Department of Medical Laboratory Sciences, Mutah University, Mutah, Jordan.
Corresponding Author E-mail:am_jaafreh@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2925
Abstract
Common Ivy (Hedera helix L.) is an ornamental plant that is known for its aesthetic qualities and ability to provide natural greenery. It is also associated with potential health benefits. When evaluating the phytochemical constituents of H. helix methanolic extracts, the fruit extract had the highest levels of total phenolic compounds (TPC) at 100 GAE mg/g extract, compared to 89.47 GAE mg/g in the leaves extract. In contrast, total flavonoid compounds and total tannin were higher in leaves extracts, 37.14 TE mg/g extract and 24.79 GAE mg/g extract respectively. fruit extracts showed the greatest level of antioxidant properties in the FRAP test 75.5 and 62.35 AscE mg/g extract also in DPPH tests, the IC50 were 3.49 and 8.79 mg/ml for fruit and leaves respectively, demonstrating their potent capacity to neutralize free radicals and high reducing power. However, when evaluated by the ABTS method, the leaves extracts indicated the strongest antioxidant activity, suggesting their potent capacity to neutralize free radicals, the IC50 were 4.54 and 8.69 mg/ml for fruit and leaves respectively, the extracts' inhibitory effects on albumin denaturation were also assessed. The findings demonstrated the potential of the extracts as anti-inflammatory, with the leaf extract having the lowest IC50 values in these tests 75.26± 3.87 µg/ml and 115.62± 56.47 µg/ml, for ripe fruit extract. Furthermore, the lowest contraction value was 81.12% for pure ointment alone, followed by drug ointment and fruit ointment at 86.43 %and 90.21%, respectively, and the extract had the highest contraction rate at 95.82%, which demonstrated the strongest wound healing activity.
Keywords
Antioxidant; Anti-Inflammatory; H. helix; In Vivo; In Vitro; Ointment; Wound-Healing
Download this article as:| Copy the following to cite this article: Al-jaafreh A. M. Anti-inflammatory, Antioxidant, and Wound-healing Properties of the Methanolic Extracts from Hedera helix Fruits and Leaves. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Al-jaafreh A. M. Anti-inflammatory, Antioxidant, and Wound-healing Properties of the Methanolic Extracts from Hedera helix Fruits and Leaves. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/4aYZJ7W |
Introduction
Herbal medicine, often called phytotherapy or botanical therapy, has been more well-liked in recent years because of its apparent therapeutic advantages and decreased risk of side effects compared to synthetic medications1. Many people view natural sources as a more holistic and sustainable approach to healthcare. Plants contain various chemical compounds, including alkaloids, flavonoids, and terpenoids, which can have medicinal properties. The complexity of these compounds allows for a broad spectrum of therapeutic effects2. In recent years, there has been an increasing interest in studying the efficacy and safety of herbal remedies. However, it’s essential to approach herbal medicine cautiously, seeking guidance from qualified practitioners and considering potential interactions with other medications or health conditions. Furthermore, further research is necessary to better understand the effectiveness and safety of different herbal remedies.
Hedera helix, commonly known as ivy or English ivy, is a member of the Araliaceae family and is native to Europe and western Asia. It is a versatile and vigorous evergreen climber, known for its attractive appearance. It is often grown as an ornamental plant for its dark green leaves and ability to scale walls and cover surfaces3.
People have topically applied the H. helix as a remedy for removing benign warts. The extract of H. helix contains compounds with antioxidant properties that protect cells from oxidative damage caused by free radicals. Antioxidants are generally associated with potential health benefits4. Additionally, it may help relax smooth muscles, potentially making it useful for conditions involving muscle spasms or bronchial constriction. This effect may help manage conditions involving bronchial spasms, such as asthma. It can help thin and loosen mucus in the respiratory tract5. This can be particularly beneficial for individuals with productive acute coughs, making it easier to expel mucus6.
Some studies have reported positive effects on respiratory functions, including improved lung function7. However, further research is necessary to validate these effects and establish suitable dosages. It may help reduce allergic responses in some cases. Preliminary studies have suggested that certain compounds found in H. helix extract may exhibit antitumor properties8. Some studies have suggested that it may have a role in improving glucose metabolism and insulin sensitivity. However, more research is necessary to determine its effectiveness in managing diabetes9.
Researchers have long explored herbal remedies for their potential anti-inflammatory and anti-cancer properties. Such a diverse array of bioactive compounds in H. helix leaves suggests its potential for various therapeutic applications, like phenolic acids and flavonoids10, known for their antioxidant properties. They can help neutralize harmful free radicals in the body and reduce oxidative stress. They have anti-inflammatory effects and can potentially strengthen blood vessels and reduce the risk of cardiovascular issues.
The current study aimed to determine the total phenolic, flavonoid, and tannin content of methanolic extracts of H. helix fruits and leaves. Additionally, the extracts’ antioxidant qualities were evaluated using DPPH, FRAP, and ABTS tests. The study also assessed the possible anti-inflammatory properties of the extracts, as well as their wound-healing qualities on adult albino rats through ointments made from the extracts.
