Siti Fairuz Ishak1 , Nor Fadilah Rajab2
, Nor Fadilah Rajab2  and Dayang Fredalina Basri1*
and Dayang Fredalina Basri1*
1Centre for Diagnostic, Therapeutic and Investigative Studies (CODTIS), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, WP Kuala Lumpur, Malaysia.
2Center for Healthy Aging and Wellness (H-CARE), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia.
Corresponding Author E-mail: dayang@ukm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2918
Abstract
Canarium odontophyllum or dabai is a natural plant found along the river banks of the Sarikei, Kapit and Kanowit in Sarawak and it comes from the 'Burseraceae' family. C. odontophyllum also known as 'dabai' and this dabai fruit is eaten by the community in Sarawak. The fruit is a seasonal natural fruit that is less used because of its short lifespan. A few studies show that acetone extract of Canarium odontophyllum stem bark (dabai) exhibit antiproliferative and cytotoxicity effect on cancer colorectal cells. However, there is currently no analysis of bioactive compounds in acetone extract of Canarium odontophyllum stem bark (dabai). The various bioactive compounds detected in acetone extract were identified using gas chromatography-mass spectrometry (GC-MS). A total of 24 phytoconstituents were detected in this acetone extract. It was found that major peaks represented bicyclo [3.1.0] hex-2-ene,2-methyl-5-(1-methylethyl)-, bicyclo [3.1.0] hexane,4-methylene-1-(1-methylethyl)-, alpha cubebene, 1H cyclopenta [1,3] cyclopropa [1,2] benzene, octahydro-7-methyl-3-methylene-4-(1-methylethyl)-,[3aS-(3a.alpha., 3b.beta., 4.beta., 7.alpha., 7aS*)]-, phenol,2,4-bis(1,1-dimethylethyl)-, spathulenol, copaene, 9-Eicosene, (E)-, hexadecane, 5-Octadecene, (E)-, hexadecane, 2,6,10,14-tetramethyl-, nonadecane, n-Hexadecanoic acid, heptacosane, 1-chloro-, 9,12-Octadecadienoic acid (Z,Z)-, 9,12-Octadecadienoic acid, ethyl ester, octadecanoic acid, 10-Heneicosene (c,t), heptafluorobutyric acid, hexadecyl ester, dehydroabietic acid, phenol, 2,4-bis(1-phenylethyl)-, beta sitosterol, beta-amyrin and alpha-amyrin. The highest peak area (%) for this acetone extract is alpha-amyrin (16.6644%) followed by beta-amyrin (4.6159%), beta sitosterol (3.3369%) and 9,12-Octadecadienoic acid (Z,Z)- (3.2045%). In conclusion, various bioactive compounds detected in acetone extract of Canarium odontophyllum stem bark (dabai) were demonstrated various medicinal properties while alpha and beta-amyrin may be responsible for the cytotoxicity and apoptotic effect against HCT 116 cell.
Keywords
Acetone extract; Canarium odontophyllum; dabai; GC-MS; Stem bark; Sarawak
Download this article as:| Copy the following to cite this article: Ishak S. F, Rajab N. F, Basri D. F. Analysis of GC-MS from Acetone Extract of Canarium odontophyllum Miq Stem Bark (Dabai). Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Ishak S. F, Rajab N. F, Basri D. F. Analysis of GC-MS from Acetone Extract of Canarium odontophyllum Miq Stem Bark (Dabai). Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3UWgZEK |
Introduction
The use of wild plants from nature for medicinal purposes is a practice that has been practiced for centuries. This practice is widely found among communities that practice traditional medicine. This is because natural plants have rich sources of biologically active compounds. 1 Various natural products exhibit different biological properties and have long been used as medicines with different applications. The use of natural products is not only used in traditional medicine but the detailed research done on natural products allows them to be used in modern medicine. Various bioactive compounds from medicinal plants exhibit stimulating actions of pharmacological such as antifungal, antibacterial, anticancer, antiinflammatory and antioxidant properties. 2,3 The capability of these bioactive compounds needs to be analyzed first before being used in various diseases treatment. 2,4 Crude plant extracts are often prepared from plant-based medicines consisting of a different phytochemicals complex mixture. 4 These phytochemicals have a complex and unique structure, used in chronic and infectious diseases treatment. 4,5 A large group of bioactive secondary metabolites exist in many species of plant, but only a small part of them has been studied and maintained as an important source of bioactive agents. In finding novel compounds and quality control is very important to develop appropriate screening methods. 6 Various extractions and characterization from bioactive compounds of many medicinal plants have led to the delivery of specific drugs with high profiles of activity. 7
Preliminary screening of medicinal plants through methods of chromatographic and spectrometric give general information about pharmacological and chemical activity and helps to choose biologically active plants. 8 Within a decade, there have been several dramatic advances in techniques of analytical including Ultraviolet-visible (UV) Spectroscopy, Thin Layer Chromatography (TLC), Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance Spectroscopy (NMR) which are the tools used for the process of isolation, identification and determination of the phytochemical structure of many bioactive therapeutic compounds found in medicinal plants. 9,10 Best combination of analytical techniques is gas chromatography-mass spectrometry (GC-MS) which is fast, sensitive and plays an important role in chemotaxonomic studies and phytochemical analysis of medicinal plants containing biologically active components. The GC-MS method can qualitatively and quantitatively identify organic compounds in extracts. 11,12
The GC-MS method is used to determine and identify compounds found in plant samples including alcohols, long chain hydrocarbons, alkaloids, steroids, nitro compounds, amino acids, esters and organic acids. In addition, this GC-MS method only need a small volume of plant extracts. 11,13 GC-MS was also performed for bioactive compounds identified based on ions of MS fragment produced, retention time and the percentage of bioactive compounds analyzed from the area of total peak. Phytochemicals were identified by comparing patterns of MS spectral with standard mass spectra that available in the Mass Spectral Database of National Institute of Standards and Technology (NIST). 12 One of the most accurate methods for identifying many secondary metabolites found in plant extracts is GC-MS. 14 Therefore, the technique of GC-MS was used in this study, for the detection and identification of phytochemical compounds found in the acetone extract of Canarium odontophyllum stem bark (dabai).
