did not respond to letrozole or anastrozole, which are drugs that lower estrogen levels. This approval was based on a phase III clinical trial called “BOLERO-2” that showed that everolimus combined with exemestane, another drug that blocks estrogen, improved the outcomes of these patients.
Mitotic Inhibitor
Mitotic inhibitors are drugs that stop cell division or mitosis by affecting the microtubules that help the chromosomes move. This makes the cell cycle pause in the G2/M phase or prevents the formation of the spindle that separates the chromosomes. Some examples of mitotic inhibitors are docetaxel, paclitaxel, and vinblastine. Docetaxel and paclitaxel make the cell cycle stop in the G2/M phase, while vinblastine stops the spindle from developing.
Docetaxel and paclitaxel are drugs that stop cell division in BC cells that are in early, advanced, or metastatic stages in women who have or have not stopped having periods. They are used as extra treatments before or after surgery, either alone or with other drugs that kill cancer cells. Paclitaxel was first found in a type of tree called pacific yew in 1971. It was used to treat a condition where the arteries become narrow again after surgery. Docetaxel and paclitaxel were first used for ovarian and prostate cancer, respectively, in 1992. After that, they were repositioned as additional drugs for BC treatment regimens. Paclitaxel was given by IV injection for 3 hours every 3 weeks for 4 times with a regimen that contained doxorubicin, another drug that stops cell division. Docetaxel was given by IV injection for 1 hour after doxorubicin and cyclophosphamide, another drug that kills cancer cells, every 3 weeks for 6 cycles 61–63. Taxanes are drugs that are often used to treat BC that has spread to other parts of the body. Doctors around the world use different combinations of drugs that include taxanes as a normal way to treat BC 64.
A naturally occurring Vinca alkaloid called vinblastine is present in Vinca rosea, the white-flowered periwinkle. In 1958, Robert Noble and Charles T. Beer made this discovery. Vinblastine’s discovery serves as a stunning illustration of serendipity in medication development. The team instead looked to assess the extract’s anti-diabetic effects in rats and discovered Pseudomonas-mediated septicemia that was followed by a sharp drop in WBC and granulocytopenia. In rats treated with Vinca rosea extract, further research in this approach revealed peripheral granulocytopenia and leukopenia. Finally, they showed vinca rosea extract and vinblastine had carcinostatic action in rats with transplantable mammary adenocarcinoma and sarcoma 65,66. In 1965, vinblastine received approval to treat lymphoma. Additionally, since the 1980s, chemotherapy for advanced and metastatic BC has included the use of vinblastine (2-4 mg/mm2 IV once weekly or every other week), mitomycin, and MVP (mitomycin C, vinblastine, and cisplatin) 67,68.
Furthermore, the study explored the biological basis behind the successful use of repurposed drugs in BC treatment. The methodology used for this is summarized below:
Methodology
Literature review
A thorough search of relevant literature on breast cancer treatment using computational analysis was conducted following PRISMA 2020 guidelines. It was achieved by searching various a list of potential repurposed drugs for breast cancer treatment based on previous studies and clinical trials were compiled. Then conducted a comprehensive literature search of the molecular targets, mechanisms of action, and clinical outcomes of these drugs in breast cancer. The various databases, including PubMed, Scopus, and Web of Science, to gather relevant literature published between 2000 and 2022. We included studies that reported the use of these drugs in breast cancer treatment and their potential anticancer properties.
Prediction of protein receptors for drugs and construction of a protein-protein interaction (PPI) networks
A bioinformatics software called as Swiss Target Prediction was used to predict the probable receptors for drugs. This information was later used to construct a PPI network of molecular targets of identified drugs for finding key nodes and interactions amongst them. Publicly available databases, such as STRING was used to construct PPI network which was further analyzed using Cytoscape.
Result and Discussion
The PRISMA 2020 guidelines were used to guide the systematic review process. The diagram (Fig. 2) represents a standardized approach to documenting the identification, screening, eligibility, and inclusion/exclusion of studies. A comprehensive search was conducted using various electronic databases and other sources, resulting in a total of 392 articles found. After removing duplicates, 392 articles remained for screening. The first screening process included reviewing titles and abstracts to assess for relevance, resulting in 133 articles that were eligible for full-text review. The full-text review further assessed for eligibility based on predefined inclusion and exclusion criteria, resulting in 97 articles that were included in the systematic review. A detailed description of the search strategy and inclusion/exclusion criteria can be found in the methods section.
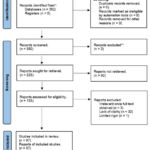 |
Figure 2: Factors Influencing the Knowledge, Attitude and Use of CAM Modalities. |
Exploring the Efficacy and Mechanisms of Existing Drugs for Breast Cancer Treatment
BC is a type of cancer that affects the breast cells and is the main cause of death for women around the world. In the US, about half of the women who got BC did not have any known risk factors for it, except for being female and older than 40 years. The risk factors are things that can increase the chance of getting BC, such as age, weight, alcohol use, past radiation exposure, family history, reproductive history (when the first period and the first pregnancy happened), smoking, and hormone therapy after menopause. However, even if these risk factors are changed, the risk of getting BC can only be reduced by up to 30%. Some women also have inherited gene mutations that make them more likely to get BC. These are genes that have a big effect on BC risk, such as BRCA1, BRCA2, PALB-2, and others like ATM, TP53, and PTEN. Women have a higher risk than men (who get BC in about 0.5-1% of cases) 69. Women who have changes in some important genes that increase their BC risk may choose to have both breasts removed to lower their risk, but this is not a choice for all women. The number of deaths and the chance of living for 5 years after diagnosis have improved lately, but this is only true for tumors that have not spread. BC that has spread to other parts of the body will affect 2.3 million women around the world in 2020 and cause 685 000 deaths. It will still be a serious health problem 70. The number of deaths from BC is increasing steadily, so there is a need for a drug that can make people live longer. Most BC cases are ER+ BC, which means that the cancer cells grow when they are exposed to estrogen, a hormone. About 65% of them are also PR-positive, which means that they grow when they are exposed to progesterone, another hormone. There is another type of BC called triple-negative, which does not have receptors for estrogen or progesterone, and does not express HER2, a protein that helps cancer cells grow 71. Various therapies have been created during the past 20 years, including chemotherapy, radiation, hormone therapy, and immunotherapy 72. But because of the illness’s development, recurrence, and treatment resistance, therapeutic failures were expected 73. The creation of an innovative treatment strategy is necessary. Drug repurposing may therefore be a useful method for developing future therapies. Several drugs have been repurposed for the breast cancer showed in Table 1 below:
Table 1: Different drugs that have been currently evaluated for breast cancer.
|
Name of Drug (Chemical Name) |
Common Name |
Drug Class |
Common Side Effects |
|
Anastrazole |
Arimidex, Adova, Stazonex |
Aromatase inhibitor (Phenylpropane Derivative) |
Hot flashes, muscle and joint discomfort, nausea, osteoporosis, rash on the skin, and weakness |
|
Aspirin |
Ecosprin, Asprin, Loprin |
NSAID’s- Non-Selective COX 1&2 Inhibitors (Salicylates) (Acylsalicylic Acid Derivative) |
Indigestion, a greater propensity to bleed, Nausea, stomach ache, and vomiting |
|
Celecoxib |
Zycel, Celedol, Cobix |
NSAID’s -Selective COX-2 Inhibitors (Pyrazole Derivative) |
Symptoms of the flu, indigestion, stomach ache, Diarrhea, Edema in the legs, and flatulence |
|
Cyclophosphamide |
Endoxan N, Endoxan, Cycloxan |
Alkylating agent (Nitrogen Mustard Compounds) |
Fever, urine with blood, loss of hair White blood cell count decline, Vomiting, Nausea, Diarrhea |
|
Doxorubicin (ADM) |
|
Anthracycline Topoisomerase Inhibitor |
Hives, a rash, itching, breathing difficulties, and seizures |
|
Everolimus |
Evermil, Rolimus, Rapact |
Immunosuppressant- mTOR inhibitors (Macrolide Lactams) |
Infection, fever, cough, exhaustion, stomatitis, otitis media, diarrhea, and upper respiratory tract illness |
|
Fluorouracil |
Flonida, Fluracil, 5FU Cbc |
Antimetabolites (Pyrimidine Analog) |
Weakening, vomiting, and nauseous decrease in appetite, a greater chance of infection, loss of hair Diarrhea, lower blood cells, oral ulcer Blisters on the hands or feet |
|
Metformin |
Glycomet, Glyciphage, Gluconorm |
Biguanides (Biguanides Derivative) |
Nausea, vomiting, a change in flavor, Diarrhea, stomach ache, reduced appetite |
|
Nitroxoline |
|
|
Nausea, headache, dizziness, anxiety, fatigue, and difficulty falling asleep |
|
Paclitaxel |
Taxonab, Intaxel, Mitotax |
Antimicrotubule agents- Taxanes (Taxanes Derivatives) |
Weakening, vomiting, nauseous rash, decreased blood platelets, urinary tract infection, upper respiratory tract infection Bleeding, Anemia, reduction in blood pressure, Flushing, neuropathy in the periphery loss of hair Diarrhea, White blood cell count decline |
|
Phenfluridol |
Flurilept, Penridol, Flumap |
Typical Antipsychotics (Diphenylbutylpiperidine Derivatives) |
Irregular voluntary motions, Constipation, tongue feeling dry increased blood prolactin levels, muscle rigidity, hypotension when standing, Sleepiness, Tremors, urinary incontinence, gaining weight |
|
Pimozide |
Atarap, Mozep, Larap |
Typical Antipsychotics (Diphenylbutylpiperidine Derivatives) |
Tiredness, hypoxemia when standing, tongue feeling dry irregular voluntary motions, gaining weight, increased blood prolactin levels, urinary incontinence, Constipation, muscle rigidity, strange vision, Tremor |
|
Tamoxifen |
Tamodex, Tamtero, Fineova |
Selective estrogen receptor modulators (SERM)- Breast Cx (Nonsteroidal Antiestrogen) |
A skin rash, hot flushes, and nausea the hair is thinning sexual dysfunction, swelling, and vaginal discharge. |
Anastrazole
Anastrazole is a drug that was used in the past to help women who could not get pregnant or who had a condition that caused many cysts in their ovaries. It is used to treat ER+ BC because it blocks the aromatase enzyme, which lowers the amount of estrogen in the body 74. Recent research, however, alerts us to the potential for this medication to bind to ER and activate the downstream pathway of malignant development, becoming an oestrogen receptor ligand 75. Therefore, it is crucial to do more research to better understand how anastrozole works and to show with certainty when it binds to the oestrogen receptor to develop effective therapies and enhance clinical results. Some studies have found that a change in the CSMD1 gene that makes more of the CSMD1 and CYP19 proteins makes cancer cells more sensitive to anastrozole, a drug that lowers estrogen levels. Anastrozole works well for people who have the rs4646 SNP in CYP19A1, a gene that makes the CYP19 protein. It is important to know which tumors are more likely to respond to this treatment method to provide effective cancer therapy. Previous studies showed that the SNPs in CSMD1 and CYP19A1 genes could be used as indicators of how well anastrozole works 76,77.