Materials and Methods
Plant material
H. helix leaves and ripe fruits were collected in September 2023 from the Mutah University campus in Al-Karak, south Jordan. After that, dust and dirt were eliminated from the ripe fruits and fresh leaves by washing them under running tap water without squeezing them. The ripe fruits and fresh leaves were air-dried in the shade for fourteen days at room temperature. An electronic blender was used- to grind the dried leaves and fruits, which were then put through a 40-mesh screen and kept in a closed glass container for later use. Using 100 ml of 95% methanol, the powdered portion (20 g) was macerated for 24 hours while being stirred periodically. After filtering, the methanol extract was placed in a rotating evaporator for three hours to evaporate the methanol and concentrate the extract.
Total Phenolic Content (TPC)
The total soluble phenolic component in the various ivy extracts was ascertained using the Folin-Ciocalteu reagent11. Half a milliliter of the extract was combined with 2.5 ml of Folin-Ciocalteu reagent (which had been diluted 10-fold with distilled water) and 2 ml of 7.5% Na2CO3 after the extract made from methanol had been diluted to a concentration of one mg/mL. After ninety minutes of incubation at thirty degrees Celsius, the samples were analyzed for absorbance at 765 nm using a spectrophotometer (HITACHI U-5100 UV-VIS) against a blank sample. The amount of phenolics in the extracts was expressed using gallic acid equivalent (mg GA/g extract). Each value was consistently stated as one gram of the relevant dry weight of the plant extract. All measurements were repeated three times
Determination of total flavonoid content:
The total amount of flavonoid in 95% methanol plant extracts was quantified using the AlCl3 technique12. Briefly, a 2% AlCl3.6H2O solution was mixed with 20 µL of the extract. The mixture was shaken vigorously, and 10 ml of water that had been double-distilled was added to dilute it. After 10 minutes of incubation, the absorbance for the reaction mixture was measured using an ultraviolet-visible spectrophotometer (HITACHI U-5100 UV-VIS) at 440 nm. The flavonoid content was quantified- in milligrams per gram of dry material, or Trolox equivalents. Three duplicates of each determination were made.
Estimation of Total Tannin Content
The Folin-Ciocalteu method was applied to ascertain the total tannin content13. About 0.1 mL of the extract was added. Then a volumetric flask (10 mL) was filled with 7.5 mL of distilled water. Half a milliliter of Folin-Ciocalteu phenol reagent, one milliliter of 35% sodium carbonate solution, and diluted to 10 mL after distilled water was added. The mixture was left at ambient temperatures for half an hour. Using the same procedure, a series of reference standard solutions of gallic acid were made. Using a spectrophotometer, the absorbance of the test and standard solutions at 700 nm against the blank was measured. Three separate measurements of the tannin content were made.
DPPH free radicals scavenging activity
The standard approach14 was applied with appropriate modifications to assess the plant extract’s ability to scavenge DPPH free radicals15. A solution of DPPH (10 mg) in 250 mL of methanol was created, yielding a 40 µg/ml concentration. A concentration of 1 mg/mL was achieved by preparing the plant extract stock solution in methanol. Ten concentrations ranging from 500 to 0.97 µg/ml were obtained via dilutions. DPPH (1 mL) was combined with diluted solutions (1 mL each). At room temperature, the absorbance was measured at 517 nm after 30 minutes in the dark. Except for the extract, the control samples contained all of the essential reagents. The formula used to compute the percentage of inhibitions was:

A non-linear regression analysis was used to estimate the IC50 values based on the curve representing the percentage inhibition vs. concentration. The information was displayed as mean values (n = 3) ± standard deviation.
ABTS free radicals scavenging activity:
The ABTS radical cation removal of the color test was also used to measure the activity of plant materials to eliminate free radicals16. After mixing 2.45 mM persulfate of potassium (1:1) with 7 mM ABTS in water, the ABTS cation radical was generated. The mixture was then left to rest for 16 hours in the dark at room temperature before being used. Then, methanol was added to the ABTS+ solution to dilute it and achieved an absorbance of 0.7 ±0.02 at 734 nm. After 30 minutes following the first mixing, 3.995 mL of diluted ABTS· solution was then followed by 5 µL of plant extract, and the absorbance was measured. For every assay, a suitable solvent blank was used. A minimum of three measurements were made for each. To find the percent of inhibition, the absorbance at 734 nm- was measured and the following formula used was:

% of inhibition={(control Abs-sample Abs)/(control Abs)}*100%
where Am represents the absorbance of the ABTS radical with methanol and Ae represents the absorbance of the ABTS radical extract. In contrast, a non-linear regression model was used to determine the IC50 values based on the curve representing the percentage of inhibition vs. concentration. The information was displayed as mean values of triplet measurements ± standard deviation.
Ferric reducing antioxidant power (FRAP)
Reduced antioxidant power due to ferric was assessed using spectrophotometry17. The process is based on reducing the colorless Fe3+ TPTZ complex to the blue-colored Fe2+-tripyridyltriazine complex, created at low pH by the action of antioxidants that donate electrons. This process was assessed by measuring an alteration in absorption at 593 nm. FRAP solution was prepared by mixing 10 mL TPTZ in 40 mM HCl, 20 mM FeCl3.6H2O, and 300 mM acetate buffer in a 10:1:1 ratio at 37°C. Five microliters of the appropriately diluted plant sample and the newly prepared working FRAP reagent (3.995 mL) were pipetted and well-mixed. After 30 min at 37°, the Fe3+ TPTZ complex reduced to Fe2+ and formed a bright blue color complex. Comparing the absorption at 593 nm to a reagent blank, 3.995 mL of FRAP reagent and 5 µL of distilled water were used in place of the sample. Ascorbic acid (As) was used as a reference substance in the concentration range of 25-500 µg/mL. Ascorbic acid equivalents (AsE) for each gram of dried ivy extract were used to express FRAP activity results. The information was displayed as mean values of triplet measurements ± standard deviation.