Canarium odontophyllum is also known as ‘dabai’ and this dabai fruit is eaten by people in Sarawak. The fruit is a seasonal natural fruit that is less used because of its short lifespan. 15 The fruit is purple in color and has one elongated seed (Figure 1). 16 The fruit of C. odontophyllum has physical characteristics similar to olives and changes from light green to dark purple when ripe enough. This fruit is oval shaped with a mass of around 10.0g -18.0g, length 3.0cm – 4.0cm and diameter around 2.2cm – 3.0cm. The fruit of C. odontophyllum has one seed located in the middle with a hard and thick endocarp. The length of this fruit seed is 3.5cm with a diameter of 1.6cm – 2.0cm while this seed is triangular in shape. 17
This C. odontophyllum tree is moderately upright to approximately 40–50 m (130–160 ft) tall and has alternate, oval, spiral, pinnate leaves that are between 9.5 cm to 28 cm long and about 4 cm to 11 cm (Figure 2). 18 While the flowers of the C. odontophyllum plant are whitish yellow (Figure 3). 19 The stems and branches of C. odontophyllum plants are greenish gray, light brown or yellowish brown, usually scaly with many small lenticels and smooth. Furthermore, the outer bark of branches and trunks is thin, gray and usually soft in nature while the inner bark is reddish grey or pinkish brown, smooth, laminated, layered, soft, moist with exudates, oily fluids, sticky, and strong produces an aromatic odor. 20-22 The stems of C. odontophyllum plants are cylindrical. 22 Dabai has wide potential to be marketed locally and exported internationally due to its high nutritional contents but still not yet exported internationally. Only food-based products available and there is still no medicinal product developed from dabai.
 |
Figure 1: Fruits and seed of C. odontophyllum |
 |
Figure 2: The stem bark of C. odontophyllum is greenish gray and scaly.18 |
 |
Figure 3: Flowers and leaves of C. odontophyllum.19 |
Materials and Methods
Preparation of the Stem Bark Extract
First, dried the samples in an oven at 40°C until they reached a constant weight for several days. Then, grinding of the dried sample is done to get the sample in powder form. Powder of C. odontophyllum stem bark was soaked in acetone solvent. The mixture was shaken using an electric shaker at room temperature for 24 hours. Then, the solution was filtered using Whatman No. 1 filter paper to collect the obtained filtrate. The filtrate was mixed and concentrated using a rotary evaporator under reduced pressure. The extract is finally allowed to dry in fume hood to obtain crude acetone extract. 21 100 mg of acetone extract was dissolved in 1 ml of absolute DMSO for the preparation of a stock solution with a concentration of 100 mg/ml. Next, mixed the stock solution using an autovortex and then centrifuged at 2500 rpm for 5 minutes to ensure that all extracts were completely dissolved. The stock was filtered using a 0.22 μm nitrocellulose filter membrane to ensure the extract was free from contamination and stored in a refrigerator at -20˚C until use.
GC–MS Analysis
The active compounds in the acetone extract from stem bark of C. odontophyllum were identified using the method of gas chromatography-mass spectrometry (GC-MS). Analysis of gas chromatography mass spectrometer (GC-MS) was carried out using an Agilent 7890A gas chromatograph (GC) coupled directly to a mass spectrometer (MS) system equipped with a DB-5MS UI column (30.0 m x 0.25 mm, film thickness 0.25 μm, 5% phenyl methylpolysiloxane) as well as connected to an inert Agilent 5975C MSD with a three-axis detector and operated in a system of electron ionization with a 70 eV impact mode used. Helium gas is used at a constant flow rate of 1ml/minute as a carrier gas. The powder form from acetone extract of C. odontophyllum stem bark was diluted with a suitable solvent which is acetone and then the solution was filtered. A particle-free dissolved acetone extract (1μl) was taken using a syringe and injected into the injector in split mode with an injector temperature of 250 C and an ion source temperature of 280 C. The temperature oven was programmed with an increase of 10C/ min from 40C (isothermal for 5 min), up to 300C/min isothermal and held for 5 minutes. Mass spectra were taken at 70 eV, scan interval 0.5 s and fragments from 45 to 450Da. The total time GC was run for 34 minutes. 13, 23
Identification of the Components
The percentage composition of the crude extract components is demonstrated as a percentage by peak area. Raw GC chromatogram was shown all peaks using MSD Chemstation. Then, combined the results in a single peak table. A library search was conducted for all peaks using NIST/EPA/NIH (version 2.0) for the name, structure of the compounds and molecular weight from the sample.
Results and Discussion
Analysis of GC-MS was carried out on the acetone extract from C. odontophyllum stem bark and a total of 24 phytoconstituents were detected. The chromatogram is displayed in Figure 4, while the constituent chemicals with molecular weight (MW), molecular formula, peak area (%) and retention time (RT) for each phytoconstituent are shown in Table 1. In Table 2 shown the phytoconstituent found in the extract and their activities. The highest peak area (%) for the acetone extract is alpha- amyrin (16.6644%) followed by beta- amyrin (4.6159%), beta sitosterol (3.3369%) and 9, 12-octadecadienoic acid (Z, Z)- (3.2045%). The lowest peak area (%) for the acetone extract is shown by bicyclo [3.1.0] hex-2-ene, 2-methyl-5-(1-methylethyl)- which is 0.0828%.