Aspirin
Aspirin is another common medicine that has been repurposed to treat triple-negative BC 78. When its anti-tumoral activity was discovered, aspirin’s therapeutic application was further broadened. Originally intended to treat cardiovascular disorders, aspirin is an antiplatelet drug. According to certain research, consistent aspirin use lowers the incidence of the disease 79. It’s significant to note that polymorphisms in PIK3CA have been linked to various effects of aspirin in the treatment 80. Aspirin’s ability to limit the development of cancerous cells in the breast with this gene mutation via activating the AMPK and mTORC1 inhibitory signaling pathways supplies evidence in favor of this 81. Consequently, Henry et al. hypothesize that PI3K inhibitors and aspirin may be used in combination therapy to treat the disease. Henry and his team suggest that to make this treatment work, it is important to first group these patients based on their PI3K gene genetic profile to show who are likely to respond to this treatment 82.
Celecoxib
NSAID which is a particular COX-2 inhibitor also shows chemo-preventive potential against malignancies like colorectal and BC. Celecoxib was found to lessen the risk of the disease start and progression when taken often 83,84. Numerous cancers, including breast, oesophageal, gastric, and liver cancers, expressed COX-2 more often. Additionally, the amount of PGE2 rose in the tumor location, which finally sparked the growth of cancer cells 85. Additionally, it was shown that this COX-2/PGE2 induces Akt phosphorylation and aids in lowering cancer cell apoptosis 86. On one more side, PGE2 stimulates the -catenin signaling pathway, which encourages cell mitosis and metastasis 87. Numerous inflammatory disorders are treated with celecoxib in a big way. Numerous preclinical and clinical investigations have shown ample evidence that celecoxib improves prognosis by reducing cancer cell growth and proliferation through a variety of pathways 88. Numerous clinical studies have showed that providing celecoxib enhances the quality and prognosis in clinical cancer patients while also preventing the development of cancer. However, it may be vital to discover which class of cancer patients it is suited for by how sensitive it is too varied populations; this must be examined 69.
By suppressing the COX-2 mediated signal axis and downstream gene targets or pathways, celecoxib may effectively restrict the spread and proliferation of cancer cells. Celecoxib’s decrease of COX-2 was discovered to reduce IDO levels in BC tests, and the management of COX-2’s later PGE2 also comprises preventing IDO’s tryptophan synthesis (2,3-dioxygenase) (2,3-dioxygenase). Celecoxib may additionally influence the tumor microenvironment, have anti-apoptotic and anti-angiogenic actions, and aid other anti-cancer treatments to become less sensitive 89. The suppression of Akt phosphorylation triggers the apoptotic activity, which then causes an increase in Bax levels and, finally, caspase activation to cause apoptosis 90. Reduced VEGF levels and COX-2 suppression govern the cancer environment and help anti-angiogenic action 91. Additionally, it has been claimed that celecoxib increases the disease sensitivity to anti-cancer treatments through the ABC pathway 92. Celecoxib has been reported to boost the sensitivity of cancerous cells in the breast through MDR1, which oversees drug resistance, and may help in the overexpression of COX-2 93,94. By finding the increased methyl residues (hypermethylation) on MDR1, celecoxib decreases the production of MDR1 and limits the DNA binding element’s ability to bind to transcription factors (NF-B and AP-1) (NF-B and AP-1). These transcription factors interact with MDR1, which causes drug resistance 95,96. As a result, celecoxib creates a new opportunity for the treatment of cancer with other medications by making cancerous cells in the breast more sensitive to those medications. This gives the use of celecoxib an edge over traditional treatment.
Cyclophosphamide
An alkylating agent with a history of efficacy in immune system control is cyclophosphamide. In the tumor microenvironment, it lowers the suppressive regulatory T cells and boosts the effector T cells 74. This medicine is being used to treat BC and other malignancies. Depending on the dosage, this medicine performs diverse activities. At lower doses, it affects immunological function; at larger quantities, it functions as an alkylating agent, producing cancer and lymphoid cell death 97. The bioactivation of this chemotherapy drug is dependent on cytochrome P450 (CYP) enzymes. Several genes, particularly the polymorphic CYP2B6 and CYP2C19, encode these enzymes. A more detailed study is necessary to understand the roles of different variants of these genes in the response to treatment. Some of these genetic variations may result in function modification or even elimination. According to specific research, the genetic polymorphisms in the CYP2B6 (rs12721655, rs3745274, *1, *6) and CYP2C19 (rs4244285, *1/*17/*2) genes have an influence on the drug’s bioactivation and thus the efficiency of cyclophosphamide therapy. These findings suggest that cancer patients should have their genotypes checked for these gene SNPs before receiving cyclophosphamide because these variants affect how the drug is transformed into its bioactive form.
Doxorubicin (ADM)
An anti-cancer antibiotic that belongs to the anthracycline class. It was made with the use of Streptomyces peucetius var (hydroxylated congener of daunorubicin). BC, Kaposi’s sarcoma, lymphoma, bladder cancer, and acute lymphocytic leukemia are among the malignancies that it has anti-cancer activity against 98. ADM enters cells directly and is also taken up by cells via organic cation transport. SLC22A16 99. ADM affects tumor cells in two ways: 1) It intercalates into DNA, interfering with DNA repair by disrupting Topo-II 2) The generation of ROS damages DNA, proteins, and cell membranes 100. The NOS3, NQO1, and XHD enzymes help doxorubicin switch back and forth between its normal and semiquinone forms. This process makes the cell produce semiquinone, which changes into doxorubicin again and lets out ROS. This ROS harms the cell by breaking down its lipids, DNA, and membrane, and triggering its self-destruction 101. By attaching to the Sp1 site, ADM boosts the production of GCS (an enzyme that makes glucosylceramide) in breast cancer cells that have ER + receptors 102. Doxorubicin is a better treatment than others, as many studies have shown. FTY720 helped lower the inflammation caused by the drug, making it more effective, and combining it with other drugs worked better for BC patients who were obese 103. Patients with BC that had spread to other parts of the body had better results after getting anthracycline before the main treatment, the PLD study found 104. According to research, combining PEGylated Liposomal Doxorubicin (PLD) with Paclitaxel and Platinum drugs works better for HER2 + patients. This type of doxorubicin is carried by liposomes, which reduce the heart damage caused by the drugs 105. The PLD is a better drug for BC patients who need more than one treatment, because it has fewer side effects and toxicity than the current anthracycline, which can harm the heart, and it is still effective.
Everolimus
Everolimus is a medicine that has been repurposed. It was originally allowed for the treatment of renal cancer in 2009, then for the immune system’s suppression during kidney transplants in 2010, and lastly for the prevention and treatment of pancreatic cancer in 2011 74. Everolimus is being used in the treatment of anastrozole- and letrozole-resistant metastatic BC. Everolimus displays its anti-neoplastic action via its impact on the mTOR pathway. This drug got approved by the FDA for this type of cancer in 2012. Later, it was tested with exemestane to treat HR+, HER2 advanced cancers that spread to other parts of the body and did not respond to letrozole or anastrozole. This was part of a phase III trial called “BOLERO-2”, which stands for “Breast Cancer Trial of Oral Everolimus-2” 106,107.” Everolimus is processed by cytochrome enzymes, just like many other medications. More study is necessary to prove this, although some of the genes involved in its metabolism, such as the CYP3A4 (rs35599367), have been connected to a lower drug metabolic rate. Some genes, especially those related to the PI3K/AKT/mTOR pathway and the one that makes the protein transporter for the drug (ABCB1), can change how the drug is processed in the body. Some studies have found that some SNPs in these genes are linked to the side effects of everolimus, such as mucositis from ABCB1 (rs1045642), low lymphocyte count from ABCB1 (rs2032582), high blood sugar and low white blood cell count from PIK3R1 (rs10515074), and lung inflammation from RAPTOR (rs9906827) 108,109. Therefore, to give therapies for each patient and prevent the toxicities linked to this medicine, doctors must be aware of these genetic variations.
Fluorouracil (5-FU)
5-FU is a type of heterocyclic aromatic compound that acts like the pyridine ring in DNA and RNA. It is a modified version of Uracil with a fluorine atom at the C-5 position instead of a hydrogen atom. This would be a more accurate term 110. The US-FDA approved 5-FU as a treatment for BC patients. The disease is often treated with 5-FU. Its anti-cancer actions have been linked to numerous methods, including progression inhibition (by inhibiting the Ras/ERK pathway) and induction of apoptosis 111. MDA-MB-231 and Tumor2 cell lines employed in in-vitro experiments with 5-FU showed apoptotic activity and lowered levels of the H-Ras gene and its expression. Ras/ERK pathway inhibition also has an antiproliferative effect 112. Additionally, 5-FU reduces the amount of the Rho-A gene, which lowers metastasis and apoptosis 113. 5-FU dramatically lowered Rac1 protein expression, which is needed for cell motility, invasiveness, and anti-apoptotic activity, in Tumor2 and MDA-MB-231 cells 112. In Tumor2, 5-FU reduced the p53 gene’s expression 114. In cancerous cells in the breast, NF-B activation is often noticed. According to research, 5-FU therapy reduced the expression of the NF-B gene, cancer cell growth, and survival 115. According to a study, 5-FU reduced protein expression in the tumor2 cell line when compared to its equivalents. Fluorouracil has a significant advantage over currently available medications in that it reduces drug resistance in human cancer cell lines, according to several publications, where drug resistance is at its highest 115,116. Several added studies back up these showing that fluorouracil is more effective as a treatment when NF-B expression is reduced 115. When used in conjunction with other treatments, fluorouracil (5-FU) enhances the likelihood that tumors with medication resistance may respond 117. Targeted treatment based on nanoparticles is used to enhance the effectiveness of 5-FU by lowering adverse effects and resistance 118. Technology advancements have led to the creation of hydrogels (Cellulose Nanofiber-Based) coated with 5-FU, which quickly trigger pyroptosis in cancerous cells in the breast and present a new avenue for BC treatment 119. Additionally, 5-FU has the benefit of being used by BC patients who have developed Tamoxifen resistance 120.
Metformin
A drug called N′, N′ -dimethyl biguanide is used worldwide to treat people with type 2 diabetes 121,122. People who took metformin for problems like obesity and diabetes had a lower risk of getting cancer, according to studies of populations 123,124. Metformin also helps BC patients respond better and live longer with other treatments. A study that combined data from different sources found that people with DM who took metformin had up to 65% more chance of survival than those who did not use the drug 125. Several studies look at the advantages of using metformin together with other medications to treat BC patients 126,127. How well metformin works depends on how much metformin gets into the cell, which is controlled by OCT1 and varies in people with diabetes 128,129. The OCT1 has in vivo anti-cancer action in cancerous cells in the breast 127. When metformin gets into breast cancer cells through OCT1, it directly affects AMPK to maintain balance. In Triple-negative BC, metformin also stops the cell cycle, blocks the pathways that lead to cell death, and slows down the breakdown of PARP 130,131. Metformin inhibited the mTOR, MAPK, and Akt pathways to produce its anti-apoptotic, anti-angiogenic, and antiproliferative effects 132. Additionally, it inhibits the primary enzymes involved in the creation of cholesterol by blocking EGFR and HER 2/3. (HMGCR, HMGCS, MVD, SQLE, and LSS). It also blocks STAT3 and TGF signals, which further reduce the formation of new blood vessels, inflammation, movement, invasion, and EMT in cells 133. Studies show that metformin reduces inflammation by lowering the levels of IL6 and TNF-, COX2, and some factors that control gene expression, such as NF-kB, STAT, Saa2 and Saa1. By reducing the expression of NF-kB, metformin can be the greatest solution for treating BC since it plays a crucial role in chemoresistance. Additionally, metformin activates SIRT1, which has many pathways for reducing inflammation and is implicated in anti-inflammatory activities 134,135. The combination of metformin and heme was found to be effective in stopping TNBC, which is very hard to treat with existing therapies. This combo study gave us a clue on how to use metformin as an additional drug to treat cancers 136. Shi et al., 2021 published a study showing that metformin can also suppress COX-2 expression, suggesting that it may be used in conjunction with another COX-2 inhibitor to target the COX-2 pathway. Metformin is one of the effective drugs that has advanced to the final stages of clinical trials for treating cancers of the prostate, mouth, breast, pancreas, and uterus 137. Metformin has some benefits over the current cancer treatments, such as its stability (no change), little or no side effects, interactions with other drugs, low price, and easy availability 138.
