Ravishankar Patil1,2,4,5, Chetan Aware1, Kavita Shinde3,6, Ruchika Kaul-Ghanekar3,6,7, Govind vyavahare1, Vishwas Bapat1 and Jyoti Jadhav1,2*
1Department of Biotechnology, Shivaji University, Vidyanagar, Kolhapur, Maharashtra, India.
2Department of Biochemistry, Shivaji University, Vidyanagar, Kolhapur, Maharashtra, India.
3Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth University, Pune, Maharashtra, India.
4Amity Institute of Biotechnology, Amity University, Mumbai, Maharashtra, India.
5Amity Centre for Nuclear Biotechnology, Amity University, Mumbai, Maharashtra, India
6Cancer Research Lab, Symbiosis School of Biological Sciences (SSBS), Symbiosis International (Deemed University), Pune Maharashtra, India
7Symbiosis Centre for Research and Innovation (SCRI), Symbiosis International (Deemed University), Pune, Maharashtra, India
Corresponding Author E-mail:profjpjadhav@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2898
Abstract
The Fabaceae (Leguminosae) plant family contains several species of the Mucuna Adans. genus possessing therapeutic potential and growing widely in tropical and sub-tropical regions. In this research, we investigated the anti-inflammatory and antioxidant properties of the extract from the Mucuna sanjappae Aitawade & S.R.Yadav seeds. Initially, we conducted an in vitro anti-inflammatory activity test using the bovine serum albumin anti-denaturation assay and found promising dose-dependent activity. Subsequently, we performed an in vivo anti-inflammatory and antioxidant study on a rat paw edema model induced by carrageenan. Three different doses of M. sanjappae seed water extract (50, 100 and 200mg/kg B/W) were used for the study (Oral administration). Edema measurement was carried out at 0, 2, 4 and 6 hr intervals. Dose dependent inhibition in edema in the M. sanjappae seed extract treatment group was observed with maximum activity for 200mg/kg B/W dose at 4 hr (53.49%). Standard drug showed maximum edema inhibition (54.94%) at 6hr. Our results also showed that, M. sanjappae seed extract inhibited pro-inflammatory cytokine TNF-α and increases anti-inflammatory cytokine IL-10 with increased level of blood serum antioxidants. Phytochemical analysis for secondary metabolites including polyphenol, flavonoids, phytic acid, proanthocyanidin, tannin and saponin was also quantified which might be the responsible component for biological activities under study.
Keywords
Antioxidant; Anti-inflammatory drug; Carrageenan; Cytokine; Inflammation; Mucuna
Download this article as:| Copy the following to cite this article: Patil R, Aware C, Shinde K, Ghanekar R. K, Vyavahare G, Bapat V, Jadhav J. Anti-Inflammatory and Antioxidant Properties of the Mucuna sanjappae Seeds in the Rat Model and In Vitro Assays. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Patil R, Aware C, Shinde K, Ghanekar R. K, Vyavahare G, Bapat V, Jadhav J. Anti-Inflammatory and Antioxidant Properties of the Mucuna sanjappae Seeds in the Rat Model and In Vitro Assays. Biomed Pharmacol J 2024;17(2). |
Introduction
Inflammation can be defined as essential response exhibited by host after tissue injury or infection. However, its longstanding persistence may result in chronic diseases like cancer, cardiovascular diseases, neurological disorders, diabetes, pulmonary diseases, and arthritis1-7. Inflammation is characterized by pro-inflammatory enzymes, chemokines, cytokines, and certain signal proteins generated in response to infection or injury. Management of inflammatory diseases today represents an important medical problem since currently used non-steroidal anti-inflammatory drugs (NSAIDs) has several other adverse effects commonly known as gastroenteropathy1,7. Hence, identification of novel and effective therapies for safer management of inflammatory diseases remains an urgent priority. Natural plant wealth is continually being investigated for novel bioactive molecules with therapeutic properties. In contrast to modern synthetic drugs, natural bioactive are cost effective and provides significant protection from diseases without secondary adverse complications. Therefore, researchers are investigating plant-based drugs which can provide promising anti-inflammatory activity with lower secondary complications8. Numerous reports endorse the use of various plants for treating inflammation1,9.
Genus Mucuna belongs to family fabaceae, popularly known as cowitch, kapikachu and atmagupta. Mucuna species are recognized for their itching properties due to hairs present on the pods. Since ancient time, Indian ayurveda system of medicine uses seeds of Mucuna for the management of different types of diseases and disorders. Remarkable research has been carried out on Mucuna pruriens particularly for its anti-Parkinson’s, anti-infertility, anti-venom, anti-inflammatory and anti-bacterial activity7,10-16. Mucuna seed powder is very common ingredient in various ayurvedic formulations marked in India and across the world. However, exploitation of M. pruriens at large scale may affect ecosystem adversely and due to limited availability, final product cost may also increase. To avoid this, there is increasing interest in investigating hidden potential of other underutilized Mucuna spp. as a promising alternative therapeutic agent. Previously, our research group have successfully reported Mucuna species including M. macrocarpa, M. bracteata, M. imbricata and M. sanjappae etc. for their nutritional and medicinal benefits15-24. In 2019, we have reported L-DOPA (L-3,4-dihydroxyphenylalanine), an FDA approved anti-Parkinson’s drug in seeds of different Mucuna species found in Indian contingent and proved that number of Mucuna species possesses higher level of L-DOPA as compared to the commonly used M. pruriens25.