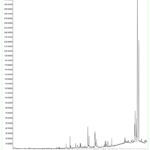 |
Figure 4: Chromatogram of acetone extract from stem bark of C. odontophyllum by GC-MS |
Table 1: Phytoconstituents found in the acetone extract of C. odontophyllum stem bark
|
No |
RT (minute) |
Name of compound |
Molecular formula |
Molecular weight (MW) |
Peak area (%) |
|
1 |
7.7554 |
Bicyclo [3.1.0] hex-2-ene, 2-methyl-5-(1-methylethyl)- |
C10H16 |
136.2340 |
0.0828 |
|
2 |
8.8972 |
Bicyclo [3.1.0] hexane, 4-methylene-1-(1-methylethyl)- |
C10H16 |
136.2340 |
0.1020 |
|
3 |
15.2437 |
Alpha Cubebene |
C15H24 |
204.3511 |
0.1329 |
|
4 |
15.6538 |
Copaene |
C15H24 |
204.3511 |
0.0921 |
|
5 |
15.8178 |
1H-Cyclopenta [1,3] cyclopropa [1,2] benzene,octahydro-7-methyl-3-methylene-4-(1-methylethyl)-,[3aS-(3a.alpha., 3b.beta., 4.beta., 7.alpha., 7aS*)]- (beta cubebene) |
C15H24 |
204.3511 |
0.1775 |
|
6 |
17.3571 |
Phenol, 2,4-bis(1,1-dimethylethyl)- |
C17H30OSi |
278.5050 |
0.5372 |
|
7 |
18.2593 |
(-)-Spathulenol |
C15H24O |
220.3505 |
0.9400 |
|
8 |
18.3413 |
9-Eicosene, (E)- |
C20H40 |
280.5316 |
0.3050 |
|
9 |
18.4233 |
Hexadecane |
C16H34 |
226.4412 |
0.2202 |
|
10 |
20.5871 |
5-Octadecene, (E)- |
C18H36 |
252.4784 |
0.4770 |
|
11 |
20.7133 |
Hexadecane,2,6,10,14-tetramethyl- (phytane) |
C20H42 |
282.5475 |
0.9387 |
|
12 |
21.6912 |
Nonadecane |
C19H40 |
268.5209 |
0.3311 |
|
13 |
22.3535 |
n-Hexadecanoic acid |
C16H32O2 |
256.4241 |
2.4496 |
|
14 |
23.6279 |
Heptacosane, 1-chloro- |
C27H55Cl |
415.179 |
0.1509 |
|
15 |
24.0254 |
9,12- Octadecadienoic acid (Z,Z)- |
C18H32O2 |
280.4455 |
3.2045 |
|
16 |
24.1894 |
9,12- Octadecadienoic acid, ethyl ester |
C20H36O2 |
308.4986 |
0.5246 |
|
17 |
24.2462 |
Octadecanoic acid |
C18H36O2 |
284.4772 |
1.1298 |
|
18 |
24.4859 |
10-Heneicosene (c,t) |
C21H42 |
294.5582 |
0.5806 |
|
19 |
26.2018 |
Heptafluorobutyric acid, hexadecyl ester |
C20H33F7O2 |
438.4636 |
0.3939 |
|
20 |
26.6497 |
Dehydroabietic acid |
C20H28O2 |
300.4351 |
0.6012 |
|
21 |
26.9715 |
Phenol, 2,4-bis(1-phenylethyl)- |
C22H22O |
302.4095 |
0.7200 |
|
22 |
33.1350 |
Beta Sitosterol |
C29H50O |
414.7067 |
3.3369 |
|
23 |
33.5703 |
Beta-Amyrin |
C30H50O |
426.7174 |
4.6159 |
|
24 |
34.0876 |
Alpha-Amyrin |
C30H50O |
426.7174 |
16.6644 |
Table 2: Phytoconstituents in the acetone extract of C. odontophyllum stem barkand their activities.