Nitroxoline (NTX)
Chemically named 5-nitro-8-hydroxy-quinoline, this antibiotic is often used in Asian, European, and African nations for the treatment of UTIs 139. Because of its powerful anti-cancer properties, nitroxoline is currently garnering a lot of popularity. The initial anti-cancer action associated with nitroxoline in human bladder cancer was anti-angiogenic 140, lymphoma, glioma, leukemia, breast, bladder, pancreatic, and ovarian cancers, as well as breast, bladder, and pancreatic cancers, were among the tumors for which NTX showed potential antineoplastic effect 141,142. According to reports, NTX decreases tumor volume in BC (60%) 143 bladder cancer growth was seen to be reduced in the orthotopic mouse model using xenografts 140. Nitroxoline slowed down the growth, spread, and blood vessel formation of tumors in living mice that had breast cancer or fibrosarcoma models. NTX inhibits the selective and reversible cathepsin B (Cat B), which is increased in BC patients. In 2D and 3D tumor models, Cat B inhibition reduces ECM breakdown and later invasion. Nitroxoline works together with MetAP2, SIRT1, and Cat B in living organisms to prevent the formation of blood vessel-like structures by endothelial cells and greatly reduces the growth and spread of tumors 144. According to a study, nitroxoline prevents cancer hallmarks including metastasis, angiogenesis, and invasion in in-vivo animals independent of the delivery method. Its superior pharmacokinetic profile and lack of systemic toxicity make it a more effective treatment for BC than existing regimens 144.
Paclitaxel
Another drug that has been repurposed; is paclitaxel was originally intended to treat arterial restenosis. In 1971, it experienced its first isolation from Pacific Yew. It was first used in 1992 for ovarian cancer 74. Currently, paclitaxel is used to treat ovarian and BC as an adjuvant or neoadjuvant therapy. Its anti-tumor method involves preventing cells from going through mitosis, which lowers the pace at which cancer cells proliferate 74,145. The genetic variability of the individuals is primarily responsible for the dispersion in the tumor response rate to paclitaxel. Some changes in the DNA of LPHN2, ROBO1, SNTG1, and GRIK1 genes may indicate that the tumor is less likely to respond to paclitaxel, according to computer-based studies. These findings are still being investigated for validation before being applied in translation 146,147.
Penfluridol (PF)/Pimozide
First-generation diphenylbutylpiperidine penfluridol is a powerful antipsychotic that was developed in 1968 by Janssen Pharmaceutical. Several types of cancer cells, such as those in the pancreas, breast, brain, and lung, can be killed by penfluridol, which works through different mechanisms. Also proved was in-vivo anti-cancer efficacy, with data proving a little alteration in animal weight and organ volume 148. In-vitro PF treatment significantly reduced ROCK1, Rac1/2/3, Fak, Integrin 4, and Integrin 6. On therapy with penfluridol in the orthotopic model of BC, TNBC growth was reduced by 49%. Additionally, PF showed increased autophagy and apoptosis in metastatic BC and ended paclitaxel resistance 149. Injecting penfluridol into the heart or brain of a TNBC model (4T1) reduced the spread of cancer by 90 and 72%, respectively. The creation of ROS and consequent decrease in cMyc in MDA-MB-231 and SKBR3 cells promote the anti-metastasis effect. A study found that giving PF to cells decreased their ability to withstand paclitaxel. Orthotropic apoptosis was increased in the BC model after PF treatment, which suggested a novel use for PF in Paclitaxel-resistant cancer 150. PF antitumor action was shown in cancerous cells in the breast lines by impairing lysosomal and mitochondrial functioning 151. PF at low doses stopped the growth of new blood vessels in living organisms and blocked the movement and formation of blood vessel-like structures of cells in the lab that were stimulated by VEGF and FGF 152. PF at low doses (the same as those used for treating mental disorders) can reduce the formation of new blood vessels around the tumor. This can replace the current treatment that needs a high dose and has more side effects. The effects of pimozide last for up to seven days. There are no problems with taking or following the treatment, and the long-term outcomes are better than those of conventional medicines 148. The anticancer dose may be larger than the antipsychotic dose, but the dose may still be further perfected in combination therapy. By formulation in a suitable carrier, the dosage can be reduced while potentially improving the bioavailability.
Tamoxifen (TAM)
It belongs to a class of drugs that block the effects of estrogen on the ER, forming a stable complex that prevents ER from activating genes, stops the cell cycle at the G1 stage in breast cancer cells, and reduces their growth 153. TAM is a medication used to treat early and advanced oestrogen receptor-positive (ER+) BC in premenopausal and postmenopausal women 154. The PAX2 protein appears to be necessary for TAM to have strong anti-cancer effects. When PAX2 levels are high, the ERBB2 protein is suppressed, which causes the TAM/ER complex to exhibit antiproliferative activity 155. In-vitro TAM boosted the expression of TGF1 and TGF2 while decreasing TGF expression 156. Studies conducted in vivo on MCF7 xenograft mice revealed that TAM prevented BC in DMBA and NMU rats and decreased ICF-1 levels. A strong dosage of TAM in cell culture reveals BC’s damaging tendency. Chronic therapy prevents carcinogenesis, which has been applied to humans when it is shown in rats 157. In BC patient samples, it reduces proliferation and raises ER expression 156. Tamoxifen is the most often used BC treatment because it has a 75% effectiveness rate (ER), which stabilizes cancer in around 50% of people with malignant BC who had not previously received treatment. Formulating the right carrier can supply a more effective defense against malignancies. In a cohort trial, TAM also showed promising results in males with BC, with an increase in disease-free survival (DFS) 158. TAM lowers the incidence in women who have an elevated risk of the disease, according to the results of a five-year follow-up NSABP research. Additionally, it results in a 43% decrease in invasive BC. A further 25-year follow-up research revealed that TAM treatment was effective for patients with big, low-grade tumors who also evaluated positive for the progesterone receptor 159. When compared to the conventional dose (20 mg), the low dose of TAM (2.5 mg) also lessens the associated adverse effects while supporting its ability to decrease tumor density 160.
Finding medications that are already being used and have also shown a link with receptors that can be a target for treating the disease were the goals of literature mining. Initially, the indicated medications were bought using the Drug Bank database 161. It was important to figure out whether any existing pathways overlapped with the target protein classes of the existing medicines’ biological processes. Results are compiled in Table 2 and were obtained using Swiss Target Prediction 162 (Supplementary file 1).
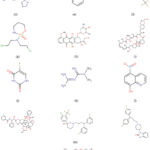 |
Figure 3: Chemical structure of clinically approved drugs repurposing for breast cancer treatment. |
The predicted protein targets (supplementary Table 1), which belonged to different protein classes, were identified. The protein interaction networks for each drug were created using Cytoscape and the network was further analysed for protein target receptors and their associated biological pathways 163. A few medications, including Anastrozole, Aspirin, Celecoxib, Cyclophosphamide, Doxorubicin, Everolimus, Fluorouracil, Metformin, Nitroxoline, Paclitaxel, Penfluridol, Pimozide, and Tamoxifen, were found to interact with unique protein targets.
Table 2: The relationship between repurposed drugs and their targeted protein classes, and the biological processes involved.