Inhibition of protein denaturation
Assay for Bovine Serum Albumin (BSA) denaturation using Williams et al. (2008), a modified form of the BSA test18, the anti-inflammatory properties of both extracts of plants were assessed. Tris-buffered saline was used to create a 0.4% w/v BSA solution. Acetic acid was used to bring the pH down to 6.4. Stock solutions were prepared in methanol for each plant at 50 micrograms per ml, Many concentrations of one µg/mL, 0.50 µg/mL, and 0.25µg/mL of the sample concentrations were reflected by equivalent aliquots of 5.0 µL, 10 µL, and 20 µL placed within test tubes that held one milliliter of 0.4%, w/v BSA solution. The negative control (methanol) was also tested in this way. After that, the solutions were heated for 10 minutes at 72 °C in a water bath and then cooled for 20 minutes in a lab setting. Using an air blank, the solutions` turbidity (or degree of protein precipitation) was obtained at 660 nm in a (HITACHI U-5100 UV-VIS) spectrophotometer. The mean values for absorbance were recorded, and the experiments were run in duplicate. The equation was used to determine the percentage reduction in precipitation (protein denaturation) about the negative control:
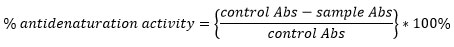
Ointment formulation
As Paju et al. (2013)19 outlined, the conventional procedure was the basis for creating the medicated unguent formulation. Neobacin, a commercial ointment containing Bacitracin Zinc and Neomycin Sulphate (5 mg)was used as routine therapy. One gram of the extract and nineteen grams of white Vaseline were combined to create the medicated ointment. The 5% therapeutic ointment ingredients were all combined in a mortar and mortar, stirring continuously until the mixture was homogeneous and formed into an ointment preparation. To create an ointment without the therapeutic components. Before adding the mixture, the mortar was preheated for between five and six minutes at fifty degrees Celsius.
Experimental animals
For the study, twenty adult albino rats of both sexes (weighing 200–250 grams) aged eight weeks were split into a total of four categories of five rats each. Acquired from the Mutah University Science College’s Animal House, Biology Department. The animals were housed in different cages in randomly assigned groups to monitor wound healing after a five-day acclimatization period. The animals were fed freely and had access to light for twelve hours daily. The care and management of the animals adhered to globally recognized standards for the ethical use of animals in laboratories, The Present work was reviewed and approved by the institutional animal ethics committee (Decision number 2012021), as per the committee for supervision of in-vivo experimentations on animal guidelines in Mutah University.
Before creating the wound, 70% ethanol had been applied as an antiseptic to the area that had been shaved. Diethyl ether was used to anesthetize the rats, and an electrical clipper removed their hair. Applying a biopsy punch to remove an entire-thickness piece of skin from a pre-shaven region resulted in circular wounds that were 10 mm in diameter. According to study20, no topical or systemic antimicrobial medications were used, and the wounds were left uncovered. On the third day after the wounds had healed, the rats were divided into four groups by randomization. Topically applied to the excised wounds were the two prepared herbal ointments (leaves and fruits), positive (drug), and negative (simple ointment alone) controls. Wounds were tracked to the four therapy groups every three days starting on the third post-wounding day. Using a digital vernier caliper, the wound diameters were measured, and the contraction ratios of the wound were computed using 100% as the starting size.
Where Di is the initial wound’s diameter while Df is the final wound diameter on the last day (day 12),
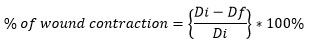
Statistical Analysis
Data analysis was performed using Microsoft Excel software (Windows 10). Significant statistical differences were evaluated using a one-way analysis of variance (ANOVA).
Results
The maceration of 100 g of dried and powdered H. helix leaves in a solution of 95% methanol for forty-eight hours yielded 4.27 g of dried raw extract. And 5.52g of dried raw extract was produced from 100 g of dried and powdered ripe H. helix fruits that were macerated in 95% methanol for 48 hours.
Table 1 displays the examined samples`- total phenolic amount (TPC). The TPC, measured in terms of GAE, was 89.47 ± 7.5 mg of GAE/g of extract for H. helix leaves and 100 ± 7.64 mg of GAE/g of extract for mature fruits. Trolox equivalents (mg) per gram of dry extract was the unit of measurement for total flavonoid content (TFC). The amounts of flavonoids in H. helix‘s leaves and mature fruits also differed greatly. With 37.14 ±1.3 mg of TE/gm of extract, leaves had the highest flavonoid content, followed by ripe fruits with 27.61 ± 0.2 mg of TE/gm of extract.