M. sanjappae is endemic plant species found in Western Ghats region of Maharashtra, India which belongs to genus Mucuna26. In previous studies we have demonstrated that M. sanjappae seeds possess promising level of nutritional components with gross energy 383 kcal and around 5.43 g of protein. Moreover, it contains about 7.3 % L-DOPA and other important primary and secondary metabolites, minerals, important phenolics etc. Furthermore, anti-Parkinson’s activity of M. sanjappae seed extract in Parkinson’s disease (PD) mice model intoxicated by MPTP is reported18,19. M. sanjappae seed extract could successfully ameliorate PD symptoms developed by MPTP toxicity. However, till date, there is no data available on the effect of M. sanjappae on inflammatory diseases and oxidative stress using in vitro or in vivo model. Carrageenan induced rat paw edema model is popular method of anti-inflammatory studies of natural as well as synthetic drugs1. Hence, present efforts have been made to examine anti-inflammatory and antioxidant properties of M. sanjappae seed on carrageenan induced rat paw edema model.
Material and Methods
Chemicals and reagents
Analytical grade solvents and chemicals were used the study. Carrageenan and enzyme-linked immunosorbent assay (ELISA) cytokine kits for TNF-α and IL-10 measurement were obtained from Sigma-Aldrich, USA. Diclofenac (Standard anti-inflammatory drug) was obtained from Recon, Bangalore, India respectively.
Plant material and preparation of drugs for administration
The pods of M. sanjappae were collected from its original location (Pune district, Western Ghats, Maharashtra, India). The herbarium was carefully prepared and stored at the herbarium center of the Botany department, Shivaji University, Kolhapur under the guidance of taxonomist Prof S. R. Yadav.
After removing the healthy seeds from the pods, fine powder was prepared. Extract of seed was produced by adding and macerating 1g M. sanjappae seed powder in 100 ml D/W in the mortar and pestle. Further, sonication for 15min and centrifugation at 10000 rpm for 10 min was carried out. Supernatant was carefully separated and stored for further use. Effective yield was calculated by evaporating water. Importantly, seed extract and standard drug diclofenac were prepared freshly at the time of dosing.
In vitro anti-inflammatory activity
Bovine serum albumin (BSA) anti-denaturation assay
This test was performed using a method defined earlier with slight modifications27. Various concentrations of seed extract and standard drug diclofenac were reacted with 1ml of 1% BSA solution prepared in 50mM Tris buffer (pH 6.5). Incubation was carried out at 37°C for 20 min and further heating at 64°C in water bath till mixture get turbid (around 5 to 10 min). Finally, tubes were cooled, and absorbance of generated turbidity was measured at 660 nm. D/W was used as a control. Following formula was used to calculate denaturation inhibition percentage:
% denaturation inhibition = A (control) – A (sample) / A (control) X 100
Where, A (control): Absorbance of the control; A (sample): Absorbance of samples.
Hypotonicity-induced HRBC membrane stabilization method
Capacity of M. sanjappae seed to protect hypotonicity encouraged human red blood cell membrane protection have been determined28. Various concentrations of M. sanjappae seed extract were made into 1 mL using distilled water in a tube. Initially, 0.5ml of 10% HRBC suspension and 0.5 mL of 0.25% hyposaline were added to each tube. Then, mixture was incubated at static condition for 30 mins at 37°C and centrifuged at 3000 rpm for 20 mins at 4°C. The amount of hemoglobin in the supernatant was performed at a wavelength of 560 nm. Working solution of standard drug aspirin was prepared in 0.2 M phosphate buffer (1 mL) at various concentration ranging from 100 µg to 500 µg. To induce complete hemolysis (100%) without any sample or drug, a control was prepared using distilled water as a replacement for hyposaline. To calculate the percentage of HRBC hemolysis and evaluate the degree of membrane stabilization or protection, below given formula was employed:
% of hemolysis = Absorbance of test/ Absorbance of control x 100
% protection = 100 – (% of hemolysis)
In vivo anti-inflammatory activity
Rats were divided into seven groups (6 rats per polypropylene cage) housed under controlled conditions (Relative humidity 44–56 %, temperature of 25±2 °C, and 12 h light/ dark cycles). Standard diet and water ad libitum was provided to the experimental animals. The experiment was started after proper acclimatization of animals in the laboratory environment after a week. Details of randomization, grouping and drug dosing are given in table 1. Seven days prior dosing was carried out by M. sanjappae seedextractin group IV, V, VI and VII. On the 8th day rats were kept fasted but water was provided ad libitum. Extract was administered 2hr before inducing the inflammation by carrageenan (0.9%) through sub plantar way and further investigation was accomplished.
Table 1: Randomization and grouping of animals for in vivo study
|
Group number |
Group name |
Dose content |
Number of animals |
|
I |
Normal control |
Vehicle solvent (D/W) |
6 |
|
II |
Carrageenan control |
100 µl (0.9% carrageenan) # |
6 |
|
III |
Diclofenac |
10mg/kg BW |
6 |
|
IV |
Test Dose-1 50mg/kg BW* |
50mg/kg BW |
6 |
|
V |
Test Dose-2 100mg/kg BW |
100mg/kg BW |
6 |
|
VI |
Test Dose-3 200mg/kg BW |
200mg/kg BW |
6 |
|
VII |
Sham Control |
200mg/kg BW |
6 |
BW* – Body Weight of animals.