|
Name of Drug |
Protein Classes targeted |
Biological process |
|
Anastrozole |
Aromatase |
Estrogen biosynthesis |
|
Estrogen receptor alpha |
Estrogen signaling pathway |
|
|
Estrogen receptor beta |
Estrogen signaling pathway |
|
|
Androgen receptor |
Androgen signaling pathway |
|
|
Insulin-like growth factor-1 receptor (IGF-1R) |
IGF-1 signaling pathway |
|
|
Mitogen-activated protein kinase (MAPK) |
MAPK signaling pathway |
|
|
Phosphoinositide 3-kinase (PI3K) |
PI3K/Akt/mTOR signaling pathway |
|
|
Mammalian target of rapamycin (mTOR) |
PI3K/Akt/mTOR signaling pathway |
|
|
Cyclin-dependent kinase 4/6 (CDK4/6) |
Cell cycle regulation |
|
|
Cyclin D1 |
Cell cycle regulation |
|
|
Aspirin |
COX-1 |
Prostaglandin synthesis |
|
COX-2 |
Prostaglandin synthesis, angiogenesis, inflammation |
|
|
NF-κB |
Inflammation, cell survival, proliferation, angiogenesis |
|
|
AMPK |
Energy metabolism, autophagy |
|
|
PPAR-γ |
Differentiation, apoptosis |
|
|
Wnt/β-catenin |
Cell proliferation, differentiation |
|
|
PI3K/Akt/mTOR |
Cell survival, proliferation, angiogenesis |
|
|
STAT3 |
Inflammation, angiogenesis, cell survival |
|
|
P53 |
Cell cycle arrest, apoptosis, DNA repair |
|
|
HIF-1α |
Angiogenesis, cell survival |
|
|
TGF-β |
Cell differentiation, apoptosis |
|
|
NFKBIA |
Inflammation, cell survival |
|
|
PTGS2 |
Prostaglandin synthesis, inflammation |
|
|
Celecoxib |
Cyclooxygenase-2 (COX-2) |
Prostaglandin Pathway |
|
Akt |
PI3K/Akt Pathway |
|
|
VEGF |
Angiogenesis |
|
|
MMPs |
Extracellular Matrix Remodeling |
|
|
NF-κB |
Inflammation |
|
|
EGFR |
EGFR Signaling Pathway |
|
|
Cyclophosphamide |
DNA alkylating agent |
Cell cycle arrest and apoptosis |
|
Prodrug |
Oxidative stress and DNA damage |
|
|
Alkylating agent |
Inhibition of DNA synthesis and cell proliferation |
|
|
Antineoplastic agent |
Induction of immunogenic cell death and anti-tumor immune response |
|
|
Doxorubicin |
Topoisomerase II |
DNA damage and repair |
|
DNA intercalation |
DNA damage and repair |
|
|
Free radical |
Induction of apoptosis |
|
|
Iron metabolism |
Induction of apoptosis |
|
|
Calcium signaling |
Induction of apoptosis |
|
|
p53 |
Cell cycle regulation |
|
|
Bcl-2 family |
Apoptosis regulation |
|
|
NF-kB |
Inflammation regulation |
|
|
HER2 |
HER2 signaling pathway |
|
|
VEGF |
Angiogenesis regulation |
|
|
Everolimus |
mTORC1 inhibitor |
PI3K/Akt/mTOR pathway |
|
Ser/Thr kinase |
Signal transduction |
|
|
Protein kinase |
Cell cycle regulation |
|
|
Apoptosis inhibitor |
Apoptosis |
|
|
Angiogenesis inhibitor |
Angiogenesis |
|
|
Fluorouracil |
Thymidylate synthase |
Pyrimidine metabolism |
|
Dihydropyrimidine |
Pyrimidine metabolism |
|
|
Dehydrogenase |
||
|
Thymidine phosphorylase |
Pyrimidine metabolism |
|
|
Ribonucleotide reductase |
Purine and pyrimidine metabolism |
|
|
Orotate phosphoribosyltransferase |
Pyrimidine metabolism |
|
|
Thymidylate phosphorylase |
Pyrimidine metabolism |
|
|
Thymidine kinase |
Pyrimidine metabolism |
|
|
DNA polymerase alpha |
DNA replication and repair |
|
|
Tyms and FH2 |
Nucleotide metabolism |
|
|
5-FU enzyme complex |
Nucleotide metabolism |
|
|
TS, DHFR, and TK |
Pyrimidine metabolism and DNA synthesis |
|
|
Metformin |
AMPK |
Inhibition of mTORC1, leading to reduced protein synthesis, cell growth, and proliferation |
|
mTORC1 |
Inhibition of cell proliferation and increased autophagy |
|
|
Insulin receptor |
Reduction in insulin-like growth factor 1 (IGF-1) signaling and downstream effects on cell growth and survival |
|
|
PI3K/Akt/mTOR |
Disruption of this pathway, which is frequently upregulated in breast cancer, leads to decreased cell proliferation and survival |
|
|
GLUT1 |
Reduction in glucose uptake, leading to decreased energy supply for cancer cells |
|
|
Nitroxoline |
Topoisomerase inhibitor |
DNA replication and repair |
|
Anti-inflammatory agent |
Inflammation |
|
|
NF-κB inhibitor |
NF-κB signaling pathway |
|
|
Histone deacetylase inhibitor |
Epigenetic regulation |
|
|
ROS inducer |
Oxidative stress |
|
|
Iron chelator |
Iron metabolism |
|
|
ERα agonist |
ER signaling pathway |
|
|
Paclitaxel |
Microtubule stabilizers |
Cell cycle arrest and apoptosis |
|
Tubulin |
Mitotic spindle formation |
|
|
Cyclin-dependent kinase inhibitors (CDKIs) |
Cell cycle regulation |
|
|
p53 |
DNA damage response |
|
|
Bcl-2 family proteins |
Apoptosis regulation |
|
|
NF-κB |
Inflammatory response regulation |
|
|
Vascular endothelial growth factor (VEGF) |
Tumor angiogenesis regulation |
|
|
Penfluridol |
Dopamine D2 receptor antagonist |
Unknown, but thought to induce apoptosis and inhibit cancer cell proliferation |
|
Sigma receptor agonist |
Unknown, but may modulate intracellular calcium levels and affect tumor growth |
|
|
Calcium channel blocker |
Unknown, but may affect calcium-dependent processes such as apoptosis and cell proliferation |
|
|
G protein-coupled receptor antagonist |
The exact mechanism is not clear, but it may interfere with some of the pathways that help cancer cells grow and survive. |
|
|
Histone deacetylase inhibitor |
Induction of cell cycle arrest, differentiation, and apoptosis |
|
|
Protein kinase C inhibitor |
By altering the pathways that control how cells divide, change, and die. |
|
|
DNA topoisomerase inhibitor |
Induction of DNA damage and apoptosis |
|
|
Microtubule inhibitor |
Inhibition of microtubule polymerization, leading to cell cycle arrest and apoptosis |
|
|
Cholesterol biosynthesis inhibitor |
By blocking the pathway that produces cholesterol, the growth of cancer cells may be slowed down or stopped. |
|
|
Glutathione S-transferase inhibitor |
Modulation of cellular redox status and potential induction of apoptosis |
|
|
Pimozide |
Dopamine receptor antagonist |
PI3K/Akt/mTOR pathway |
|
Calcium channel blocker |
Calcium signaling pathway |
|
|
Histamine receptor antagonist |
Not well-established in breast cancer |
|
|
Sigma receptor antagonist |
Not well-established in breast cancer |
|
|
Tamoxifen |
Estrogen receptor |
ER signaling pathway |
|
G protein-coupled receptors |
MAPK/ERK signaling pathway |
|
|
Protein kinases |
PI3K/Akt/mTOR signaling pathway |
|
|
Nuclear receptor co-regulators |
Transcriptional regulation |
|
|
Apoptosis-related proteins |
Apoptosis pathway |
|
|
Growth factor receptors |
Growth factor signaling pathway |
|
|
Cyclin-dependent kinases |
Cell cycle regulation |
|
|
DNA repair proteins |
DNA damage response pathway |
Challenges of proposed repurposed drugs for novel applications
Drug repurposing is not always successful, several of the instances that have already been examined failed when tested for repurposing (Table 3). One aspect of all these failures in phase III seems to be common. Due to the candidates’ established safety profiles, it should be less probable that these failures were caused by toxicity. Before repurposing medications, there are a variety of issues and problems that contributed to the failure of these candidates. These difficulties include organizational obstacles, patent problems, and regulatory considerations. Let’s go over each of the problems with medication repurposing individually:
Table 3: Drugs that were tested for repurposing but did not show efficacy in the intended conditions.
|
Name of Drug |
Repurposed for |
The strategy employed for repurposing |
Year |
Reference |
|
Favipiravir |
Coronavirus |
Efforts to combat SARS-CoV-2 (In-vitro) |
2020 |
164 |
|
Hydroxychloroquine |
Coronavirus |
RCTs and animal pharmacological analysis |
2020 |
165 |
Regulation-Related Factors
Any drug that is repurposed must follow the regulatory standards set by regulatory bodies for any drug testing. Many repurposed drugs are evaluated according to the European Medicines Agency and undergo a common process to obtain marketing approval in all EEA member states of the European Economic Area 166. In some cases, such as for older drugs, national approval is possible at the country level. There are different legal ways to get new therapeutic uses approved and added to the label. The main legal basis for drug applications for reprofiled drugs is Directive 2001/83/EC, including Sections 6, 8(3), 10(3), and 10(5). The researchers must provide enough pre-clinical evidence for any new indications, which can be supported by available literature references.
Patent Problems
When a patent holder’s drug must be used to treat cancer, they must be given a significant advantage. Intellectual property laws, however, prohibit the patenting of any of these alternatives. Because of the low profit potential, only charitable organizations are interested in researching these drugs. An example of this is the Repurposing Drugs in Oncology project, which is funded by drug companies in collaboration with NPO, a group of hospitals and universities that accounts for less than 5% of the total and covers about 70 drugs with 190 registered CTs 167. Drugs that have been repurposed for new uses after their patents have expired may apply for a new method-of-use patent. However, this patent application process is very strict and requires a lot of evidence that the drug can treat a specific disease or condition using new, inventive dosage forms and formulations. Moreover, the main challenges to drug repurposing are patent disputes by generic drug manufacturers and market exclusivity incentives by patent holders.
Disproportionate Prescription
Theoretically, generic or reprofiled medications should be used wherever possible, and prescription medications should be provided based on scientific evidence from CT. However, by making substantial efforts in patient marketing, physician marketing, and consumer advertising, the pharmaceutical industry may have an influence on this. Because of this, patients rather than biopharma win when doctors prescribe less expensive medicines that have the same impact as more expensive medications.
The landscape of drugs used for treating breast cancer is vast and diversified, encompassing both established treatments and repurposed medications. Established drugs for breast cancer treatment typically include a range of therapies categorized into hormone-based treatments, chemotherapy, targeted therapy, and immunotherapy. Hormone-based treatments like tamoxifen, aromatase inhibitors (such as anastrozole), and selective estrogen receptor modulators (SERMs) have been cornerstones in managing hormone receptor-positive breast cancer. Chemotherapeutic agents such as anthracyclines (e.g., doxorubicin), taxanes (like paclitaxel), and antimetabolites (fluorouracil) are frequently employed for various breast cancer stages. Targeted therapies, like CDK4/6 inhibitors such as palbociclib and mTOR inhibitors like everolimus, have emerged as valuable additions to the treatment arsenal. These drugs are pivotal in targeting specific molecular pathways, controlling cell cycle progression, and inhibiting growth factors, contributing to improved outcomes in specific breast cancer subtypes. Immunotherapies are also under exploration for breast cancer, particularly in the domain of triple-negative breast cancer.
On the other hand, repurposed drugs for breast cancer treatment are medications that were initially developed and utilized for different medical conditions but have demonstrated potential efficacy in treating breast cancer. Drugs like aspirin, metformin, and nitroxoline, typically employed for different health concerns, are being reconsidered for their potential role in breast cancer management. Their repurposing arises from observations suggesting possible anti-cancer effects or supportive actions in conjunction with traditional breast cancer treatments. These repurposed drugs span various classes—ranging from NSAIDs like aspirin to antimalarials and antifungals. Their mechanisms of action often differ from the conventional breast cancer treatments, reflecting a diverse array of pathways they may influence within the cancer microenvironment. For instance, aspirin, primarily used for pain relief, shows potential in influencing inflammation and potentially impacting cancer progression.
The key distinction between established breast cancer treatments and repurposed drugs lies in their development history and primary medical indications. Established treatments undergo rigorous clinical trials and are specifically formulated for treating breast cancer, backed by substantial evidence and regulatory approval. Repurposed drugs, on the other hand, lack this direct targeting and are often used off-label or under investigation through clinical trials to assess their efficacy and safety in breast cancer. While repurposed drugs may offer the advantage of potential cost-effectiveness and established safety profiles due to their prior use, they require extensive investigation to validate their efficacy in the context of breast cancer. Both categories contribute to the evolving landscape of breast cancer treatment, with established drugs forming the cornerstone of evidence-based care, while repurposed drugs present opportunities for novel approaches and potential adjunct therapies. The efficacy, safety, and compatibility of repurposed drugs alongside established treatments are subjects of ongoing research to ascertain their roles in optimizing breast cancer management.
Conclusion
The pursuit of repurposing existing drugs for breast cancer treatment presents an invaluable avenue in the realm of drug research. It offers a cost-effective and expeditious approach, significantly alleviating the burden of therapy for cancer patients. Through this process, a multitude of novel anti-breast cancer medications are being unearthed, diversifying the therapeutic arsenal. By leveraging drug repurposing, the drug development trajectory gains acceleration, promising potential in discovering cures within condensed time frames and with fewer resource allocations compared to de novo drug production. This research journey has spotlighted the promising prospect of pharmaceuticals with analogous biological and molecular mechanisms, when complementing existing therapies, showcasing encouraging effectiveness. Despite certain inherent limitations, this approach holds tremendous potential, especially when supported by in silico techniques, bolstering the identification of optimal therapeutic candidates. The integration of a comprehensive approach, involving precise protein modeling through systems biology and bioinformatics, demonstrates a high potential in formulating safer, more efficient, and economically viable chemotherapeutics for even the most severe conditions of breast cancer.