Another class of bioactive chemicals is known as total tannin content. Tannins in extracts should be considered despite their lower levels than other bioactive ingredients. Table 1 expresses the overall tannin content of the different extracts. This investigation found the highest total tannin concentration was 17.5 ± 2.54 mg GAE/g in ripe fruit extract and 24.79± 2.27 mg GAE/g in leaf extract.
Regarding the DPPH measurement of antioxidant activity in each research extract, the extract, including ripe fruits, had the highest activity (IC50 3.49±0.26 mg/mL). In contrast, the leaves extract had the lowest value (IC50 8.79± 1.26 mg/ml). Regarding ABTS antioxidant activity, the ripe fruit extract had a lower scavenging ability and higher IC50 values (8.7 ± 1.85 mg/mL), but the leaf extract had a more significant scavenging activity (4.54±0.99). Finally, the FRAP values indicate that the lower antioxidant properties were similar to those of the DPPH scavenging experiment, which found that the antioxidant activity of ripe fruit extracts was higher than that of leaf extracts; the IC50 values were 62.35± 4.41 and 75.5± 7.53, respectively.
The current study compared the anti-inflammatory effects of mature fruits and leaves extracts to those of bovine serum albumin that had been denatured In vitro. Table 2 presents the findings. The current results showed that both test extracts inhibited 50% of protein (albumin) denaturation in a dose-dependent manner at concentrations that ranged from 31.25 to 200 μg/ml. The IC50 values of 75.26± 3.87 and 115.62± 6.47 for the leaf and mature fruit extracts indicated that the leaf extract was more active.
Table 1: Phytochemical content and total Yield of the Crude Extract of 95% methanol leaf extract and ripe fruit extract of H.helix
|
|
Polyphenol GAE mg/g extract |
Flavonoid TE mg/g extract |
Tannin GAE mg/g extract |
Extract yield % g/100 g dry powder |
|
Leaves |
89.47±7.5 |
37.14±1.3 |
24.79±0.27 |
4.27 % |
|
Fruit |
100±7.64 |
27.61±0.2 |
17.5±0.54 |
5.52 % |
Table 2: Antioxidant activity and anti-albumin denaturation activity of 95% methanol leaf extract and ripe fruit extract of H.helix
|
|
ABTS IC50 mg/ml |
DPPH IC50 mg/ml |
FRAP AscE mg/g |
albumin denaturation |
|
Leaves |
4.54± 0.99 |
8.79±1.26 |
62.35± 4.41 |
75.26±3.87 |
|
Fruit |
8.696 ± 1.85 |
3.49± 0.65 |
75.5±7.53 |
115.62±6.47 |
Table 3: Effect of 95% methanol leaf extract and ripe fruit extract of H.helix on wound contraction in excision model
|
Treatment day |
-ve group Pure Ointment |
5% Ointment Leaves |
5% Ointment fruit |
Drug |
|
3 |
21.64 |
14.12 |
10.47 |
16.51 |
|
6 |
30,4 |
46.29 |
43.42 |
33.96 |
|
9 |
52.13 |
62.78 |
68.31 |
68.64 |
|
12 |
81.12 |
95.82 |
90.21 |
86.43 |
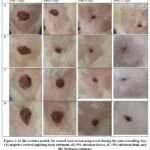 |
Figure 1: In the excision model, the wound contraction progressed during the post-wounding days (A) negative control applying basic ointment, (B) 5% ointment leaves, (C) 5% ointment fruit, and (D) Neobacin ointment. |
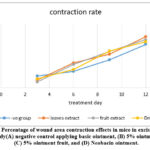 |
Figure 2: Percentage of wound area contraction effects in mice in excision mode, in vivo study(A) negative control applying basic ointment, (B) 5% ointment leaves, (C) 5% ointment fruit, and (D) Neobacin ointment. |
Discussion
Research concerning plant varieties’ antioxidant properties has exploded in recent years due to the use of several of them as sources of phytotherapeutic compounds21–23. Some of these methods include preventing oxidative stress, limiting reactive oxygen species (ROS) to safe levels24,25, and using them for effective signaling. Studying the antioxidant properties of plant species is essential because secondary metabolites, especially phenols, can change the amount of reactive oxygen species (ROS) and start a chain of metabolic reactions that promote tolerance. Because of their aromatic ring, which stabilizes and repositions the unpaired electrons in their structure to allow for the exchanging of hydrogen atoms and electrons to their hydroxyl groups, phenols are the main compounds in plants that show antioxidant activity26,27. The total phenol content is influenced by temperature, water stress, light conditions, plant tissue, developmental stage, and other environmental factors28,29.
H. helix‘s phytochemical screening revealed the presence of many kinds of secondary metabolites, including tannins, terpenoids, alkaloids, and saponins. Alkaloids were detected throughout the methanol, n-hexane, chloroform, and ethyl acetate extracts, whilst tannins, terpenoids, and saponins were found in the chloroform and methanolic extracts30. β-amyrin, stigmasterol, and hexadecanoic acid were identified as the active ingredients in H. helix leaves extract extracted by methylene chloride31. Chromatographic techniques were used to identify the flavonoids quercetin, kaempferol, apigenin, and rutin32. With the use of reversed-phase high-HPLC, the saponins α-hederin and hederacoside C from various ivy leaf extracts were identified33.