# – 0.9% carrageenan were prepared in saline solution
Rat paw edema measurement
The inflammation in terms of swelling of carrageenan induced foot of animal was measured using plethysmometer (UGO Basile, Italy) as a water displacement in ml. The measurement was done at 0, 2, 4 and 6 hr. The decrease in paw volume was compared to the vehicle control. The percentage of inhibition in seed water extract and diclofenac treated group was compared with carrageenan induced group.
Inhibition Percentage (%) = [Carrageenan treated group-test drug group / Carrageenan treated group] X 100
Inflammatory biomarkers and Oxygen radical absorbance capacity (ORAC) assay
TNF-α and IL-10 concentration in serum of control and drugs treated respective groups was determined using ELISA kit. The experiment was performed as per instructions of manufacturer. TNF-α and IL-10 level was expressed as picogram per milligram (pg/mg).
Antioxidant level of serum samples in treated and control animal group was carried out by ORAC method29. Shortly, 25 μl of serum sample added in fresh 150 μl of 10nM fluorescein solution and allowed to stand at 37 0C for 30 min. After incubation, 25 ml of AAPH substrate (500mM) mixed and the fluorescence was calculated for 150 min at 485 and 520nm (Excitation and emission wavelengths respectively) using microplate reader. Standard trolox was used as a and results were expressed as micromoles of Trolox equivalents (TE) per liter of sample.
Phytochemical analysis
The total polyphenol level of M. sanjappae seeds was determined spectrophotometrically30 and represented as mg of gallic acid equivalent per gram (mg GAE g–1) of dry mass. Theflavonoids content was analyzed31 and results represented as milligram of quercetin equivalents per gram (mg QUE g-1) of dry weight. Proanthocyanidin examined and reported as catechin equivalents per gram (mg CAE g-1) of dry weight32. The phytic acid was determined and absorbance was measured at 500 nm33. Tannin and saponin level also studied according to methods reported earlier34,35.
Statistical Analysis
GraphPad Prism 5 is used for data analysis. All the results were represented Mean ± SEM. P-values of less than 0.05 were considered as significant.
Results and Discussion
Nature has gifted us with medicinally important, inexhaustible sources of secondary metabolites, including alkaloids, terpenoids, phenolics, saponins, and other classes of organic compounds. These phytometabolites have tremendous health benefits for the overall growth and the management of diseases. Over time, experimental procedures and tools for the isolation, characterization, validation, and development of drugs for disease have been well established36. The present attempt was aimed to find out the anti-inflammatory and antioxidant properties of M. sanjappae seeds for future natural drug development.
In vitro anti-inflammatory activity
Preliminary screening of anti-inflammatory potential was performed using table assay as given below:
Bovine serum albumin (BSA) anti-denaturation potential:
Denaturation of proteins causes several inflammatory responses in the body. Many synthetic drug molecules show promising anti-inflammatory activity37 but are known to cause secondary complications after long-term use38. Hence, the use of plant-mediated drugs may prove more advantageous than their synthetic counterparts. M. sanjappae seed extract has shown inhibition of heat-induced albumin denaturation activity at different concentrations, as shown in Fig. 1. M. sanjappae extracts showed strong inhibition of albumin denaturation (87.73±3.81%) at 500μg/ml concentration. The standard drug diclofenac showed 94.82± 1.79% inhibition at 500 μg concentration. The results suggested good anti-inflammatory activity of M. sanjappae seed extract.
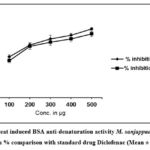 |
Figure 1: Heat induced BSA anti-denaturation activity M. sanjappae seed water extract in % comparison with standard drug Diclofenac (Mean ± D, n=3). |
HRBC Membrane stability potential
Stabilisation of lysosomal membrane is an essential process in the regulation of inflammation process. It occurs by preventing the release of lysosomal components of active neutrophils including bactericidal enzymes and proteases. Red blood cell membrane bears resemblance to lysosomal membrane28, therefore understanding the stability of RBC membrane by our drug of interest gives idea about its potential to protect lysosomal membrane to prevent release of inflammatory response markers. Haemoglobin released in the supernatant in the control tube (without std drug or plant extract) due to bursting of red blood cells, while the yellow supernatant in the plant extract tube indicates the stabilisation of HRBCs. Our results demonstrated that M. sanjappae seed extract could protect the HRBC membrane with maximum protection at 500ug M. sanjappae seed extract (60.47±2.1 %) as depicted in Fig. 2. As concentration of M. sanjappae seed extract was increased, HRBC membrane stability was also increased suggesting concentration dependent stabilization activity by M. sanjappae seed. Standard drug aspirin showed superior HRBC membrane protection capacity as compared to M. sanjappae seed extract. The maximum protection by aspirin at 500ug concentration was 99.56±1.01 and it also represented dose dependent increase in activity.
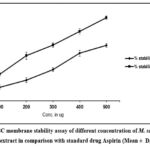 |
Figure 2: HRBC membrane stability assay of different concentration of M. sanjappae seed water extract in comparison with standard drug Aspirin (Mean ± D, n=3) |
In vivo anti-inflammatory activity
Carrageenan is a sulfonated polysaccharide widely used in food industries. It is extracted from red seaweed algae. It does not have any nutritional potential but is preferentially used as thickening, gelling and emulsifying agent39. Several studies have successfully reported use of carrageenan as an inflammatory agent to induce acute paw edema1. It is a simple and effective mean of assessing anti-inflammatory properties of drug.