|
No |
Name of compound |
Natural compound |
Activity |
|
1. |
Alpha cubebene |
Sesquiterpene |
Antioxidant,antiproliferative, antigenotoxic, antitumor. 24 |
|
2. |
Copaene |
Sesquiterpene |
Antipyretic, Antifungal, antioxidant properties, antigenotoxic and antioxidant against human lymphocytes. 24, 25 |
|
3. |
Spathulenol |
Sesquiterpene |
Anticholinesterase, antimycobacterial, antioxidant, antiproliferative, cytotoxicity. 26,27 |
|
4. |
Phenol,2,4-bis(1,1-dimethylethyl)- |
Phenol |
Antioxidant, anti-inflammatory, Cytotoxicity, antibacterial, antifungal, antiviral, antimicrobial. 28 |
|
5. |
9-Eicosene, (E)- |
Alkene |
Antimicrobial, Cytotoxicity. 29 |
|
6. |
Bicyclo [3.1.0] hex-2-ene, 2-methyl-5-(1-methylethyl)- (alpha thujene) |
Monoterpene |
Antiproliferative, cytotoxic against cell line of breast cancer. 30 |
|
7. |
Bicyclo [3.1.0] hexane, 4-methylene-1-(1-methylethyl)- (sabinene) |
Monoterpene |
Antifungal, antiinflammatory, antioxidant, anticancer activity against colon cancer and melanoma cells, causing apoptosis in B16F10 melanoma cells. 31-33 |
|
8. |
Hexadecane |
Alkane |
Cytotoxicity, Antioxidant, Antimicrobial, Antidiabetic, Antiinflammatory. 34,35 |
|
9. |
Nonadecane |
Alkane |
AntiHIV, Antioxidant, Antibacterial, Antimicrobial, Cytotoxic effect. 36,37 |
|
10. |
n-Hexadecanoic acid |
Palmitic acid, Fatty acid |
Hypercholesterolemia, antioxidants, Antiandrogenic, Antibacterial, Antiinflammatory, anticancer effects. 38,39 |
|
11. |
Heptacosane, 1-chloro- |
Alkyl halide |
Antioxidant. 40 |
|
12. |
9,12-Octadecadienoic acid (Z,Z)- 9,12-Octadecadienoic acid, ethyl ester |
Linoleic acid, ethyl ester |
Antioxidant, Antitumor, Antiinflammatory, hypocholesterolemic, antiandrogenic, antiarthritic, anticoronary, antihistamine, hepatoprotective. 41-43 |
|
13. |
Octadecanoic acid |
Stearic acid, Fatty acid |
Antifungal, Antitumor, Antibacterial, lowers LDL cholesterol, Anti-inflammatory,hypocholesterolemic, cancer prevention, hepatoprotective, antiviral, antioxidant. 29,44 |
|
14. |
Dehydroabietic acid |
Diterpenoid |
Act pharmacologically against aging, inflammation, bacterial infection, cancer and increased cell death in human epithelia and fibroblast cells. 45-47 |
|
15. |
Phenol,2,4-bis(1-phenylethyl)- |
Phenol |
Effects of apoptosis on human breast cancer cells. 48 |
|
16. |
Beta Sitosterol
|
Phytosterols |
Anti-diabetic, anti-inflammatory, anti-atherosclerotic, lipid-lowering and hepatoprotective, protection against oxidative damage, anti-tumor effect against lung, breast, prostate, kidney and colorectal cancer. 49-51 |
|
17. |
Beta-amyrin |
Triterpenoid |
Antitumor effect against Hep-G2 liver carcinoma cells, antiinflammatory, antifibrogenic antioxidant, antihyperglycemic, hypolipidemic effect, analgesic, antidepressant, gastroprotective, hepatoprotective, antipancreatic, cytotoxicity against several cancer cells such as promyelocytic leukemia HL-60, colon cancer HCT-8, glioblastoma SF-295, MDAMB-435 melanoma, colorectal and breast adenocarcinoma HCT 116, lung and liver carcinoma, pancreatic adenocarcinoma, kidney and prostate carcinoma. 52-57 |
|
18. |
Alpha-amyrin |
Triterpenoid |
Antiinflammatory, antioxidant, antihyperglycemic, hypolipidemic effect, analgesic, antidepressant, gastroprotective, hepatoprotective, antipancreatic, antileukemic agent, cytotoxicity against leukemic cells, cytotoxicity against several cancer cells such as promyelocytic leukemia HL-60, colon cancer HCT-8, glioblastoma SF-295, MDAMB-435 melanoma, HCT 116 colorectal and breast adenocarcinoma, lung and liver carcinoma, pancreatic adenocarcinoma, kidney and prostate carcinoma. 52,54,56,58 |
Different phytochemicals contain in plants, known as secondary metabolites. 59,60 Phytochemicals are important in the industry of pharmaceutical for the preparation of therapeutic agents and the new drugs development.61 The new drugs development begins with the identification of active principles from natural sources. New approach to find therapeutically active compounds in various species of plants is by screening of plant extract. 38,62 Phytochemicals such as saponins, alkaloids, flavonoids, terpenoids and tannins have some of biological properties including antiinflammatory, antioxidant, antiulcer, anticancer, and antidiarrhea activity. 38 Exposure through diet or drug administration to phytochemicals can stop or delay process of carcinogenic. Phytochemicals may play an important role in decreasing the incidence of colon cancer. 63 Polyphenols, such as phenolic acids, sesquiterpenoids and flavonoids are known for their capability to induce apoptosis in colon cancer cells. 64,65 The results of phytochemical screening in previous studies shown that the phytochemicals found in acetone extract of C. odontophyllum stem bark are flavonoids, saponins, tannins, phenolic compounds and terpenoids may exhibit cytotoxicity and apoptosis effect against HCT 116 and function as anticancer agents. 21,66 So, this study was conducted to identify which bioactive compound in this acetone extract may be responsible for cytotoxicity and apoptosis effect against HCT 116.
The highest peak area (%) for the acetone extract is alpha-amyrin followed by beta-amyrin. Alpha-amyrin and beta-amyrin are triterpenoids which are active compounds that belong to the terpenoid group and have antimicrobial, antifungal and antiinflammatory effects. 67 Triterpenoids have also been reported to act as a potential source of cytotoxicity in cancer cells. Calotroposide A isolated from the ethyl acetate extract of the roots of Calotropis gigantea is a triterpenoid glycoside and reported can inhibit the growth of WiDr colon cancer cells. 68 Triterpenoids are a vital group of terpenoids with bioactivity. 69 Plant terpenoids have important functions in respiration, growth regulation and development, membrane fluidity and photosynthesis. 70 Terpenoids are also found in Campsis grandiflora leaves, cuticle wax of apple skin and Lantana camara L. aerial parts. 71 In addition, terpenoids are also detected in Fusarium sp. 72, Laurencia sp (marine algae) 73 and endophytic fungi Huperzia serrata. 74 Terpenoid biosynthesis is important for agriculture, industry and living organisms. Approximately 80,000 terpenoid compounds as secondary metabolites have been found in nature and have performed various roles (functional and structural). 75 Based on this study, the main bioactive compound of this acetone extract which are alpha and beta-amyrin may be responsible for the cytotoxicity and apoptotic effect against colorectal cancer cell, HCT 116 and capable of being developed as an anticancer agent.