Furthermore, this pursuit necessitates a continued expansion of current pharmaceutical databases, multi-omics technologies, and bioinformatics tools through collaborative efforts among scientists, researchers, and medical professionals. This collective expansion is pivotal in fostering the development of innovative treatments, ultimately advancing the trajectory toward combatting and potentially eradicating the life-threatening grip of breast cancer. A comprehensive narrative synthesis of the findings from the selected studies is imperative. This synthesis should encapsulate a detailed depiction of the discovered drugs, their respective mechanisms of action, and their potential implications in breast cancer treatment. Additionally, such a synthesis should illuminate existing gaps in the literature, inconsistencies in findings, and areas ripe for further exploration and research. This multifaceted approach, fortified by the integration of in silico techniques and collaborative advancements, stands as a beacon of hope in the relentless pursuit of more effective, safer, and accessible treatments for breast cancer.
Acknowledgement
None to declare
Conflict of interest
There is no conflict of interest
Funding Sources
There is no funding Sources
References
- Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1). doi:10.1186/s40659-017-0140-9
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/CAAC.21660
- Sharma S, Deep A, Sharma AK. Current Treatment for Cervical Cancer: An Update. Anticancer Agents Med Chem. 2020;20(15):1768-1779. doi:10.2174/1871520620666200224093301
- Houzé de l’Aulnoit A, Rogoz B, Pinçon C, Houzé de l’Aulnoit D. Metastasis-free interval in breast cancer patients: Thirty-year trends and time dependency of prognostic factors. A retrospective analysis based on a single institution experience. Breast. 2018;37:80-88. doi:10.1016/J.BREAST.2017.10.008
- Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, Jiang J, Yao PP, Zhu HP. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci. 2017;13(11):1387-1397. doi:10.7150/IJBS.21635
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673-683. doi:10.1038/NRD1468
- Chen YJ, Huang WC, Wei YL, Hsu SC, Yuan P, Lin HY, Wistuba II, Lee JJ, Yen CJ, Su WC, Chang KY, Chang WC, Chou TC, Chou CK, Tsai CH, Hung MC. Elevated BCRP/ABCG2 Expression Confers Acquired Resistance to Gefitinib in Wild-Type EGFR-Expressing Cells. PLoS One. 2011;6(6):e21428. doi:10.1371/JOURNAL.PONE.0021428
- De Jonge ME, Huitema ADR, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44(11):1135-1164. doi:10.2165/00003088-200544110-00003
- Eid RA, Razavi GSE, Mkrtichyan M, Janik J, Khleif SN. Old-School Chemotherapy in Immunotherapeutic Combination in Cancer, A Low-cost Drug Repurposed. Cancer Immunol Res. 2016;4(5):377-382. doi:10.1158/2326-6066.CIR-16-0048
- Singh JC, Mamtani A, Barrio A, Morrow M, Sugarman S, Jones LW, Yu AF, Argolo D, Smyth LM, Modi S, Schweber S, Boafo C, Patil S, Norton L, Baselga J, Hudis CA, Dang C. Pathologic Complete Response with Neoadjuvant Doxorubicin and Cyclophosphamide Followed by Paclitaxel with Trastuzumab and Pertuzumab in Patients with HER2-Positive Early Stage Breast Cancer: A Single Center Experience. Oncologist. 2017;22(2):139-143. doi:10.1634/THEONCOLOGIST.2016-0268
- Nakatsukasa K, Koyama H, Oouchi Y, Imanishi S, Mizuta N, Sakaguchi K, Fujita Y, Fujiwara I, Kotani T, Matsuda T, Fukuda K, Morita M, Kawakami S, Kadotani Y, Konishi E, Yanagisawa A, Taguchi T. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer. 2017;24(1):63-68. doi:10.1007/S12282-016-0666-7
- Sykes MP, Karnofsky DA, Philips FS, Burchenal JH. CLINICAL STUDIES ON TRIETHYLENEPHOSPHORAMIDE AND DIETHYLENEPHOSPHORAMIDE, COMPOUNDS WITH NITROGEN-MUSTARD-LIKE ACTIVITY. doi:10.1002/1097-0142
- Kim KW, Roh JK, Wee HJ, Kim C. Cancer drug discovery: Science and history. Cancer Drug Discovery: Science and History. Published online January 1, 2016:1-276. doi:10.1007/978-94-024-0844-7/COVER
- Lyons A, Edelstyn G. Thiotepa in treatment of advanced breast cancer. Br Med J. 1962;2(5315):1280-1283. doi:10.1136/BMJ.2.5315.1280
- Perloff M, Hart RD, Holland JF. VINBLASTINE, ADRIAMYCIN, THIOTEPA, AND HALOTESTIN (VATH) Therapy for Advanced Breast Cancer Refractory to Prior Chemotherapy. Cancer. 1978;42:2534-2537. doi:10.1002/1097-0142(197812)42:6
- Soni H. Martindale: The Complete Drug Reference Brayfield Alison (Ed) Martindale: The Complete Drug Reference £459 4,688pp Pharmaceutical Press 9780857111395 0857111396 [Formula: see text]. Emerg Nurse. 2014;22(5):12-12. doi:10.7748/EN.22.5.12.S13
- Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with Drug Administered in free form: Changing toxicity profiles. Drug Saf. 2001;24(12):903-920. doi:10.2165/00002018-200124120-00004/METRICS
- Yen Moore A. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J Dermatolog Treat. 2009;20(6):328-335. doi:10.3109/09546630902789326
- Ansfield FJ, Schroeder JM, Curreri AR. Five years clinical experience with 5-fluorouracil. JAMA. 1962;181(4):295-299. doi:10.1001/JAMA.1962.03050300015003
- Lokich JJ, Phillips D, Green R, Paul S, Sonneborn H, Zipoli TE, Curt G. 5-Fluorouracil and Methotrexate Administered Simultaneously as a Continuous Infusion A Phase I Study. Published online 2395. doi:10.1002/1097-0142
- Cameron DA, Gabra H, Leonard RCF. Continuous 5-fluorouracil in the treatment of breast cancer. Br J Cancer. 1994;70(1):120-124. doi:10.1038/BJC.1994.259
- Klaassen U, Wilke H, Harstrick A, Seeber S. Fluorouracil-based combinations in the treatment of metastatic breast cancer. Oncology. Published online 1998.
- Zoli W, Ulivi P, Tesei A, Fabbri F, Rosetti M, Maltoni R, Giunchi DC, Ricotti L, Brigliadori G, Vannini I, Amadori D. Addition of 5-fluorouracil to doxorubicin-paclitaxel sequence increases caspase-dependent apoptosis in breast cancer cell lines. Breast Cancer Research. 2005;7(5). doi:10.1186/BCR1274
- Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997;23(4):739-755. doi:10.1016/S0889-857X(05)70358-6
- Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The Pharmacology and Clinical Use of Methotrexate. http://dx.doi.org/101056/NEJM198311033091805. 2010;309(18):1094-1104. doi:10.1056/NEJM198311033091805
- WRIGHT JC, PRIGOT A, WRIGHT B, WEINTRAUB S, WRIGHT LT. An evaluation of folic acid antagonists in adults with neoplastic diseases: a study of 93 patients with incurable neoplasms. J Natl Med Assoc. 1951;43(4):211-240. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/14850976/?tool=EBI
- Fracchia AA, Farrow JH, Facs +, Adam YG, Monroy J, Knapper WH. SYSTEMIC CHEMOTHERAPY FOR ADVANCED BREAST CANCER. doi:10.1002/1097-0142
- Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, De Lena M, Tancini G, Bajetta E, Musumeci R, Veronesi U. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294(8):405-410. doi:10.1056/NEJM197602192940801
- Bonadonna G, Moliterni A, Zambetti M, Daidone MG, Pilotti S, Gianni L, Valagussa P. 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330(7485):217-220. doi:10.1136/BMJ.38314.622095.8F
- Radu AF, Bungau SG. Management of Rheumatoid Arthritis: An Overview. Cells. 2021;10(11). doi:10.3390/CELLS10112857
- Tabata T, Katoh M, Tokudome S, Nakajima M, Yokoi T. IDENTIFICATION OF THE CYTOSOLIC CARBOXYLESTERASE CATALYZING THE 5′-DEOXY-5-FLUOROCYTIDINE FORMATION FROM CAPECITABINE IN HUMAN LIVER. Drug Metabolism and Disposition. 2004;32(10):1103-1110. doi:10.1124/DMD.104.000554
- Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34(8):1274-1281. doi:10.1016/S0959-8049(98)00058-6
- Pivot X, Asmar L, Buzdar AU, Valero V, Hortobagyi G. A unified definition of clinical anthracycline resistance breast cancer. Br J Cancer. 2000;82(3):529-534. doi:10.1054/BJOC.1999.0958
- Villanueva C, Awada A, Campone M, Machiels JP, Besse T, Magherini E, Dubin F, Semiond D, Pivot X. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: a phase I/II study. Eur J Cancer. 2011;47(7):1037-1045. doi:10.1016/J.EJCA.2011.01.001
- Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil Delgado MA, Kerbrat P, Garcia-Giralt E, Keiling R, Namer M, Closon MT, Goudier MJ, Chollet P, Lecourt L, Montcuquet P. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993;11(7):1245-1252. doi:10.1200/JCO.1993.11.7.1245
- Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, Da Costa SCV, Ro J, Rubio G, Rondinon M, Manga GP, Peck R, Poulart V, Conte P. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28(20):3256-3263. doi:10.1200/JCO.2009.24.4244
- Alvarellos ML, Lamba J, Sangkuhl K, Thorn CF, Wang L, Klein DJ, Altman RB, Klein TE. PharmGKB summary: gemcitabine pathway. Pharmacogenet Genomics. 2014;24(11):564-574. doi:10.1097/FPC.0000000000000086
- Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17 Suppl 5(SUPPL. 5). doi:10.1093/ANNONC/MDJ941
- Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6 Suppl 6(SUPPL. 6):7-13. doi:10.1097/00001813-199512006-00002
- Lee K, Kim D eun, Jang KS, Kim SJ, Cho S, Kim C. Gemcitabine, a broad-spectrum antiviral drug, suppresses enterovirus infections through innate immunity induced by the inhibition of pyrimidine biosynthesis and nucleotide depletion. Oncotarget. 2017;8(70):115315-115325. doi:10.18632/ONCOTARGET.23258
- Rs K. Gemcitabine. New first-line therapy for pancreatic cancer. Cancer Pract. Published online 1996.
- Takada M, Negoro SI, Kudo S, Furuse K, Nishikawa H, Takada Y, Kamei T, Niitani H, Fukuoka M. Activity of gemcitabine in non-small-cell lung cancer: results of the Japan gemcitabine group (A) phase II study. Cancer Chemother Pharmacol. 1997;41(3):217-222. doi:10.1007/S002800050731
- Spielmann M, Llombart-Cussac A, Kalla S, Espié M, Namer M, Ferrero JM, Diéras V, Fumoleau P, Cuvier C, Perrocheau G, Ponzio A, Kayitalire L, Pouillart P. Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology. 2001;60(4):303-307. doi:10.1159/000058524
- Triggle DJ. Comprehensive Medicinal Chemistry II Volume 1 : Global Perspective. 2006;1:355-403.