The study relied on this information to compare the effects of the ripe H.helix fruit extract and the H. helix leaf extract. While ripe ivy leaves have been the subject of numerous studies, as far as we know, none of them have examined the significance of ripe H. helix fruits in wound healing.
The investigators are now utilizing a variety of antioxidant tests to find the antioxidant potential in natural extracts. More than a single experiment is required to measure the antioxidant capacity of natural extracts due to the complex structure of the bioactive chemicals present3,34,35. However, DPPH, ABTS, and FRAP are the most commonly utilized techniques36. Using phenolic compounds with antioxidant activity is preferable when creating pharmaceutical formulations from any natural resource5,37. Several studies have supported the link between phenolic chemicals and antioxidant capability38,39. Table 2 shows the antioxidant potential of many different extracts that exhibit different antioxidant properties from one another. Phenolic compounds are among the main chemical classes that are known to act as principal antioxidants or harmful free radical terminators.
These earlier results suggest that any of the techniques employed in this investigation can determine and categorize the antioxidant activity of these different extracts. According to study40, wound healing is a complicated process that begins during the fibroblastic phase, when the wound’s surrounding tissue begins to shrink, and concludes with the cellular structures and layers of tissue of wounded tissue being returned to their initial state. Contracture, granulation, epithelization, and collagenation are some of its stages41. The therapeutic benefits of herbal treatments are mostly caused by a variety of circumstances. Because polyphenolic compounds may control and modify inflammatory reactions, they are useful as therapeutic agents in the healing of wounds. By promoting fibroblast proliferation and/or collagen formation, a variety of phytochemicals found in medicinal plants are significant regulators of homeostasis, re-epithelialization, and regeneration. Many studies have shown that medicinal plants and the phytochemicals they contain have a potent effect on wound healing through several interconnected pathways42,43.
There are three stages of wound healing: the inflammatory, proliferative, and maturational/remodeling phases. Collagen deposition, angiogenesis, and epithelialization occur after the proliferative phase.
It is well known that wound healing throughout the regeneration process requires the carefully regulated presence of defense cells44,45. Inflammation and hemostasis are the distinctive characteristics of the inflammatory phase. So, controlling inflammation is the beginning of treating wounds without causing scarring. Significant alterations were validated by the examination of data obtained from polymorphonuclear cells. In the chronic stage, the H. helix extract first decreases the number of polymorphonuclear cells and then encourages the exchange of polymorphonuclear cells, which is essential for the completion of the regenerative and inflammatory mechanisms46.
According to Gülçin et al. (2004)47, H. helix constituents such as α-hederin completely drain intracellular glutathione during In vitro tests, hence preventing the generation of oxygen species that are reactive (ROS). Therefore, a decrease in nitric oxide release leads to a decrease in the NF-kappa B expression, the transcription factor of inflammatory cytokines (e.g., IL1-β, TNF-α), and the chemokines that cause the migration of inflammatory cells34. This indicates that the H. helix extract can function in both polymorphonuclear migration control and reducing the activation of macrophages. Reduced visible scar tissue results from wounds contracting throughout the maturation48. The granulation tissue that develops at the end of the proliferative phase mainly comprises new tiny blood vessels, fibroblasts, collagen, and edema. These phytochemical ingredients powerfully and efficiently prevent the release or action of prostaglandin and bradykinin during the third stage of edema production. Its secondary metabolites may contribute to its anti-inflammatory properties. Terpenoids prevent the metabolism of arachidonic acid and decrease the activity of phospholipase A249,50. Also, Terpenoids help wounds heal by having antibacterial and astringent abilities that seem to have been the cause of wound contraction and an increased rate of epithelial development25,51.
Fibroblasts relocate to the wound site throughout the healing process, where they proliferate and go on to create collagen, the primary extracellular matrix component. Fibroblast stimulation is one of the ways that herbal extracts may speed up the healing of wounds. Put differently, some phytochemicals stimulate the growth, migration, and activity of fibroblasts52,53. The lifespan of collagen fibrils will rise with any medication that suppresses these processes. Therefore, by increasing the antioxidant state, several herbal extracts promote improved collagenation54. Several researchers have acknowledged that antioxidants can influence the process of oxidation by their ability to scavenge oxygen, chelate catalytic metals, and react with free radicals. It is believed that reactive oxygen species (ROS) and free radicals play a significant role in impeding the healing process20. Research has demonstrated the antioxidant properties of terpenoids30, flavonoids55, tannins56,57, and saponins58. The existence of saponins with antioxidant activities that are isolated from the H. helix has been shown, such as hederacolchisides E and F, α-hederin, and hederasaponin-c47. In addition, it is well known that flavonoids and their derivatives inhibit the death of cells by increasing vascularity and reducing lipid peroxidation. By blocking prostaglandin formation, polyphenols and flavonoids have antimicrobial and anti-inflammatory properties28. Glycosides have anti-inflammatory, antibacterial, and antioxidant properties. Because of their astringent and antioxidant qualities, tannins improve the efficiency and development of new tissue, speeding up the wound recovery process. It is therefore plausible that the phytochemical component present in the raw extract promotes wound healing59,60. Applying plant extracts and components topically onto a wound will not cause the plant to produce the desired effect, to ensure a sustained release of the medication, it is necessary to apply ointment at the site of application. The ointment’s base facilitated the formation of a moisture barrier across the wound region, mainly due to the presence of hard, white, and soft paraffin in large part49.