Paw edema measurement
Determination of paw edema level after carrageenan toxicity and its furth treatment using oral dose of M. sanjappae seed extract was evaluated. Carrageenan generates acute paw edema which can be seen as redness and swelling at the site of injection. In the present study, paw edema was induced by using carrageenan and measured at 0hr, 2hr, 4hr and 6hr post injection. Carrageenan toxicity successfully developed inflammation in the rat paw which was evident from redness and swelling at the site of induction with highest paw thickness at 4hr (9.44±0.25). Vehicle control animal group does not show edema. Animal group treated with different doses of M. sanjappae seed extract exhibited significant reduction in the paw edema with maximum result at 200mg/kg body weight (Table 2). It showed 53.49% of edema inhibition after 4 hr treatment. Standard drug showed maximum edema inhibition (54.94%) at 6hr.The anti-inflammatory potential of M. sanjappae seed extract was observed very close to the standard drug Diclofenac control group. Dose dependent increase in the anti-inflammatory activity of M. sanjappae seed extract in respect to edema reduction was clear from the study. Sham control group was given the highest dose of M. sanjappae seed extract (200mg/kg body weight) and does not show any inflammatory symptoms during the experiments. Our finding supports anti-inflammatory potential of M. sanjappae seed and its traditional use in the management of inflammation related disorders.
Table 2: Effect of M. sanjappae on carrageenan induced rat paw edema.
|
0hr |
2hr |
4hr |
6hr |
|
|
Group I: Vehicle control |
4.11±0.04 |
4.16±0.05 |
3.97±0.06 |
4.05±0.04 |
|
Group II: Carrageenan Control |
4.41±0.08 |
8.23±0.07 |
9.44±0.25 |
8.9±0.39 |
|
Group III: Diclofenac control |
3.89±0.04 |
4.51±0.06*** (47.0) |
4.26±0.21*** (54.87) |
4.01±0.18*** (54.94) |
|
Group IV: 50mg test drug$ |
3.91±0.14 |
6.02±0.36*** (26.85) |
5.24±0.19*** (44.49) |
5.04±0.2*** (43.37) |
|
Group V: 100mg test drug |
4.28±0.09 |
5.99±0.25*** (27.21) |
5.27±0.33*** (44.17) |
5.11±0.29*** (42.58) |
|
Group VI: 200mg test drug |
3.81±0.02 |
4.53±0.15*** (44.95) |
4.39±0.22*** (53.49) |
4.18±0.31*** (53.03) |
|
Group VII: SHAM control |
4.49±0.05 |
4.47±0.08 |
4.52±0.1 |
4.36±0.11 |
$: M. sanjappae seed water extract. Values have been represented as mean±SEM, n=6 in each group. * p<0.05; ** p<0.01; *** p<0.001 when compared with carrageenan induced control. Values shown in parentheses represent the percent (%) reduction in paw edema in comparison with carrageenan induced control.
Effect of M. sanjappae treatment on pro-inflammatory cytokine TNF-α
TNF-α is a mediator of inflammatory responses playing a key role in the development of innate immune system via activating macrophages, T cells and secretion of other inflammatory cytokines. In the carrageenan induced acute inflammatory model, complement system stimulation along with inflammatory mediators’ synthesis are major events 40. Serum TNF-α level was determined after 6hr post carrageenan treatment (Fig 3a). There was considerable increase in TNF-α (89±8.13 pg) in serum due to inflammatory response after carrageenan injection confirming its pro-inflammatory properties (Group II). However, treatment of M. sanjappae seed extract at various doses significantly decreased TNF-α level. Among tested doses, 100 and 200mg dose significantly reduced (p<0.01) TNF-α level (59.5±4.48 pg and 57±1.96 pg respectively) in animal (Fig 3a) and we found superior results as compared to standard drug diclofenac (61.3±6.61 pg). Vehicle control and Sham control represented normal TNF- α level. According to the results, it can be concluded that, M. sanjappae has potential of reducing the inflammation caused by external toxic compounds and possesses potential to use for managing inflammatory diseases.
 |
Figure 3a: Effect of M. sanjappae seed extract on TNF- α level in serum of carrageenan induced rat at 6hr. |
Values are represented as Mean±SEM (n=6). **p<0.01, **p<0.01, ***p<0.001 when compared with carrageenan induced group. ##p<0.01, ###<0.001 when compared with vehicle control group (X axis indicates group number which are shown in detail in Table no. 1)
Effect of M. sanjappae treatment on anti-inflammatory cytokine IL-10
IL-10 is an important anti-inflammatory cytokine which attenuates the activity of pro-inflammatory markers including TNF- α41. In the present study, in contrast to TNF- α, IL-10 level was increased in Diclofenac and M. sanjappae seed extract treated animal groups (Fig. 3b). Reduction in IL-10 in carrageenan induced group (II), suggests, carrageenan exerts its anti-inflammatory response through suppression of anti-inflammatory cytokines. Positive effect of M. sanjappae seed extract was validated from considerable augmented IL-10 level (p<0.001) with maximum activity at 100 and 200mg M. sanjappae seed extract dose. Vehicle control (I) and SHAM control (VII) exhibited normal level of IL-10 which was higher than carrageenan induced group II. Overall, our study signifies M. sanjappae exerts its anti-inflammatory action by suppressing pro-inflammatory cytokines and expressing anti-inflammatory cytokines in the serum of carrageenan toxicated rat.
 |
Figure 3b: Effect of M. sanjappae seed extract on IL-10 level in serum of carrageenan induced rat at 6hr. |
Results are represented as Mean±SEM (n=6). ***p<0.001 when compared with carrageenan induced group. ###p<0.001 in comparison with vehicle control group. (X axis indicates group number which are shown in detail in Table no. 1).