- Stemmler HJ, Digioia D, Freier W, Tessen HW, Gitsch G, Jonat W, Brugger W, Kettner E, Abenhardt W, Tesch H, Hurtz HJ, Rösel S, Brudler O, Heinemann V. Randomised phase II trial of gemcitabine plus vinorelbine vs gemcitabine plus cisplatin vs gemcitabine plus capecitabine in patients with pretreated metastatic breast cancer. Br J Cancer. 2011;104(7):1071-1078. doi:10.1038/BJC.2011.86
- Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Diéras V, Slamon DJ. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925-1936. doi:10.1056/NEJMOA1607303
- Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, Harbeck N, Castrellon A, Iyer S, Lu DR, Mori A, Gauthier ER, Bartlett CH, Gelmon KA, Slamon DJ. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719-729. doi:10.1007/S10549-018-05125-4
- Masuda N, Inoue K, Nakamura R, Rai Y, Mukai H, Ohno S, Hara F, Mori Y, Hashigaki S, Muramatsu Y, Nagasawa T, Umeyama Y, Huang X, Iwata H. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24(3):262-273. doi:10.1007/S10147-018-1359-3
- Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, André F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379(20):1926-1936. doi:10.1056/NEJMOA1810527
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. doi:10.1016/S1470-2045(15)00613-0
- Vogel CL, Johnston MA, Capers C, Braccia D. Toremifene for breast cancer: a review of 20 years of data. Clin Breast Cancer. 2014;14(1):1-9. doi:10.1016/J.CLBC.2013.10.014
- Provinciali N, Suen C, Dunn BK, DeCensi A. Raloxifene hydrochloride for breast cancer risk reduction in postmenopausal women. http://dx.doi.org/101080/1751243320161231575. 2016;9(10):1263-1272. doi:10.1080/17512433.2016.1231575
- Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR, Mautalen CA, Zanchetta JR, Hooper MJ, Ng KW, Prince RL, Nicholson G, Roberts AP, Seeman E, Williamson M, Boschitsch E, Leb G, Body JJ, Devogelaer JP, Geusens P, Kaufman JM, Peretz A, Adachi J, Bensen W, Brown JP, Cheung A, Chik C, Gee S, Hanley D, Hawker GA, Hodsman AB, Joyce C, Monchesky TC, Olszynski WP, Roe B, Senikas V, Seminoski K, Wall J, Stepan J, Hyldstrup L, Langdahl B, Sorensen TH, Alhava E, Kormano M, Salmela P, Salmi J, Valimaki M, Audran M, Briancon D, Delmas P, Fardellone P, Ribot C, De Vernejoul MC, Balogh A, Julesz J, Szuecs J, Karsik A, Fiore C, Genazzani AR, Gennari C, Isaia GC, Melis GB, Nuti R, Oriente P, Passeri M, Sartori L, Corea-Rotter R, Gonzalez S, Murillo A, Jonker JJ, Lips P, Mulder H, Pols HA, Halse JI, Hoiseth A, Jorde R, Olford ES, Skag A, Stakkestad JA, Wist E, Badurski JE, Hoszowski K, Ogonowski J, Bose K, Lee KO, Dzurik R, Kocijancic A, Cannata Andia JB, Collado RC, Carranza FH, Diez-Perez A, Escobar-Jimenez F, Minguella JF, Solan XN, Torres MM, Larsson K, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751-1761. doi:10.1093/JNCI/DJH319
- Melinda LT, Kirsten MT, Julia R, Bryan H, Gordon BM, Kristin CJ, Zoltan S, William TB, Eric PW, Nadine MT, Steven JI, Paula DR, April GC, Alexander G, Zaina S, Diana I, Chris N, Victor A, Joshua TJ, Jerry SL, Anne-Renee H, Judy EG, James MF, Daniel PS, Andrea LR. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. 2016;22(15):3764-3773. doi:10.1158/1078-0432.CCR-15-2477
- Pistelli M, Della Mora A, Ballatore Z, Berardi R. Aromatase inhibitors in premenopausal women with breast cancer: the state of the art and future prospects. Curr Oncol. 2018;25(2):e168-e175. doi:10.3747/CO.25.3735
- Carlson RW. The history and mechanism of action of fulvestrant. Clin Breast Cancer. 2005;6 Suppl 1(SUPPL. 1):S5. doi:10.3816/CBC.2005.S.008
- Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, Lew DL, Hayes DF, Gralow JR, Linden HM, Livingston RB, Hortobagyi GN. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N Engl J Med. 2019;380(13):1226-1234. doi:10.1056/NEJMOA1811714
- Nathan MR, Schmid P. A Review of Fulvestrant in Breast Cancer. Oncol Ther. 2017;5(1):17-29. doi:10.1007/S40487-017-0046-2
- Cheer SM, Plosker GL, Simpson D, Wagstaff AJ. Goserelin: a review of its use in the treatment of early breast cancer in premenopausal and perimenopausal women. Drugs. 2005;65(18):2639-2655. doi:10.2165/00003495-200565180-00011
- Carbognin L, Furlanetto J, Vicentini C, Nortilli R, Pilotto S, Brunelli M, Pellini F, Pollini G, Bria E, Tortora G. Neoadjuvant strategies for triple negative breast cancer: “state-of-the-art” and future perspectives. Anticancer Agents Med Chem. 2015;15(1):15-25. doi:10.2174/1871520614666141019191616
- Chan JM, Rhee JW, Drum CL, Bronson RT, Golomb G, Langer R, Farokhzad OC. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci U S A. 2011;108(48):19347-19352. doi:10.1073/PNAS.1115945108
- Sparano JA. Taxanes for breast cancer: an evidence-based review of randomized phase II and phase III trials. Clin Breast Cancer. 2000;1(1). doi:10.3816/cbc.2000.n.002
- Nabholtz JM, Gligorov J. The role of taxanes in the treatment of breast cancer. Expert Opin Pharmacother. 2005;6(7):1073-1094. doi:10.1517/14656566.6.7.1073
- Crown J, O’Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist. 2004;9 Suppl 2(S2):24-32. doi:10.1634/THEONCOLOGIST.9-SUPPL_2-24
- Noble RL, Beer CT, Cutts JH. Role of chance observations in chemotherapy: Vinca rosea. Ann N Y Acad Sci. 1958;76(3):882-894. doi:10.1111/J.1749-6632.1958.TB54906.X
- Brambilla C, Zambetti M, Ferrari L, Bonadonna G. Mitomycin C and vinblastine in advanced refractory breast cancer. Tumori. 1989;75(2):141-144. doi:10.1177/030089168907500212
- Sedlacek SM. First-line and salvage therapy of metastatic breast cancer with mitomycin/vinblastine. Oncology. 1993;50 Suppl 1:16-23. doi:10.1159/000227243
- Urruticoechea A, Archer CD, Assersohn LA, Gregory RK, Verrill M, Mendes R, Walsh G, Smith IE, Johnston SRD. Mitomycin C, vinblastine and cisplatin (MVP): an active and well-tolerated salvage regimen for advanced breast cancer. Br J Cancer. 2005;92(3):475-479. doi:10.1038/SJ.BJC.6602367
- Turnbull C, Rahman N. Genetic Predisposition to Breast Cancer: Past, Present, and Future. https://doi.org/101146/annurev.genom9081307164339. 2008;9:321-345. doi:10.1146/ANNUREV.GENOM.9.081307.164339
- Kim EE, Yukihiro M. Breast cancer. Clinical PET and PET/CT: Principles and Applications. Published online October 1, 2013:227-233. doi:10.1007/978-1-4419-0802-5_18/COVER
- Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, Schnall M, Alavi A. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer. 2008;112(5):995-1000. doi:10.1002/CNCR.23226
- Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart MJ, Thürlimann B, Senn HJ, André F, Baselga J, Bergh J, Bonnefoi H, Burstein H, Cardoso F, Castiglione-Gertsch M, Colleoni M, Curigliano G, Davidson NE, Leo A Di, Ejlertsen B, Forbes JF, Galimberti V, Goodwin P, Harbeck N, Hayes DF, Huober J, Hudis CA, Ingle JN, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Kent Osborne C, Partridge AH, de la Peña L, Pritchard KI, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao ZM, Smith I, Toi M, Tutt A, Viale G, von Minckwitz G, Watanabe T, Whelan T, Xu B. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Annals of Oncology. 2015;26(8):1533-1546. doi:10.1093/ANNONC/MDV221
- Brand TC, Sawyer MM, King TA, Bolton JS, Fuhrman GM. Understanding patterns of failure in breast cancer treatment argues for a more thorough investigation of axillary lymph nodes in node negative patients. Am J Surg. 2000;180(6):424-427. doi:10.1016/S0002-9610(00)00507-9
- Aggarwal S, Verma SS, Aggarwal S, Gupta SC. Drug repurposing for breast cancer therapy: Old weapon for new battle. Semin Cancer Biol. 2021;68:8-20. doi:10.1016/J.SEMCANCER.2019.09.012
- Ingle JN, Cairns J, Suman VJ, Shepherd LE, Fasching PA, Hoskin TL, Singh RJ, Desta Z, Kalari KR, Ellis MJ, Goss PE, Chen BE, Volz B, Barman P, Carlson EE, Haddad T, Goetz MP, Goodnature B, Cuellar ME, Walters MA, Correia C, Kaufmann SH, Weinshilboum RM, Wang L. Anastrozole has an Association between Degree of Estrogen Suppression and Outcomes in Early Breast Cancer and is a Ligand for Estrogen Receptor α. Clin Cancer Res. 2020;26(12):2986-2996. doi:10.1158/1078-0432.CCR-19-3091
- Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C, Macleod SL, Kadlubar FF, Ambrosone CB. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. doi:10.1007/s10549-004-7751-x
- Cairns J, Ingle JN, Dudenkov TM, Kalari KR, Carlson EE, Na J, Buzdar AU, Robson ME, Ellis MJ, Goss PE, Shepherd LE, Goodnature B, Goetz MP, Weinshilboum RM, Li H, Bari MG, Wang L. Pharmacogenomics of aromatase inhibitors in postmenopausal breast cancer and additional mechanisms of anastrozole action. JCI Insight. 2020;5(16). doi:10.1172/JCI.INSIGHT.137571
- Spini A, Donnini S, Pantziarka P, Crispino S, Ziche M. Repurposing of drugs for triple negative breast cancer: An overview. Ecancermedicalscience. 2020;14. doi:10.3332/ECANCER.2020.1071
- Ma S, Guo C, Sun C, Han T, Zhang H, Qu G, Jiang Y, Zhou Q, Sun Y. Aspirin Use and Risk of Breast Cancer: A Meta-analysis of Observational Studies from 1989 to 2019. Clin Breast Cancer. 2021;21(6):552-565. doi:10.1016/J.CLBC.2021.02.005
- Zhou Y, Simmons J, Jordan CD, Sonbol MB, Maihle N, Tang SC. Aspirin Treatment Effect and Association with PIK3CA Mutation in Breast Cancer: A Biomarker Analysis. Clin Breast Cancer. 2019;19(5):354-362.e7. doi:10.1016/J.CLBC.2019.05.004