The findings of the excision model demonstrated that each extract significantly promoted wound healing.
Comparatively, various plants with compositions similar to those of H. helix, which has anti-inflammatory and wound-healing characteristics, have also been seen and described in analogous ways during these situations61–63.
Quercetin, kaempferol, glucosides, epicatechin, and catechin are the principal chemicals found in leaves that are recognized as wound-healing agents64. Also, punicalin, kaempferol, quercetin-3-O-xylopyranoside, Punicalagin, quercitrin, and ellagic acid, were found in the plant, also,65 reported that these compounds may be linked to better cell proliferation, re-epithelialization, and the remodeling phase of wounds, as many types of research had shown that the presence of these components in H. helix extract This can explain the healing of wounds in samples treated with extracts in less time than untreated samples.
Conclusions
The study conducted highlights the antioxidant, anti-inflammatory, and wound-healing qualities of extracts from H. helix ripe fruits and leaves. This traditional herbal medicine has piqued the interest of researchers as it can be used to treat various illnesses. The findings could inspire the pharmaceutical industry to develop treatments using these plant extracts and encourage the use of these natural products in an ecologically and socioeconomically sustainable way. The revealed bioactive components of the extracts include flavonoids, phenolic ingredients, and tannins, which could be responsible for the abovementioned healing benefits. Therefore, it can be inferred that H. helix’s healing potential can have benefits for both medicinal and cosmetic applications.
Acknowledgemet
None to declare
Conflict of Interest
The author declares no conflict of interest.
Funding Source
No external funding for this study.
References
- Okba MM, Baki PMA, Abu-Elghait M, et al. UPLC-ESI-MS/MS profiling of the underground parts of common Iris species in relation to their anti-virulence activities against Staphylococcus aureus. J Ethnopharmacol. 2022;282:114658.
CrossRef - Ramadan A, Kamel G, Shokry AA, El-Shiekh RA. Tropical Journal of Natural Product Research.”. Published online 2020.
CrossRef - Sen NB, Guzelmeric E, Vovk I, Glavnik V, Kırmızıbekmez H, Yesilada E. Phytochemical and bioactivity studies on Hedera helix L.(ivy) flower pollen and ivy bee pollen. Antioxidants. 2023;12(7):1394.
CrossRef - Rashed KNZ. Comparsion of Antioxidant Potential of Dimocarpus Longan Lour. Extracts and the main Phytoconstituents. Int J Sci Invent Today. 2013;2(6):487-491.
- Shokry A, El-Shiekh R, Kamel G, Ramadan A. Phytochemical contents, biological activities and therapeutic applications of hedera helix (ivy leaf) extracts: a review. Nat Prod J. 2022;12(4):22-32.
CrossRef - Parvu M, Vlase L, Parvu AE, Rosca-Casian O, Gheldiu AM, Parvu O. Phenolic compounds and antifungal activity of Hedera helix L.(Ivy) flowers and fruits. Not Bot Horti Agrobot Cluj-Napoca. 2015;43(1):53-58.
CrossRef - Völp A, Schmitz J, Bulitta M, Raskopf E, Acikel C, Mösges R. Ivy leaves extract EA 575 in the treatment of cough during acute respiratory tract infections: meta-analysis of double-blind, randomized, placebo-controlled trials. Sci Rep. 2022;12(1):20041.
CrossRef - Yenigün VB, Kocyigit A, Kanımdan E, Durmus E, Koktasoglu F. Hedera helix (Wall Ivy) leaf ethanol extract shows cytotoxic and apoptotic effects in glioblastoma cells by generating reactive oxygen species. Acta Medica Cordoba. 2023;54(4):295-303.
CrossRef - Khan S, Adil A, Naeem S, et al. Evaluation of Acute and Chronic Antidiabetic Activity of Ivy (Hedera helix L.) Aqueous Leaf Extract in Rat Model. Pak J Biol Sci. 2020;23(11):1357-1368.
CrossRef - Erdogdu M, Atilgan A, Erdogdu Y, Yildiz A. Flavonoid from Hedera helix fruits: A promising new natural sensitizer for DSSCs. J Photochem Photobiol A Chem. 2024;448:115288.
CrossRef - Vl S. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152-178.
CrossRef - Ordonez AAL, Gomez JD, Vattuone MA. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97(3):452-458.
CrossRef - Galvão MAM, Arruda AO de, Bezerra ICF, Ferreira MRA, Soares LAL. Evaluation of the Folin-Ciocalteu method and quantification of total tannins in stem barks and pods from Libidibia ferrea (Mart. ex Tul) LP Queiroz. Brazilian Arch Biol Technol. 2018;61:e18170586.
CrossRef - Kumarasamy Y, Byres M, Cox PJ, Jaspars M, Nahar L, Sarker SD. Screening seeds of some Scottish plants for free radical scavenging activity. Phyther Res An Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv. 2007;21(7):615-621.
CrossRef - Altarawneh RM, Al‐Jaafreh AM, Qaralleh H, Al‐Qaralleh OS. Chemical profiling of Punica granatum peels from Jordan using LC–MS/MS and study on their biological activities. Int J Food Sci Technol. 2022;57(8):5256-5267.