Conclusion
The present study of this analysis in acetone extract of C. odontophyllum stem bark has suggested the presence of 24 of phytocompouds. Thus, this acetone extract was found to possess significant phytoconstituents and considered to have various medicinal properties. Alpha-amyrin and beta-amyrin are triterpenoid detected in acetone extract of Canarium odontophyllum stem bark that may contribute to the cytotoxicity and effect of apoptotic on HCT 116 cell. In conclusion, acetone extract of C. odontophyllum stem bark capable of being developed as an anticancer agent against HCT 116 colorectal cancer cell.
Acknowledgement
Instrument used in this study has been supported by Makmal Pencirian Struktur Molekul (MPSM), Centre for Research and Instrumentation Management (CRIM), Universiti Kebangsaan Malaysia (UKM).
Conflict of Interest
No conflict of interest.
Funding Sources
This project was funded by research code grant Research University Grant DIP-2018-034.
Author Contributions
Assoc. Prof Dr Dayang Fredalina Basri designed and lead the project. Siti Fairuz Ishak performed the experiment and prepare the manuscript. Prof. Dr Fadilah Rajab supervised the experiment.
References
- Abdul Aziz M. W. H, Masre S. F, Basri D. F, & Ghazali A. R. Canarium odontophyllum Miq.(Dabai) Leaf Phytoextracts and Their Medicinal Properties. Pertanika Journal of Science & Technology., 2022; 30(3): 2115 – 2125.
CrossRef - Anand U, Jacobo-Herrera N, Altemimi A, & Lakhssassi N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites., 2019; 9(11): 1-13.
CrossRef - Malongane F, Mcgaw L. J, & Mudau F. N. The Synergistic Potential of Various Teas, Herbs and Therapeutic Drugs in Health Improvement: A Review. Journal of the Science of Food and Agriculture., 2017; 97(14): 4679-4689.
CrossRef - Pandey M. M, Rastogi S, & Rawat A. Indian Herbal Drug for General Healthcare: An Overview.The Internet Journal of Alternative Medicine., 2008; 6(1): 1-10.
CrossRef - Sahoo N, & Manchikanti P. Herbal Drug Regulation and Commercialization: An Indian Industry Perspective. The Jounal of Alternative & Complementary Medicine., 2013; 19(12): 957-963.
CrossRef - Keskes H, Belhadj S, Jlail L, El Feki A, Damak M, Sayadi S, et al. LC-MS-MS and GS-MS Analyses of Biologically Active Extracts and Fractions from Tunisian Juniperus Phoenice Leaves. Pharmaceutical Biology., 2017; 55(1): 88-95.
CrossRef - Yadav R, Khare R. K, & Singhal A. Qualitative Phytochemical Screening of Some Selected Medicinal Plants of Shivpuri District (M.P.). The International Journal of Life-Sciences Scientific Research., 2017; 3(1): 844-847.
CrossRef - Juszczak A. M, Zovko-Končić M, & Tomczyk M. Recent Trends in the Application of Chromatographic Techniques in the Analysis of Luteolin and Its Derivatives. Biomolecules., 2019; 9(11): 1-38.
CrossRef - Satapute P, Paidi M. K, Kurjogi M, & Jogaiah S. Physiological Adaptation and Spectral Annotation of Arsenic and Cadmium Heavy Metal-Resistant and Susceptible Strain Pseudomonas taiwanensis. Environmetal Pollution., 2019; 251: 555-563.
CrossRef - Fan S, Chang J, Zong Y, Hu G, & Jia J. GC-MS Analysis of the Composition of the Essential Oil from Dendranthema Indicum Var. Aromaticum Using Three Extraction Methods and Two Columns. Molecules., 2018; 23(3): 1-11.
CrossRef - Qiao Y, Bi J.-F, Chen Q, Wu X, Gou M, Hou H, et al. Volatile Profile Characterization of Winter Jujube from Different Regions via HS-SPME-GC/MS and GC-IMS. Journal of Food Quality., 2021; 2021: 1-15.
CrossRef - Chirumamilla P, Dharavath S. B, & Taduri S. GC-MS Profiling and Antibacterial Activity of Solanum Khasianum Leaf and Root Extracts. Bulletin of the National Research Centre., 2022; 46(1): 127.
CrossRef - Olivia N. U., Goodness U. C., & Obinna O. M. Phytochemical Profiling and GC-MS Analysis of Aqueous Methanol Fraction of Hibiscus Asper Leaves. Future Journal of Pharmaceutical Sciences., 2021; 7(1): 1-5.
CrossRef - Garg K., Shrivastava B., & Bhargava A. GC-MS Analysis of Methanol and Ethyl Acetate Extract of Fruits of Sphaeranthus Indicus. Journal of Drug Delivery and Therapeutics., 2019; 9(2): 28-30.
CrossRef - Hamzah M. H, Mohd Basri M. S, Maringgal B, Mohd Ali M, Wondi M. H, Che Man H, et al. Exploring Dabai (Canarium odontophyllum), Indigenous Fruit of Borneo: A Review of Nutritional Values, Potential Uses, Emerging Application in Essential Oil Processing, and Health Benefits. Plants (Basel)., 2022; 11(19): 1-17.
CrossRef - Prasad K. N, Chew L. Y, Khoo H. E, Kong K. W, Azlan A, & Ismail A. Antioxidant Capacities of Peel, Pulp, and Seed Fractions of Canarium odontophyllum Miq. Fruit. Journal of Biomedical and Biotechnology., 2010;2010: 1-8.