- Henry WS, Laszewski T, Tsang T, Beca F, Beck AH, McAllister SS, Toker A. Aspirin Suppresses Growth in PI3K-Mutant Breast Cancer by Activating AMPK and Inhibiting mTORC1 Signaling. Cancer Res. 2017;77(3):790-801. doi:10.1158/0008-5472.CAN-16-2400
- Banno K, Iida M, Yanokura M, Irie H, Masuda K, Kobayashi Y, Tominaga E, Aoki D. Drug repositioning for gynecologic tumors: A new therapeutic strategy for cancer. Scientific World Journal. 2015;2015. doi:10.1155/2015/341362
- Woditschka S, Haag JD, Mau B, Lubet RA, Gould MN. Chemopreventive effects of celecoxib are limited to hormonally responsive mammary carcinomas in the neu-induced retroviral rat model. Breast Cancer Research. 2008;10(1):1-9. doi:10.1186/BCR1864/FIGURES/3
- Saxena P, Sharma PK, Purohit P. A journey of celecoxib from pain to cancer. Prostaglandins Other Lipid Mediat. 2020;147:106379. doi:10.1016/J.PROSTAGLANDINS.2019.106379
- Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through AKT activation: Evidence for AKT inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38(3):756-768. doi:10.1053/JHEP.2003.50380
- Kern MA, Schubert D, Sahi D, Schöneweiß MM, Schöneweiß S, Moll I, Haugg M, Dienes HP, Breuhahn K, Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36(4):885-894. doi:10.1053/JHEP.2002.36125
- Simon LS, Weaver AL, Graham DY, Kavitz AJ, Lipsky PE, Hubbard RC, Isakson PC, Verburg KM, Yu SS, Zhao WW, Geis GS. Anti-inflammatory and Upper Gastrointestinal Effects of Celecoxib in Rheumatoid Arthritis: A Randomized Controlled Trial. JAMA. 1999;282(20):1921-1928. doi:10.1001/JAMA.282.20.1921
- Curry JM, Besmer DM, Erick TK, Steuerwald N, Roy L Das, Grover P, Rao S, Nath S, Ferrier JW, Reid RW, Mukherjee P. Indomethacin enhances anti-tumor efficacy of a MUC1 peptide vaccine against breast cancer in MUC1 transgenic mice. PLoS One. 2019;14(11):e0224309. doi:10.1371/JOURNAL.PONE.0224309
- Baumann KH, Klusmeier E, Eggemann I, Reinartz S, Almeroth A, Kalder M, Wagner U. Effects of celecoxib and Ly117018 combination on human breast cancer cells in vitro. Breast Cancer (Auckl). 2009;3(1):23-34. doi:10.4137/BCBCR.S2291/ASSET/IMAGES/LARGE/10.4137_BCBCR.S2291-FIG2.JPEG
- Fife RS, Stott B, Carr RE. Effects of a Selective Cyclooxygenase-2 Inhibitor on Cancer Cells In Vitro. http://dx.doi.org/104161/cbt32692. 2003;3(2):228-232. doi:10.4161/CBT.3.2.692
- Kalalinia F, Elahian F, Mosaffa F, Behravan J. Celecoxib Up Regulates the Expression of Drug Efflux Transporter ABCG2 in Breast Cancer Cell Lines. Iran J Pharm Res. 2014;13(4):1393-1401. Accessed February 19, 2023. https://europepmc.org/articles/PMC4232806
- Sorokin A. Cyclooxygenase-2: Potential Role in Regulation of Drug Efflux and Multidrug Resistance Phenotype. Curr Pharm Des. 2005;10(6):647-657. doi:10.2174/1381612043453117
- Kalalinia F, Elahian F, Behravan J. Potential role of cyclooxygenase-2 on the regulation of the drug efflux transporter ABCG2 in breast cancer cell lines. J Cancer Res Clin Oncol. 2011;137(2):321-330. doi:10.1007/S00432-010-0893-9/METRICS
- Xia W, Zhao T, Lv J, Xu S, Shi J, Wang S, Han X, Sun Y. Celecoxib enhanced the sensitivity of cancer cells to anticancer drugs by inhibition of the expression of P-glycoprotein through a COX-2-Independent Manner. J Cell Biochem. 2009;108(1):181-194. doi:10.1002/JCB.22239
- Chen C, Shen HL, Yang J, Chen QY, Xu WL. Preventing chemoresistance of human breast cancer cell line, MCF-7 with celecoxib. J Cancer Res Clin Oncol. 2011;137(1):9-17. doi:10.1007/S00432-010-0854-3/METRICS
- Grochow LB, Colvin M. Clinical Pharmacokinetics of Cyclophosphamide. Clin Pharmacokinet. 1979;4(5):380-394. doi:10.2165/00003088-197904050-00004/METRICS
- Helsby NA, Yong M, van Kan M, de Zoysa JR, Burns KE. The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br J Clin Pharmacol. 2019;85(9):1925-1934. doi:10.1111/BCP.14031
- Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T, Mizoi T, Shiiba K, Takanaga H, Terasaki T, Matsuno S, Sasaki I, Ito S, Abe T. Characterization of the organic cation transporter SLC22A16: A doxorubicin importer. Biochem Biophys Res Commun. 2005;333(3):754-762. doi:10.1016/J.BBRC.2005.05.174
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727-741. doi:10.1016/S0006-2952(98)00307-4
- Doroshow JH. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proceedings of the National Academy of Sciences. 1986;83(12):4514-4518. doi:10.1073/PNAS.83.12.4514
- Zhang X, Wu X, Su P, Gao Y, Meng B, Sun Y, Li L, Zhou Z, Zhou G. Doxorubicin Influences the Expression of Glucosylceramide Synthase in Invasive Ductal Breast Cancer. PLoS One. 2012;7(11):e48492. doi:10.1371/JOURNAL.PONE.0048492
- Katsuta E, Yan L, Nagahashi M, Raza A, Sturgill JL, Lyon DE, Rashid OM, Hait NC, Takabe K. Doxorubicin effect is enhanced by sphingosine-1-phosphate signaling antagonist in breast cancer. Journal of Surgical Research. 2017;219:202-213. doi:10.1016/j.jss.2017.05.101
- Al-Batran SE, Bischoff J, Von Minckwitz G, Atmaca A, Kleeberg U, Meuthen I, Morack G, Lerbs W, Hecker D, Sehouli J, Knuth A, Jager E. The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: a multicentre phase II trial. British Journal of Cancer 2006 94:11. 2006;94(11):1615-1620. doi:10.1038/sj.bjc.6603158
- Sparano JA, Makhson AN, Semiglazov VF, Tjulandin SA, Balashova OI, Bondarenko IN, Bogdanova N V., Manikhas GM, Oliynychenko GP, Chatikhine VA, Zhuang SH, Xiu L, Yuan Z, Rackoff WR. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: Results from a randomized phase III study. Journal of Clinical Oncology. 2009;27(27):4522-4529. doi:10.1200/JCO.2008.20.5013
- Khallaf SM, Roshdy J, Ibrahim A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J Egypt Natl Canc Inst. 2020;32(1). doi:10.1186/S43046-020-00034-4
- Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520-529. doi:10.1056/NEJMOA1109653
- Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer. 2009;9(8):576-586. doi:10.1038/NRC2683
- Pascual T, Apellániz-Ruiz M, Pernaut C, Cueto-Felgueroso C, Villalba P, Álvarez C, Manso L, Inglada-Pérez L, Robledo M, Rodríguez-Antona C, Ciruelos E. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS One. 2017;12(7). doi:10.1371/JOURNAL.PONE.0180192
- Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer 2003 3:5. 2003;3(5):330-338. doi:10.1038/nrc1074
- Gómez Del Pulgar T, Benitah SA, Valerón PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27(6):602-613. doi:10.1002/BIES.20238
- Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Communication and Signaling 2010 8:1. 2010;8(1):1-14. doi:10.1186/1478-811X-8-23
- Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Laboratory Investigation. 2003;83(6):861-870. doi:10.1097/01.LAB.0000073128.16098.31
- Osaki M, Tatebe S, Goto A, Hayashi H, Oshimura M, Ito H. 5-Fluorouracil (5-FU) induced apoptosis in gastric cancer cell lines: Role of the p53 gene. Apoptosis. 1997;2(2):221-226. doi:10.1023/A:1026476801463/METRICS
- Ponce-Cusi R, Calaf GM. Apoptotic activity of 5-fluorouracil in breast cancer cells transformed by low doses of ionizing α-particle radiation. Int J Oncol. 2016;48(2):774-782. doi:10.3892/IJO.2015.3298/HTML
- Uetsuka H, Haisa M, Kimura M, Gunduz M, Kaneda Y, Ohkawa T, Takaoka M, Murata T, Nobuhisa T, Yamatsuji T, Matsuoka J, Tanaka N, Naomoto Y. Inhibition of inducible NF-κB activity reduces chemoresistance to 5-fluorouracil in human stomach cancer cell line. Exp Cell Res. 2003;289(1):27-35. doi:10.1016/S0014-4827(03)00223-4
- Entezar-Almahdi E, Mohammadi-Samani S, Tayebi L, Farjadian F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int J Nanomedicine. 2020;15:5445-5458. doi:10.2147/IJN.S257700
- Sethy C, Kundu CN. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomedicine & Pharmacotherapy. 2021;137:111285. doi:10.1016/J.BIOPHA.2021.111285
- Balahura LR, Dinescu S, Balaș M, Cernencu A, Lungu A, Vlăsceanu GM, Iovu H, Costache M. Cellulose Nanofiber-Based Hydrogels Embedding 5-FU Promote Pyroptosis Activation in Breast Cancer Cells and Support Human Adipose-Derived Stem Cell Proliferation, Opening New Perspectives for Breast Tissue Engineering. Pharmaceutics 2021, Vol 13, Page 1189. 2021;13(8):1189. doi:10.3390/PHARMACEUTICS13081189
- Watanabe T, Oba T, Tanimoto K, Shibata T, Kamijo S, Ito KI. Tamoxifen resistance alters sensitivity to 5-fluorouracil in a subset of estrogen receptor-positive breast cancer. PLoS One. 2021;16(6):e0252822. doi:10.1371/JOURNAL.PONE.0252822
- Wang YW, He SJ, Feng X, Cheng J, Luo YT, Tian L, Huang Q. Metformin: a review of its potential indications. Drug Des Devel Ther. 2017;11:2421-2429. doi:10.2147/DDDT.S141675
- Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. Journal of Clinical Oncology. 2016;34(35):4203-4216. doi:10.1200/JCO.2016.68.4480
- Sharma AK, Sharma A, Lal S, Kumar A, Yadav NK, Tabassum F, Sayeed Akhtar M, Tarique Imam M, Saeed Almalki Z, Mukherjee M. Dysbiosis versus diabesity: Pathological signaling and promising therapeutic strategies. Drug Discov Today. 2023;28(5). doi:10.1016/J.DRUDIS.2023.103558
- Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JAE, Aragaki AK, Rohan T, Ipp E, Kaklamani VG, Vitolins M, Wallace R, Gunter M, Phillips LS, Strickler H, Margolis K, Euhus DM. Diabetes, metformin, and breast cancer in postmenopausal women. Journal of Clinical Oncology. 2012;30(23):2844-2852. doi:10.1200/JCO.2011.39.7505
- Xu H, Chen K, Jia X, Tian Y, Dai Y, Li D, Xie J, Tao M, Mao Y. Metformin Use Is Associated With Better Survival of Breast Cancer Patients With Diabetes: A Meta-Analysis. Oncologist. 2015;20(11):1236-1244. doi:10.1634/THEONCOLOGIST.2015-0096