CrossRef - Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9-10):1231-1237.
CrossRef - Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70-76.
CrossRef - Williams LAD, O’connar A, Latore L, et al. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals. West Indian Med J. 2008;57(4).
- Paju N, Yamlean PVY, Kojong N. Uji efektivitas salep ekstrak daun binahong (Anredera cordifolia (Ten.) Steenis) pada kelinci (Oryctolagus cuniculus) yang terinfeksi bakteri Staphylococcus aureus. Pharmacon. 2013;2(1).
- Cedillo-Cortezano M, Martinez-Cuevas LR, López JAM, Barrera López IL, Escutia-Perez S, Petricevich VL. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals. 2024;17(3):303.
CrossRef - Manilal A, Sabu KR, Shewangizaw M, et al. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon. 2020;6(1).
CrossRef - Ghildiyal S, Gautam MK, Joshi VK, Goel RK. Wound healing and antimicrobial activity of two classical formulations of Laghupanchamula in rats. J Ayurveda Integr Med. 2015;6(4):241.
CrossRef - Karumi EW, Maitai CK, Okalebo FA, et al. Anthelmintic and antibacterial activity of Hagenia abyssinica (Bruce) JF Gmel (rosaceae). East Cent African J Pharm Sci. 2013;16(3):75-80.
- Mechesso AF, Tadese A, Tesfaye R, Tamiru W, Eguale T. Experimental evaluation of wound healing activity of Croton macrostachyus in rat. African J Pharm Pharmacol. 2016;10(39):832-838.
CrossRef - Hossain MA, AL-Raqmi KAS, Al-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705-710.
CrossRef - Zeng Q, Xie H, Song H, et al. In vivo wound healing activity of Abrus cantoniensis extract. Evidence-Based Complement Altern Med. 2016;2016.
CrossRef - Upadhyay A, Chattopadhyay P, Goyary D, Mitra Mazumder P, Veer V. Ixora coccinea enhances cutaneous wound healing by upregulating the expression of collagen and basic fibroblast growth factor. Int Sch Res Not. 2014;2014.
CrossRef - Sharma RK, Kundra R, Samant SS, Nandi SK. Antioxidant properties of methanol extracts from Olea ferruginea Royle seeds. Natl Acad Sci Lett. 2017;40:379-382.
CrossRef - Diljkan M, Škondrić S, Hasanagić D, et al. The antioxidant response of Hedera helix leaves to seasonal temperature variations. Bot Serbica. 2022;46(2):295-309.
CrossRef - Ndezo Bisso B, Njikang Epie Nkwelle R, Tchuenguem Tchuenteu R, Dzoyem JP. Phytochemical screening, antioxidant, and antimicrobial activities of seven underinvestigated medicinal plants against microbial pathogens. Adv Pharmacol Pharm Sci. 2022;2022.
CrossRef - Medeiros JR, Medeiros H, Mascarenhas C, Davin LB, Lewis NG. Bioactive components of Hedera helix. Published online 2002.
- Ferrara L, Naviglio D, Faralli S. Identification of active principles of Hedera helix L. in aqueous extracts. J Phytochem. 2013;114:170-175.
- Demirci B, Goppel M, Demirci F, Franz G. HPLC profiling and quantification of active principles in leaves of Hedera helix L. Die Pharm Int J Pharm Sci. 2004;59(10):770-774.
- Shokry AA, El-Shiekh RA, Kamel G, Bakr AF, Ramadan A. Bioactive phenolics fraction of Hedera helix L.(Common Ivy Leaf) standardized extract ameliorates LPS-induced acute lung injury in the mouse model through the inhibition of proinflammatory cytokines and oxidative stress. Heliyon. 2022;8(5).
CrossRef - Suica-Bunghez IR, SORESCU AA, DONCEA SM, Constantin M, Raut I, Rodica Mariana ION. Phytochemical, antioxidant and antimicrobial characterization of Hedera helix L. extract. J Plant Dev. 2020;27.
CrossRef - Qabaha K, Abbadi J, Yaghmour R, Hijawi T, Naser SA, Al-Rimawi F. Unveiling the antibacterial and antioxidant potential of Hedera helix leaf extracts: recent findings. Can J Physiol Pharmacol. 2023;102(1):26-32.
CrossRef - Gavrila AI, Zalaru CM, Tatia R, et al. Green Extraction Techniques of Phytochemicals from Hedera helix L. and In Vitro Characterization of the Extracts. Plants. 2023;12(22):3908.
CrossRef - Punia S, Sandhu KS. FUNCTIONAL AND ANTIOXIDANT PROPERTIES OF DIFFERENT MILLING FRACTIONS OF INDIAN BARLEY CULTIVARS. Carpathian J Food Sci Technol. 2015;7(4).
- Sandhu KS, Godara P, Kaur M, Punia S. Effect of toasting on physical, functional and antioxidant properties of flour from oat (Avena sativa L.) cultivars. J Saudi Soc Agric Sci. 2017;16(2):197-203.