CrossRef - Chew L. Y, Khoo H. E, Amin I, Azrina A, & Lau C. Y. Analysis of Phenolic Compounds of Dabai (Canarium odontophyllum Miq.) Fruits by High-Performance Liquid Chromatography. Food Analytical Methods., 2012; 5: 126-137.
CrossRef - Ding P. 3 – Dabai (Canarium odontophyllum Miq.). In Yahia, E. M. (Ed.), Postharvest Biology and Technology of Tropical and Subtropical Fruits. Woodhead Publishing. 2011: pp. 34-42e.
CrossRef - Lim T. Canarium odontophyllum, Edible Medicinal and Non Medicinal Plants. Springer. 2012; Volume 1, Fruits: pp. 624-629.
CrossRef - Rashid N. A. H. A, Shamsudin R, Arifin S. H, & Abdullah W. N. Z. Z. Morphological and Quality Characteristics of Genus of Canarium L.: A Review. IOP Conference Series: Earth and Environmental Science., 2021; 733(11): 012015.
CrossRef - Basri D. F, Mohd M. A. A, Chan K. M, Latif E, & Huyop F. Cytotoxic Activity of Stem Bark Extracts from Canarium odontophyllum Miq (Dabai) against Human Colorectal Carcinoma HCT 116 Cell Line. American Journal of Plant Sciences., 2014; 5: 3925-3933.
CrossRef - Mogana R, & Wiart C. Canarium L.: A Phytochemical and Pharmacological Review. Journal of Pharmacy Research., 2011; 4(8): 2482-2489.
CrossRef - Deepak P, & Gopal G. GC-MS Analysis of Various Solvent Extracts of Bark in Solanum Verbascifolium Linn. American Journal of Advanced Drug Delivery., 2014; 2(4): 484-492.
- Maduabuchi E. K, & Tobechukwu O. P. Advanced Phytochemistry and Chemo-Metric Profiling of the Bioactive Medicinal Components of N-Hexane Seed Extract of Xylopia Aethiopica Using FTIR and GC-MS Techniques. GSC Biological and Pharmaceutical Sciences., 2023; 22(1): 247-256.
CrossRef - Türkez H, Çelik K, & Toğar B. Effects of Copaene, a Tricyclic Sesquiterpene, on Human Lymphocytes Cells in Vitro. Cytotechnology., 2014; 66(4): 597-603.
CrossRef - Dzul-Beh A. d, García-Sosa K, Uc-Cachón A. H, Bórquez J, Loyola L. A, Barrios-García H. B, et al. In Vitro Growth Inhibition and Bactericidal Activity of Spathulenol against Drug-Resistant Clinical Isolates of Mycobacterium Tuberculosis. Revista Brasileira de Farmacognosia., 2019; 29(6): 798-800.
CrossRef - Karakaya S, Yilmaz S. V, Özdemir Ö, Koca M. S, Pınar N. M, Demirci B, et al. A Caryophyllene Oxide and Other Potential Anticholinesterase and Anticancer Agent in Salvia Verticillata Subsp. Amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae). Journal of Essential Oil Research., 2020; 32(6): 512 – 525.
CrossRef - Zhao F, Wang P, Lucardi R. D, Su Z, & Li S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins (Basel)., 2020; 12(1): 1-26.
CrossRef - Arora S, Kumar G, & Meena S. Screening and Evaluation of Bioactive Components of Cenchrus Ciliaris L. By GC-MS Analysis. International Research Journal of Pharmacy., 2017; 8(6): 69-76.
CrossRef - Suhail M. M, Wu W, Cao A, Mondalek F. G, Fung K. M, Shih P. T, et al. Boswellia sacra Essential Oil Induces Tumor Cell-Specific Apoptosis and Suppresses Tumor Aggressiveness in Cultured Human Breast Cancer Cells. BMC Complementary & Alternative Medicine., 2011; 11(129): 1-14.
CrossRef - Krifa M, El Mekdad H, Bentouati N, Pizzi A, Ghedira K, Hammami M, et al. Immunomodulatory and Anticancer Effects of Pituranthos Tortuosus Essential Oil. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine., 2015; 36(7): 5165-5170.
CrossRef - Quiroga P. R, Asensio C. M, & Nepote V. Antioxidant Effects of the Monoterpenes Carvacrol, Thymol and Sabinene Hydrate on Chemical and Sensory Stability of Roasted Sunflower Seeds. Journal of The Science of Food and Agriculture., 2015; 95(3): 471-479.
CrossRef - Zheljazkov V. D, Astatkie T, Jeliazkova E. A, Heidel B, & Ciampa L. Essential Oil Content, Composition and Bioactivity of Juniper Species in Wyoming, United States. Natural Product Communications.,2017; 12(2): 201-204.
CrossRef - Mimica-Dukic N, Bozin B, Sokovic M, & Simin N. Antimicrobial and Antioxidant Activities of Melissa Officinalis L. (Lamiaceae) Essential Oil. Journal of Agricultural and Food Chemistry., 2004; 52(9): 2485-2489.
CrossRef - Gnanavel V, & Saral A. M. GC-MS Analysis of Petroleum Ether and Ethanol Leaf Extracts from Abrus Precatorius Linn. International Journal of Pharma and Bio Sciences., 2013; 4(3): 37-44.
- Akpuaka A, Ekwenchi M, Dashak D, & Dildar A. Biological Activities of Characterized Isolates of N-Hexane Extract of Azadirachta Indica A. Juss (Neem) Leaves. Nature and Science., 2013; 11(5): 141-147.