Oxygen radical absorbance capability (ORAC) assay
Serum antioxidant levels of different animal groups is studied by oxygen radical absorbance capacity method1. Serum antioxidant activity was significantly (p<0.001) elevated in M. sanjappae seed extract treated animal group. The higher activity was found for 100 and 200mg/kg B/W dose. There was also significant increase in serum antioxidant capacity in sham control animal suggesting M. sanjappae seed possesses secondary metabolites which are enhancing antioxidant properties in the animal. Carrageenan control showed lower level of serum antioxidant activity (468±31.6µM Trolox equiv/L) suggesting oxidative stress is get generated due to toxicity of carrageenan. Different doses of M. sanjappae seed extract showed 560±22.4, 905±53.8 and 1105±42.4 µM Trolox equiv/L respectively showing concentration dependent increase in oxygen radical absorbance potential (Fig 4).
 |
Figure 4: Effect of M. sanjappae seed extract on serum oxygen radical absorbance capacity in carrageenan induced rat at 6hr. |
Results are represented as Mean±SEM (n=6). ***p<0.001 when compared with carrageenan induced group. ###p<0.001 when compared with vehicle control group
Human disorders show pathogenesis through cellular inflammation and oxidative stress mediated cell degeneration. An inbuilt antioxidant system comprising antioxidant molecules and enzymes performs a crucial role in removing free radicals7,42. But increased oxidative stress because of environmental toxins, genetic changes, or unknown causes results in cell components/organelle to degenerate or alter leading to apoptosis of cell. In such cases, supplementary antioxidants through food or drugs become essential way of disease management. Present study demonstrated M. sanjappae seeds contains vital phyto-metabolites which have capacity to induce anti-inflammatory and antioxidant properties required in the disease treatment.
Phytochemical analysis
Phenolics and flavonoids are considered as major secondary metabolites with prominent role as antioxidant and anti-inflammatory agents20,21,22,24. These secondary metabolites are with diverse biological activities combating diseases through multiple pathways. Their role in managing diseases including neurodegenerative diseases is via unstable superoxide radicals scavenging and cellular inflammation decreasing pathway43. M. sanjappae showed 80.78±2.56 mg GAE g-1 of phenolics and 419.5±7.18 mg QAE g-1 of flavonoids. Earlier we have analyzed gallic acid, tannic acid, p-hydroxybenzoic acid and p-coumaric acid as major phenolic compounds present in M. sanjappae seeds using HPLC20. M. sanjappae seed possesses higher levels of flavonoids than polyphenols which may be attributed to the specificity, and accuracy of reaction and respective standard used for the reaction. Proanthocyanidin level was 2.14 ± 0.13 mg CAE g-1. Proanthocyanidin is flavan-3-ol group having compound with strong antioxidant, anti-inflammatory, antihypertensive, antimicrobial and antiallergic activity44-46. M. sanjappae seed contains a higher concentration of phytic acid 197.23±0.11 mg g-1. Phytic acid is metal chelating and anti-inflammatory in nature 47,48. Phytic acid decreased inflammation by reducing the level of NF-κB and p-ERK in MPTP intoxicated PD mice model48. It is also natural iron chelating agent which prevents iron induced dopaminergic neuron degeneration in Parkinson’s disease49. M. sanjappae beans showed 0.52±0.11 mg g-1 and 18.71 ± 0.13 mg g-1 of tannin and saponin content respectively. Both of these compounds possess anti-inflammatory and antioxidant activity50, 51.
Investigation of traditional ayurveda knowledge with respect to disease management has lain to find novel drug molecules and related molecular pathways responsible for it. In modern medicine, single synthetic drug is preferred for targeted and quick action. But on the other side, those synthetic molecules usually have several side effects to the patients. In this connection, plant-based drug therapy which comprises several effective drug molecules proves to be most effective and moreover exerts minimum side effects. Based on the present results, M. sanjappae may prove as a promising lead for treating inflammatory diseases.
Conclusion
Natural drugs isolated from plants show promising anti-inflammatory properties with little to no side effects. Local indigenous peoples have been using Mucuna species as a staple food and medicinal purposes, mainly for Parkinson’s disease, male infertility, and snake bite treatment. However, there has been no elaborative investigation of the anti-inflammatory properties of M. sanjappae using in vivo or in vitro model. The present study revealed the anti-inflammatory potential of M. sanjappae seed extract by inhibiting pro-inflammatory cytokines and upregulating anti-inflammatory cytokines. The carrageenan-induced edema was reduced after treatment with M. sanjappae seeds’ water extract. Furthermore, the antioxidant level was elevated after the treatment by M. sanjappae extract. Phytochemical analysis confirmed presence of active secondary metabolites such as phenolics, flavonoids, phytic acid, saponins etc. Thus, the study strongly supported therapeutic potential of M. sanjappae and suggests further molecular-level investigations for its future exploration as a pharmacological agent in the management of inflammation and oxidative stress-related diseases.