CrossRef - Yadav JP, Verma A, Pathak P, Kumar V, Patel DK. Wound healing, antidiabetic and antioxidant activity of Neolamarckia cadamba, quercetin rich, extract. Pharmacol Res Chinese Med. 2024;11:100417.
CrossRef - Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition. 2010;26(9):862-866.
CrossRef - Maver T, Maver U, Stana Kleinschek K, Smrke DM, Kreft S. A review of herbal medicines in wound healing. Int J Dermatol. 2015;54(7):740-751.
CrossRef - Artem Ataide J, Caramori Cefali L, Machado Croisfelt F, Arruda Martins Shimojo A, Oliveira‐Nascimento L, Gava Mazzola P. Natural actives for wound healing: A review. Phyther Res. 2018;32(9):1664-1674.
CrossRef - Gepdiremen A, Mshvildadze V, Süleyman H, Elias R. Acute and chronic antiinflammatory effects of Hedera colchica in rats. J Ethnopharmacol. 2004;94(1):191-195.
CrossRef - de Moraes Palma FA, da Mata AR, de Almeida Rocha I, et al. Study of wound healing in rat skin treated with extract of Hedera helix, L. Brazilian J Dev. 2021;7(12):115126-115139.
CrossRef - Vieira AE, Repeke CE, Ferreira Junior S de B, et al. Intramembranous bone healing process subsequent to tooth extraction in mice: micro-computed tomography, histomorphometric and molecular characterization. PLoS One. 2015;10(5):e0128021.
CrossRef - Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: α-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004;70(06):561-563.
CrossRef - Phillips GD, Whitehead RA, Knighton DR. Initiation and pattern of angiogenesis in wound healing in the rat. Am J Anat. 1991;192(3):257-262.
CrossRef - Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638-646.
CrossRef - Gebrehiwot M, Asres K, Bisrat D, Mazumder A, Lindemann P, Bucar F. Evaluation of the wound healing property of Commiphora guidottii Chiov. ex. Guid. BMC Complement Altern Med. 2015;15(1):1-11.
CrossRef - Ugochukwu SC, Arukwe Uche I, Ifeanyi O. Available online at www. pelagiaresearchlibrary. com. Asian J Plant Sci Res. 2013;3(3):10-13.
- Amin ZA, Ali HM, Alshawsh MA, Darvish PH, Abdulla MA. Application of Antrodia camphorata promotes rat’s wound healing in vivo and facilitates fibroblast cell proliferation in vitro. Evidence-Based Complement Altern Med. 2015;2015.
CrossRef - Bueno FG, Panizzon GP, de Leite Mello EVS, et al. Hydrolyzable tannins from hydroalcoholic extract from Poincianella pluviosa stem bark and its wound-healing properties: phytochemical investigations and influence on in vitro cell physiology of human keratinocytes and dermal fibroblasts. Fitoterapia. 2014;99:252-260.
CrossRef - Umadevi S, Mohanta G, Kalaichelvan V, Manavalan R. Studies on wound healing effect of Flaveria trinervia leaf in mice. Indian J Pharm Sci. 2006;68(1).
CrossRef - Ghasemzadeh A, Jaafar HZE, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) RM Sm grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15:1-10.
CrossRef - Jain S, Jain A, Jain S, Malviya N, Jain V, Kumar D. Estimation of total phenolic, tannins, and flavonoid contents and antioxidant activity of Cedrus deodara heart wood extracts. Egypt Pharm J. 2015;14(1):10-14.
CrossRef - Costa G, González-Manzano S, González-Paramás A, Figueiredo IV, Santos-Buelga C, Batista MT. Flavan hetero-dimers in the Cymbopogon citratus infusion tannin fraction and their contribution to the antioxidant activity. Food Funct. 2015;6(3):932-937.
CrossRef - Nadeem A, Shahzad H, Ahmed B, Muntean T, Waseem M, Tabassum A. Phytochemical profiling of antimicrobial and potential antioxidant plant: Nepeta cataria. Front Plant Sci. 2022;13:969316.
CrossRef - Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J.(Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):1-10.
CrossRef - Lin PH, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J. Zinc in wound healing modulation. Nutrients. 2017;10(1):16.
CrossRef - Lopes AI, Pintado MM, Tavaria FK. Plant-Based Films and Hydrogels for Wound Healing. Microorganisms. 2024;12(3):438.
CrossRef - Paswan SK, Verma P, Mohapatra L, Rao CV, Srivastava S, Kumar S. Assessment of wound healing activity and safety profile of Amaranthus spinosus extract using incision wound healing model. Curr Res Biotechnol. 2023;6:100151.
CrossRef - Yuniarsih N, Hidayah H, Gunarti NS, et al. Evaluation of Wound-Healing Activity of Hydrogel Extract of Sansevieria trifasciata Leaves (Asparagaceae). Adv Pharmacol Pharm Sci. 2023;2023.
CrossRef - Fujishima MAT, Sá DMC, Lima CM de S, et al. Chemical profiling of Curatella americana Linn leaves by UPLC-HRMS and its wound healing activity in mice. PLoS One. 2020;15(1):e0225514.
CrossRef - Pereira LOM, Vilegas W, Tangerina MMP, et al. Lafoensia pacari A. St.-Hil.: Wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J Ethnopharmacol. 2018;219:337-350.
CrossRef