- Banakar P, & Jayaraj M. GC-MS Analysis of Bioactive Compounds from Ethanolic Leaf Extract of Waltheria Indica Linn. And Their Pharmacological Activities. International Journal of Pharmaceutical Sciences and Research., 2018; 9(5): 2005-2010.
- Starlin T, Prabha P. S, Thayakumar B. K. A, & Gopalakrishnan V. K. Screening and GC-MS Profiling of Ethanolic Extract of Tylophora pauciflora. Bioinformation., 2019; 15(6): 425-429.
CrossRef - Korbecki J, & Bajdak-Rusinek K. The Effect of Palmitic Acid on Inflammatory Response in Macrophages: An Overview of Molecular Mechanisms. Inflammation Research., 2019; 68(11): 915-932.
CrossRef - Phillips S. T, Rao M. R. K, Prabhu K. V, Priya M. D. Kalaivani S, & Ravi A, et al. Preliminary GC-MS Analysis of an Ayurvedic Medicine “Kulathadi Kashayam”. Journal of Chemical and Pharmaceutical Research., 2015; 7(9): 393-400.
- Duke J, & Bogenschutz M. J. Dr. Duke’s Phytochemical and Ethnobotanical Database. U.S Department of Agriculture 1994.
- Tian C, Gao X, Yang J, Guo Y, Wang H, & Liu M. Chemical Compositions, Extraction Technology, and Antioxidant Activity of Petroleum Ether Extract from Abutilon theophrasti Medic. Leaves. International Journal of Food Properties., 2018; 21: 1789 – 1799.
CrossRef - Adeoye-Isijola M. O, Olajuyigbe O. O, Jonathan S. G, & Coopoosamy, R. M. Bioactive Compounds in Ethanol Extract of Lentinus Squarrosulus Mont-a Nigerian Medicinal Macrofungus. African Journal of Traditional, Complementary and Alternative Medicines., 2018; 15(2): 42-50.
CrossRef - Beschi D. A, Appavoo M. R, & Wilsy, J. I. GC-MS Analysis Collected from Kavalkinaru Area, Tirunelveli District, Tamil Nadu, India. European Journal of Molecular & Clinical Medicine., 2021; 8(11): 4287-4292.
- Kim E, Kang Y. G, Kim Y. J, Lee T. R, Yoo B. C, Jo M., et al. Dehydroabietic Acid Suppresses Inflammatory Response Via Suppression of Src-, Syk-, and TAK1-Mediated Pathways. International Journal of Molecular Sciences., 2019; 20(7): 1-14.
CrossRef - Zhang M, Xie Y, Su X, Liu K, Zhang Y, Pang W, et al. Inonotus Sanghuang Polyphenols Attenuate Inflammatory Response Via Modulating the Crosstalk between Macrophages and Adipocytes. Frontiers in Immunology., 2019; 10: 1-12.
CrossRef - Söderberg T. A, Johansson A, & Gref R. Toxic Effects of Some Conifer Resin Acids and Tea Tree Oil on Human Epithelial and Fibroblast Cells. Toxicology., 1996; 107(2): 99-109.
CrossRef - Ashraf V, Kalaichelvan V, Ragunathan R, & Venkatachalam V. Apoptosis Induction and Anticancer Activity of 2, 4-Bis (1-Phenylethyl) Phenol from Clerodendrum Thomsoniae Balf. F. In Vitro. International Journal of Pharmaceutical Investigation., 2020; 10(4): 542-547.
CrossRef - Abdou E. M, Fayed M. A. A, Helal D, & Ahmed K. A. Assessment of the Hepatoprotective Effect of Developed Lipid-Polymer Hybrid Nanoparticles (LPHNPS) Encapsulating Naturally Extracted Β-Sitosterol against CCL4 Induced Hepatotoxicity in Rats. Scientific Reports., 2019; 9(1): 1-14.
CrossRef - Lin F, Xu L, Huang M, Deng B, Zhang W, Zeng Z, et al. Β-Sitosterol Protects against Myocardial Ischemia/Reperfusion Injury Via Targeting PPARγ/NF-Κb Signalling. Evidence Based Complementary and Alternative Medicine., 2020; 2020: 1-9.
CrossRef - Bao X, Zhang Y, Zhang H, & Xia L. Molecular Mechanism of Β-Sitosterol and Its Derivatives in Tumor Progression. Frontiers in Oncology., 2022; 12: 1-12.
CrossRef - Mishra T, Arya R. K, Meena S, Joshi P, Pal M, Meena B, et al. Isolation, Characterization and Anticancer Potential of Cytotoxic Triterpenes from Betula Utilis Bark. PLoS One., 2016; 11(7): 1-14.
CrossRef - Thirupathi A, Silveira P. C, Nesi R. T, & Pinho R. A. Β-Amyrin, a Pentacyclic Triterpene, Exhibits Anti-Fibrotic, Anti-Inflammatory, and Anti-Apoptotic Effects on Dimethyl Nitrosamine-Induced Hepatic Fibrosis in Male Rats. Human & Experimental Toxicology., 2017; 36(2): 113-122.
CrossRef - Victor M. M, David J. M, Santos M. A. S, Barreiros A. L. B. S, Barreiros M. L, Andrade F. S. M, et al. Synthesis and Evaluation of Cytotoxic Effects of Amino-Ester Derivatives of Natural Α,Β-Amyrin Mixture. Journal of the Brazilian Chemical Society., 2017; 28(11): 2155-2162.
CrossRef - Wen S, Gu D, & Zeng H. Antitumor Effects of Beta-Amyrin in Hep-G2 Liver Carcinoma Cells Are Mediated Via Apoptosis Induction, Cell Cycle Disruption and Activation of Jnk and P38 Signalling Pathways. Journal of the Balkan Union of Oncology (B.U.O.N)., 2018; 23(4): 965-970.