Acknowledgements
We acknowledge Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth, Pune for providing animal house facility for the research work
Conflict of Interest
Authors declare no conflict of interest.
Funding source
This work was supported by Department of Biotechnology, Govt. of India for funding through DBT-IPLS program (Ref. No.: BT/PR4572/INF/22/147). Mr. Ravishankar Patil thanks SERB, DST for providing financial support through N-PDF (PDF/2016/002075). Mr. Chetan Aware acknowledges SUK-DBT IPLS program for the fellowship. Mr. Govind Vyavahare acknowledges Shivaji University for DRS. Dr. Ruchika Kaul-Ghanekar would like to acknowledge Director, IRSHA and ministry of AYUSH for providing financial support for the study. Prof. Vishwas Bapat is thankful to Indian National Science Academy, New Delhi, India for senior scientist fellowship.
References
- Choudhari A, Raina P, Deshpande M, Wali A, Zanwar A, Bodhankar S, Kaul-Ghanekar R. Evaluating the anti-inflammatory potential of Tectaria cicutaria L. rhizome extract in vitro as well as in vivo. Journal of Ethnopharmacology 2013; 150, 215–222. https://doi.org/10.1016/ j.jep.2013.08.025
CrossRef - Liu X, Yin L, Shen s, Hou Y. Inflammation and cancer: paradoxical roles in tumorigenesis and implications in immunotherapies. Genes & Diseases 2023; 10, 151-164.
CrossRef - Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, Jones SR, Toth PP. Inflammation and cardiovascular disease: From mechanisms to therapeutics. American Journal of Preventive Cardiology 2020; 21; 4:100130. doi: 10.1016/j.ajpc.2020.100130.
CrossRef - Marriott E, Singanayagam A, El-Awaisi J. Inflammation as the nexus: exploring the link between acute myocardial infarction and chronic obstructive pulmonary disease. Frontiers in Cardiovascular Medicine 2024; 11:2024 | https://doi.org/10.3389/fcvm.2024.1362564
CrossRef - Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochemical Pharmacology 2020; 180:114147. doi: 10.1016/j.bcp.2020.114147
CrossRef - van den Bosch MHJ, Blom AB, van der Kraan PM. Inflammation in osteoarthritis: Our view on its presence and involvement in disease development over the years. Osteoarthritis Cartilage 2024; 32:355-364. doi: 10.1016/j.joca.2023.12.005
CrossRef - Rai SN, Birla H, Singh S, Zahra W, Patil R, Jadhav J, Rao GM, SinghSP. Mucuna pruriens protects against MPTP intoxicated neuroinflammation in Parkinson’s disease through NF-κB /pAKT signaling pathways. Frontiers in Aging Neuroscience 2017;19 (9) https://doi.org/10.3389/fnagi.2017.00421
CrossRef - Nunes CdR, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, Vieira IJC, Barros de Oliveira D. Plants as Sources of Anti-Inflammatory Agents. Molecules. 2020; 25(16):3726. https://doi.org/10.3390/ molecules25163726
CrossRef - Zhen J, Guo Y, Villani T, Carr S, Brendler, Mumbengegwi D, Kong AT, Simon J, Wu W. Phytochemical Analysis and Anti-Inflammatory Activity of the Extracts of the African Medicinal Plant Ximenia caffra. Journal of Analytical Methods in Chemistry. 2015, https://doi.org/ 10.1155/2015/948262
CrossRef - Kumar N, Singh SK, Lal RK, Sunita Singh Dhawan An insight into dietetic and nutraceutical properties of underutilized legume: Mucuna pruriens (L.) DC. Journal of Food Composition and Analysis. 2024; 129, 106095.
CrossRef - Kumar A, Gupta C, Nair DT, Salunke DM. MP-4 Contributes to Snake Venom Neutralization by Mucuna pruriens Seeds through an Indirect Antibody-mediated Mechanism. Journal of Biological Chemistry 2016; 291(21), 11373-84. doi: 10.1074/jbc.M115.699173
CrossRef - Boniface F, Washa WB, and Nnungu S. Comparison of nutritional values of Mucuna pruriens L. (velvet bean) seeds with the most preferred legume pulses. Food Production, Processing and Nutrition.2024; 6, 17. https://doi.org/10.1186/s43014-023-00187-4
CrossRef - Fadilaturahmah F, Resti R, Putra S. Anti-inflammatory effects of velvet bean (Mucuna pruriens L. (DC.), Fabaceae) leaf ethanolic extract against carrageenan in male mice. Journal of Research in Pharmacy 2023; 27, 1524-33 http://dx.doi.org/10.29228/jrp.438
CrossRef - Ganesh MK, Lakshmanan G, Khan MZI, Prakash S. Aging induced testicular damage: analyzing the ameliorative potential of Mucuna pruriens seed extract. 3 Biotech 2023; 13(6):206. 10.1007/s13205-023-03618-8
CrossRef - Kajal K and Pandey RK. Ethnopharmacological uses, phytochemistry and therapeutic potential of Mucuna pruriens: a comprehensive review on current status of knowledge. Journal of Population Therapeutics and Clinical Pharmacology 2024; 31 (3):1184-94. https://doi.org/10.53555/jptcp.v31i3.5101.