- Roshan MH, Shing YK, Pace NP. Metformin as an adjuvant in breast cancer treatment. SAGE Open Med. 2019;7:205031211986511. doi:10.1177/2050312119865114
- Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, Moulder-Thompson S, Dewar JA, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: A pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128(3):783-794. doi:10.1007/S10549-011-1612-1/METRICS
- Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117(5):1422-1431. doi:10.1172/JCI30558
- Jin HE, Hong SS, Choi MK, Maeng HJ, Kim DD, Chung SJ, Shim CK. Reduced antidiabetic effect of metformin and down-regulation of hepatic oct1 in rats with ethynylestradiol-induced cholestasis. Pharm Res. 2009;26(3):549-559. doi:10.1007/S11095-008-9770-5/METRICS
- Hsieh Li SM, Liu ST, Chang YL, Ho CL, Huang SM. Metformin causes cancer cell death through downregulation of p53-dependent differentiated embryo chondrocyte 1 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J Biomed Sci. 2018;25(1):1-13. doi:10.1186/S12929-018-0478-5/FIGURES/8
- Zhuang Y, Keith WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3(0). doi:10.1186/1750-2187-3-18
- Zhu P, Davis M, Blackwelder AJ, Bachman N, Liu B, Edgerton S, Williams LL, Thor AD, Yang X. Metformin selectively targets tumor-initiating cells in ErbB2-overexpressing breast cancer models. Cancer Prevention Research. 2014;7(2):199-210. doi:10.1158/1940-6207.CAPR-13-0181/35990/AM/METFORMIN-SELECTIVELY-TARGETS-TUMOR-INITIATING
- Wahdan-Alaswad RS, Thor AD, Wahdan-Alaswad RS, Thor AD. Metformin Activity against Breast Cancer: Mechanistic Differences by Molecular Subtype and Metabolic Conditions. Metformin [Working Title]. Published online February 12, 2020. doi:10.5772/INTECHOPEN.91183
- Faria J, Negalha G, Azevedo A, Martel F. Metformin and Breast Cancer: Molecular Targets. J Mammary Gland Biol Neoplasia. 2019;24(2):111-123. doi:10.1007/S10911-019-09429-Z/METRICS
- Lee J, Yesilkanal AE, Wynne JP, Frankenberger C, Liu J, Yan J, Elbaz M, Rabe DC, Rustandy FD, Tiwari P, Grossman EA, Hart PC, Kang C, Sanderson SM, Andrade J, Nomura DK, Bonini MG, Locasale JW, Rosner MR. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature. 2019;568(7751):254-258. doi:10.1038/S41586-019-1005-X
- Singh J, Bisht P, Srivastav S, Kumar Y, Sharma V, Kumar A, Akhtar MS, Khan MF, Aldosari SA, Yadav S, Yadav NK, Mukherjee M, Sharma AK. Amelioration of endothelial integrity by 3,5,4’-trihydroxy-trans-stilbene against high-fat-diet-induced obesity and -associated vasculopathy and myocardial infarction in rats, targeting TLR4/MyD88/NF-κB/iNOS signaling cascade. Biochem Biophys Res Commun. 2024;705. doi:10.1016/J.BBRC.2024.149756
- Shi B, Hu X, He H, Fang W. Metformin suppresses breast cancer growth via inhibition of cyclooxygenase-2. Oncol Lett. 2021;22(2):1-14. doi:10.3892/OL.2021.12876/HTML
- Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res. 2019;11:3295-3313. doi:10.2147/CMAR.S200059
- Shim JS, Liu JO. Recent Advances in Drug Repositioning for the Discovery of New Anticancer Drugs. Int J Biol Sci. 2014;10(7):654-663. doi:10.7150/IJBS.9224
- Shim JS, Matsui Y, Bhat S, Nacev BA, Xu J, Bhang HEC, Dhara S, Han KC, Chong CR, Pomper MG, So A, Liu JO. Effect of Nitroxoline on Angiogenesis and Growth of Human Bladder Cancer. JNCI: Journal of the National Cancer Institute. 2010;102(24):1855-1873. doi:10.1093/JNCI/DJQ457
- Jiang H, Taggart JE, Zhang X, Benbrook DM, Lind SE, Ding WQ. Nitroxoline (8-hydroxy-5-nitroquinoline) is more a potent anti-cancer agent than clioquinol (5-chloro-7-iodo-8-quinoline). Cancer Lett. 2011;312(1):11-17. doi:10.1016/J.CANLET.2011.06.032
- Lazovic J, Guo L, Nakashima J, Mirsadraei L, Yong W, Kim HJ, Ellingson B, Wu H, Pope WB. Nitroxoline induces apoptosis and slows glioma growth in vivo. Neuro Oncol. 2015;17(1):53-62. doi:10.1093/NEUONC/NOU139
- Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C, Man YG, Chen T. Antibiotics for cancer treatment: A double-edged sword. J Cancer. 2020;11(17):5135-5149. doi:10.7150/JCA.47470
- Mirković B, Markelc B, Butinar M, Mitrović A, Sosič I, Gobec S, Vasiljeva O, Turk B, Čemažar M, Serša G, Kos J. Nitroxoline impairs tumor progressionin vitroandin vivoby regulating cathepsin B activity. Oncotarget. 2015;6(22):19027-19042. Accessed February 20, 2023. https://www.academia.edu/44786710/Nitroxoline_impairs_tumor_progression_in_vitro_and_in_vivo_by_regulating_cathepsin_B_activity
- Ruiz-Garcia E, Astudillo-de la Vega H. Translational research and onco-omics applications in the era of cancer personal genomics. :171.
- Perez-Ortiz AC, Ramírez I, Cruz-López JC, Villarreal-Garza C, Luna-Angulo A, Lira-Romero E, Jiménez-Chaidez S, Díaz-Chávez J, Matus-Santos JA, Sánchez-Chapul L, Mendoza-Lorenzo P, Estrada-Mena FJ. Pharmacogenetics of response to neoadjuvant paclitaxel treatment for locally advanced breast cancer. Oncotarget. 2017;8(63):106454-106467. doi:10.18632/ONCOTARGET.22461
- Eng L, Ibrahim-Zada I, Jarjanazi H, Savas S, Meschian M, Pritchard KI, Ozcelik H. Bioinformatic analyses identifies novel protein-coding pharmacogenomic markers associated with paclitaxel sensitivity in NCI60 cancer cell lines. BMC Med Genomics. 2011;4. doi:10.1186/1755-8794-4-18
- Tuan NM, Lee CH. Penfluridol as a Candidate of Drug Repurposing for Anticancer Agent. Molecules 2019, Vol 24, Page 3659. 2019;24(20):3659. doi:10.3390/MOLECULES24203659
- Ranjan A, Gupta P, Srivastava SK. Penfluridol: An Antipsychotic Agent Suppresses Metastatic Tumor Growth in Triple-Negative Breast Cancer by Inhibiting Integrin Signaling Axis. Cancer Res. 2016;76(4):877-890. doi:10.1158/0008-5472.CAN-15-1233
- Hedrick E, Li X, Safe S. Penfluridol represses integrin expression in breast cancer through induction of reactive oxygen species and downregulation of Sp transcription factors. Mol Cancer Ther. 2017;16(1):205-216. doi:10.1158/1535-7163.MCT-16-0451/86846/AM/PENFLURIDOL-REPRESSES-INTEGRIN-EXPRESSION-IN
- Varalda M, Antona A, Bettio V, Roy K, Vachamaram A, Yellenki V, Massarotti A, Baldanzi G, Capello D. Psychotropic Drugs Show Anticancer Activity by Disrupting Mitochondrial and Lysosomal Function. Front Oncol. 2020;10:2148. doi:10.3389/FONC.2020.562196/BIBTEX
- Srivastava S, Zahra FT, Gupta N, Tullar PE, Srivastava SK, Mikelis CM. Low Dose of Penfluridol Inhibits VEGF-Induced Angiogenesis. International Journal of Molecular Sciences 2020, Vol 21, Page 755. 2020;21(3):755. doi:10.3390/IJMS21030755
- Yao J, Deng K, Huang J, Zeng R, Zuo J. Progress in the Understanding of the Mechanism of Tamoxifen Resistance in Breast Cancer. Front Pharmacol. 2020;11:1848. doi:10.3389/FPHAR.2020.592912/BIBTEX
- Jordan VC. A current view of tamoxifen for the treatment and prevention of breast cancer. Br J Pharmacol. 1993;110(2):507-517. doi:10.1111/J.1476-5381.1993.TB13840.X
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor–PAX2 determines response to tamoxifen. Nature 2008 456:7222. 2008;456(7222):663-666. doi:10.1038/nature07483
- Dhingra K. Antiestrogens – Tamoxifen, SERMs and beyond. Invest New Drugs. 1999;17(3):285-311. doi:10.1023/A:1006348907994/METRICS
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nature Reviews Drug Discovery 2003 2:3. 2003;2(3):205-213. doi:10.1038/nrd1031
- Bekele RT, Venkatraman G, Liu RZ, Tang X, Mi S, Benesch MGK, MacKey JR, Godbout R, Curtis JM, McMullen TPW, Brindley DN. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: implications for tamoxifen therapy and resistance. Scientific Reports 2016 6:1. 2016;6(1):1-17. doi:10.1038/srep21164
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-1662. doi:10.1093/JNCI/DJI372
- Eriksson M, Eklund M, Borgquist S, Hellgren R, Margolin S, Thoren L, Rosendahl A, Lång K, Tapia J, Bäcklund M, Discacciati A, Crippa A, Gabrielson M, Hammarström M, Wengström Y, Czene K, Hall P. Low-dose tamoxifen for mammographic density reduction: A randomized controlled trial. Journal of Clinical Oncology. 2021;39(17):1899-1908. doi:10.1200/JCO.20.02598
- Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36(Database issue). doi:10.1093/NAR/GKM958
- Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014;42(Web Server issue). doi:10.1093/NAR/GKU293
- Verbaanderd C, Rooman I, Meheus L, Huys I. On-Label or Off-Label? Overcoming Regulatory and Financial Barriers to Bring Repurposed Medicines to Cancer Patients. Front Pharmacol. 2020;10. doi:10.3389/FPHAR.2019.01664
- Sharma AK, Bisht P, Gupta B, Sayeed Akhtar MD, Shaik Alavudeen S, Afzal O, SA Altamimi A. Investigating miRNA subfamilies: Can they assist in the early diagnosis of acute myocardial infarction? Drug Discov Today. 2023;28(10):103695. doi:10.1016/J.DRUDIS.2023.103695
- Martinez MA. Lack of Effectiveness of Repurposed Drugs for COVID-19 Treatment. Front Immunol. 2021;12:653. doi:10.3389/FIMMU.2021.635371/BIBTEX
- Martinez MA. Clinical Trials of Repurposed Antivirals for SARS-CoV-2. Antimicrob Agents Chemother. 2020;64(9). doi:10.1128/AAC.01101-20
- Dewangan J, Srivastava S, Mishra S, Divakar A, Kumar S, Rath SK. Salinomycin inhibits breast cancer progression via targeting HIF-1α/VEGF mediated tumor angiogenesis in vitro and in vivo. Biochem Pharmacol. 2019;164:326-335. doi:10.1016/J.BCP.2019.04.026
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience. 2014;8(1). doi:10.3332/ECANCER.2014.442