- Nogueira A. O, Oliveira Y. I. S, Adjafre B. L, De Moraes M. E. A, & Aragão G. F. Pharmacological Effects of the Isomeric Mixture of Alpha and Beta Amyrin from Protium Heptaphyllum: A Literature Review. Fundamental & Clinical Pharmacology., 2019; 33(1): 4-12.
CrossRef - Anburaj J, Elango T, Swapna S, & Amuthavalli K. Beta-Amyrin Modulates P38 Mapk and Jnk Pathway to Inhibit Cell Proliferation and Induce Ros-Mediated Apoptosis in Hela Cells. Indian Journal of Pharmaceutical Sciences., 2020; 82(3): 420-428.
CrossRef - Neto S. F, Prada A. L, Achod L. D. R, Torquato H. F. V, Lima C. S, Paredes-Gamero E. J, et al. Α-Amyrin-Loaded Nanocapsules Produce Selective Cytotoxic Activity in Leukemic Cells. Biomedicine & Pharmacotherapy., 2021; 139(8): 111656.
CrossRef - Patel D. Plants as a Source of Medicine. Medicinal & Aromatic Plants Research Journal., 2015; S: 3.
CrossRef - Mahomoodally F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evidence-Based Complementary and Alternative Medicine., 2013; 2013: 1-14.
CrossRef - Nisha K, Mehta D, Madhu G, & Mehta B. GC-MS Analysis and Anti-Microbial Activity of Psidium Guajava (Leaves) Grown in Malva Region of India. International Journal of Drug Development and Research., 2011; 3(4): 237-245.
- Gopalakrishnan K, & Udayakumar R. GC-MS Analysis of Phytocompounds of Leaf and Stem of Marsilea Quadrifolia (L.). International Journal of Biochemistry Research & Review., 2014; 4(6): 517-526.
CrossRef - George B. P, Chandran R, & Abrahamse H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants (Basel)., 2021; 10(9): 1-23.
CrossRef - Abu-Izneid T, Rauf A, Shariati M. A, Khalil A. A, Imran M, Rebezov M, et al. Sesquiterpenes and Their Derivatives-Natural Anticancer Compounds: An Update. Pharmacological Research., 2020; 161: 105165.
CrossRef - Hazafa A, Iqbal M. O, Javaid U, Tareen M. B. K, Amna D, Ramzan A, et al. Inhibitory Effect of Polyphenols (Phenolic Acids, Lignans, and Stilbenes) on Cancer by Regulating Signal Transduction Pathways: A Review. Clinical and Translational Oncology., 2022; 24(3): 432-445.
CrossRef - Ishak S. F, Rajab N. F, & Basri D. F. Antiproliferative Activities of Acetone Extract From Canarium odontophyllum (Dabai) Stem Bark Against Human Colorectal Cancer Cells. Dose Response., 2023; 21(2): 1-10.
CrossRef - Vazquez L, Palazon J, & Navarro-Ocaa A. The Pentacyclic Triterpenes Alpha, Beta-Amyrins: A Review of Sources and Biological Activities. In (Ed.), Phytochemicals – A Global Perspective of Their Role in Nutrition and Health., 2012: pp 487-502.
- Mutiah R, Widyawaruyanti A, & Sukardiman S. Calotroposid A: A Glycosides Terpenoids from Calotropis Gigantea Induces Apoptosis of Colon Cancer WiDr Cells through Cell Cycle Arrest G2/M and Caspase 8 Expression. Asian Pacific Journal of Cancer Prevention., 2018; 19(6): 1457-1464.
- Chandra M, Kushwaha S, Mishra B, & Sangwan, N. Molecular and Structural Insights for the Regulation of Terpenoids in Ocimum Basilicum and Ocimum Tenuiflorum.Plant Growth Regulation., 2022; 97(1): 61-75.
CrossRef - Tholl D. Biosynthesis and Biological Functions of Terpenoids in Plants. Advances in Biochemical Engineering/Biotechnology., 2015; 148: 63-106.
CrossRef - Abdjul D. B, Yamazaki H, Maarisit W, Rotinsulu H, Wewengkang D. S, Sumilat D. A, et al. Oleanane Triterpenes with Protein Tyrosine Phosphatase 1b Inhibitory Activity from Aerial Parts of Lantana Camara Collected in Indonesia and Japan. Phytochemistry., 2017; 144: 106-112.
CrossRef - Ibrahim S. R, Abdallah H. M, Mohamed G. A, & Ross S. A. Integracides H-J: New Tetracyclic Triterpenoids from the Endophytic Fungus Fusarium Sp. Fitoterapia., 2016; 112: 161-167.
CrossRef - Singh A, Kumar A, & Singh, I. K. Marine Flora: Source of Drugs from the Deep-Sea Environment. Marine Niche: Applications in Pharmaceutical Sciences: Translational Research., 2020; 161-181.
CrossRef - Cui L, Noushahi H. A, Zhang Y, Liu J, Cosoveanu A, Liu Y, et al. Endophytic Fungal Community of Huperzia Serrata: Diversity and Relevance to the Production of Huperzine a by the Plant Host. Molecules., 2021; 26(4): 1-19.
CrossRef - Pemberton T. A, Chen M, Harris G. G, Chou W. K, Duan L, Köksal M, et al. Exploring the Influence of Domain Architecture on the Catalytic Function of Diterpene Synthases. Biochemistry., 2017; 56(14): 2010-2023.
CrossRef