CrossRef - Neshige R, Neshige S. Mucuna beans administered through hydrogen-infused superheated steam in advanced Parkinson’s disease. Clinical parkinsonism & related disorders 2024; 10:100252
CrossRef - Lu K, Lee H, Huang M, Lai S, Ho Y, Chang Y, Chi C. Synergistic Apoptosis-Inducing Antileukemic Effects of Arsenic Trioxide and Mucuna macrocarpa Stem Extract in Human Leukemic Cells via a Reactive Oxygen Species-Dependent Mechanism. Evidence-Based Complementary and Alternative Medicine 2012; 921430. https://doi.org/10.1155/2012/921430
CrossRef - Patil RR, Gholave AR, Jadhav JP, Yadav SR, Bapat VA. Mucuna sanjappae Aitawade et Yadav: a new species of Mucuna with promising yield of anti-Parkinson’s drug L-DOPA. Genetic Resources and Crop Evolution 2015; 62, 155–162. https://doi.org/10.1007/s10722-014-0164-8
CrossRef - Patil RR, Rai SN, Jadhav JP, Singh SP. Mucuna sanjappae shows promising anti-Parkinson’s activity by reducing oxidative stress in MPTP induced mouse model. European Journal of Pharmaceutical and Medical Research 2016a; 3(11), 452-463.
- Patil RR, Rane MR, Bapat VA, Jadhav JP. Phytochemical Analysis and Antioxidant Activity of Mucuna sanjappae: A Possible Implementation in the Parkinson’s Disease Treatment. Journal of Pharmaceutical and Medicinal Research 2016b; 2(1), 48–51.
- Aware CB, Patil RR, Vyavahare GD, Gurme ST & Jadhav JP. Ultrasound-Assisted Aqueous Extraction of Phenolic, Flavonoid Compounds and Antioxidant Activity of Mucuna macrocarpa Beans: Response Surface Methodology Optimization. Journal of the American College of Nutrition 2019a; 38(4), 364–372. https://doi.org/10.1080/ 07315724. 2018.1524315
CrossRef - Aware C, Patil R, Bapat V, Gaikwad S, Yadav S, Jadhav J. Evaluation of L-DOPA, proximate composition with in vitro anti- the inflammatory and antioxidant activity of Mucuna macrocarpa beans: A future drug for Parkinson’s treatment. Asian Pacific Journal Tropical Biomedicine 2017; 7 (12), 1097-1106. https://doi.org/10.1016/j.apjtb.2017.10.012
CrossRef - Aware C, Patil R, Vyavahare G, Gurav R, Bapat V & Jadhav J. Processing Effect on L-DOPA, In Vitro Protein and Starch Digestibility, Proximate Composition, and Biological Activities of Promising Legume: Mucuna macrocarpa. Journal of the American College of Nutrition 2019b; 38(5), 447–456. https://doi.org/10.1080/07315724.2018.1547230
CrossRef - Rane M, Suryawanshi S, Patil R, et al. Exploring the proximate composition, antioxidant, anti-Parkinson’s and anti-inflammatory potential of two neglected and underutilized Mucuna species from India. South African Journal of Botany 2019; 124:304–310. https://doi.org/10.1016/j.sajb.2019.04.030
CrossRef - Patil RR, Aware CB, Gaikwad S et al. RP-HPLC Analysis of Anti-Parkinson’s Drug L-DOPA Content in Mucuna Species from Indian Subcontinent. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci 2019; 89, 1413–1420. https://doi.org/10.1007/s40011-018-01071-9
CrossRef - Aitawade MM, Yadav SR. Mucuna sanjappae, a new species from the north-Western Ghats, India. Kew Bulletin 2012; 67, 539–543. https://doi.org/10.1007/s12225-012-9369-1
CrossRef - Sreewardhini S, Sankari D, Vijayalakshmi V, Mangalagowri A, Veeramuthu A. Phytochemical analysis, anti-inflammatory, antioxidant activity of Calotropis gigantea and its therapeutic applications. Journal of Ethnopharmacology. 2023; 303, 115963. https://doi.org/10.1016/j.jep.2022.115963
CrossRef - Yesmin, S., Paul, A., Naz, T. et al. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clinical Phytoscience 2020; 6, 59. https://doi.org/10.1186/s40816-020-00207-7
CrossRef - Ola A, Mourad J, Hanen N, Nacim Z, Hichem S, Moncef N. Sulfated polysaccharides from the viscera of Mustelus shark: Characterization and antioxidant, anticoagulant and anti-proliferative activities. Bioactive Carbohydrates and Dietary Fibre 2024. 100399. https://doi.org/10.1016/j.bcdf.2023.100399
CrossRef - Hudz N, Yezerska O, Shanaida M, Horčinová Sedláčková V, Wieczorek PP. Application of the Folin-Ciocalteu method to the evaluation of Salvia sclarea extracts. Pharmacia 2019; 66, 209-215. https://doi.org/10.3897/pharmacia.66.e38976
CrossRef - Chandra S, Khan S, Avula B, Lata H, Yang MH, Elsohly MA, Khan IA. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evidence-Based Complementary and Alternative Medicine. 2014; 2014, 253875. doi: 10.1155/2014/253875.
CrossRef - Ladhari A, Corrado G, Rouphael Y, Carella F, Nappo GR, Di Marino C, De Marco A, Palatucci D. Chemical, Functional, and Technological Features of Grains, Brans, and Semolina from Purple and Red Durum Wheat Landraces. Foods. 2022; 25, 11:1545. doi: 10.3390/foods11111545
CrossRef - Soares JC, Zimmermann L, Zendonadi Dos Santos N, Muller O, Pintado M, Vasconcelos MW. Genotypic variation in the response of soybean to elevated CO2. Plant Environment Interactions. 2021; 2, 263-276. doi: 10.1002/pei3.10065
CrossRef - Kavitha Chandran CI and Indira G. Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). Journal of Medicinal Plants Studies 2016; 4 (4), 282-286. https://www.plantsjournal.com/archives/ 2016/vol4issue4/PartD/4-4-4-759.pdf
- Alam F, Us Saqib QN. Pharmacognostic study and development of quality control parameters for fruit, bark and leaf of Zanthoxylum armatum (Rutaceae). Ancient Science of Life, 2015; 34(3), 147-55. doi: 10.4103/0257-7941.157159.
CrossRef - Najmi A, Javed SA, Al Bratty M, Alhazmi HA. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules. 2022; 27(2), 349. https://doi.org/10.3390/molecules27020349
CrossRef - Nedeljković N, Dobričić V, Bošković J, Vesović M, Bradić J, Anđić M, Kočović A, Jeremić N, Novaković J, Jakovljević V, Vujić Z, Nikolić M. Synthesis, and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen. Pharmaceuticals (Basel). 2023; 16(5):666. doi: 10.3390/ph16050666
CrossRef - Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochemical Pharmacology, 2020; 180, 114147. doi: 10.1016/j.bcp.2020.114147.
CrossRef - Pradhan B, Jang-Seu K Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease. International Journal of Biological Macromolecules. 2023, 238, 124085. https://doi.org/10.1016/j.ijbiomac.2023.124085
CrossRef - Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ, Ojha S, Patil CR. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. International Journal of Molecular Science. 2019; 20, 4367. doi: 10.3390/ijms20184367.
CrossRef - Mondello S, Hayes R Biomarkers Handbook of Clinical Neurology 2015; 127, Pages 245-265. https://doi.org/10.1016/B978-0-444-52892-6.00016-7
CrossRef - Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biology 2019; 25, 101084, 10.1016/j.redox.2018.101084
CrossRef - Oluwole O, Fernando WMAD, Lumanlan J, Ademuyiwa O, Jayasena V. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health – a review. International Journal of Food Science & Technology; 2022, 57, 6326-6335. https://doi.org/10.1111/ijfs.15936.
CrossRef - Verma P, Sen R, Bamanna A, Elhindawy M, Nagpal K, Krishnan V. Structural chemistry to therapeutic functionality: A comprehensive review on proanthocyanidins. Biocatalysis and Agricultural Biotechnology 2024; 55, 102963.
CrossRef - Salinas-Sánchez DO, Jiménez-Ferrer E, Sánchez-Sánchez V, Zamilpa A, González-Cortazar M, Tortoriello J, Herrera-Ruiz M. Anti-Inflammatory Activity of a Polymeric Proanthocyanidin from Serjania schiedeana. Molecules 2017; 22(6), 863. https://doi.org/10.3390/molecules22060863
CrossRef - Limtrakul P, Yodkeeree S, Pitchakarn P, Punfa W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutrition Research and Practice 2016; 10(3), 251-258. https://doi.org/10.4162/ nrp.2016.10.3.251
CrossRef - Abdulwaliyu I, Arekemase SO, Adudu JAA, Batari ML, Egbule MN, Okoduwa.SIR. Investigation of the medicinal significance of phytic acid as an indispensable anti-nutrient in diseases, Clinical Nutrition Experimental 2019; 28, 42-61. https://doi.org/10.1016/ j.yclnex.2019.10.002
CrossRef - Lv Yuqiang, Zhang Zheng, Hou Lin, Zhang Li. Phytic acid attenuates inflammatory responses and the levels of NF-κB and p-ERK in MPTP-induced Parkinson’s disease model of mice. Neuroscience Letters 2015; 597, 132-136. https://doi.org/10.1016/j.neulet.2015.04.040
CrossRef - Chen Y, Yuan W, Xu Q, Reddy M. Neuroprotection of phytic acid in Parkinson’s and Alzheimer’s disease, Journal of Functional Foods 2023; 110, 105856. https://doi.org/10.1016/j.jff.2023.105856
CrossRef - Wijesekara T, Luo J, Xu B. Critical review on anti-inflammation effects of saponins and their molecular mechanisms. Phytotherapy Research. 2024; 38(4), 2007-2022. doi: 10.1002/ptr.8164.
CrossRef - Park M, Cho H, Jung H, Lee H, Hwang K. Antioxidant and Anti-Inflammatory Activities of Tannin Fraction of the Extract from Black Raspberry Seeds Compared to Grape Seeds. Journal of food Biochemistry 2014; 38 (3), 259–270. https://doi.org/10.1111/jfbc.12044
CrossRef
Abbreviations
BSA: Bovine serum albumin
ELISA: Enzyme-linked immunosorbent assay
HRBC:Human Red Blood Cell
IL-10: Interleukin 10
L-DOPA: L-3,4-dihydroxyphenylalanine
MPTP: N-methyl-4-phenyl-l,2,3,6-tetrahydropyridine
Mucuna sanjappae water extract
NSAIDs: Non-steroidal anti-inflammatory drugs
NF-κB: Nuclear factor-κB
ORAC assay: Oxygen radical absorbance capability
p-ERK: Phosphorylated extracellular signal-related kinase
PD: Parkinson’s disease
RBC: Red blood cell
TNF-α: Tumor Necrosis Factor alpha







