Eka Pramyrtha Hestianah1 , Widjiati Widjiati1*
, Widjiati Widjiati1* , Juliano Mwenda Ntoruru2
, Juliano Mwenda Ntoruru2 , Muhammad Yohanes Ardianta Widyanugraha3
, Muhammad Yohanes Ardianta Widyanugraha3 and Epy Muhammad Luqman1
and Epy Muhammad Luqman1
1Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia
2Research Assistant, Meru University of Science and Technology, Meru, Kenya.
3Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
Corresponding Author E-mail:widjiati@fkh.unair.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2914
Abstract
The purpose of this study is to ascertain how nanocurcumin 0-50 nm affects malondialdehyde (MDA) levels, folliculogenesis, and the quantity of corpus luteum (CL) in a mice model of endometriosis. 24 mice were used in this scientific experiment, and they were split up into 4 treatment groups; positive control (C+) as mice model of endometriosis, Treatment 1 (T1) as mice model of endometriosis given nanocurcumin 0-50 nm (2.5 mg/KgBW) PO, Treatment 2 (T2) mice given nanocurcumin (5 mg/KgBW) and Treatment 3 (T3) as mice model of endometriosis treated with nanocurcumin (10 mg/KgBW). The nanocurcumin was administered for 14 days. The findings demonstrated that, out of all treatment groups, T3 had the lowest MDA level (20.78±4.58 ng/ml) and statistically significant (p<0.05) compared to C+, T1 and T2. Significant differences (p<0.05) were observed in the number of primary, secondary, and tertiary follicles at T3, from C+, T1, and T2, according to the folliculogenesis profile. Although the number of Graafian follicles tended to grow, there was no discernible difference between the number of Graafian follicles and the CL. It can be concluded that the administration of nanocurcumin for 14 days decreased MDA levels and increased the folliculogenesis profile. Administration of nanocurcumin at a dose of 10 mg/KgBW caused a decrease in MDA levels and an improvement in the folliculogenesis profile. Thus, administering nanocurcumin could improve the quality of folliculogenesis in endometriosis sufferers and improve reproductive health.
Keywords
Endometriosis; Folliculogenesis; Nanocurcumin MDA; Reproductive health
Download this article as:| Copy the following to cite this article: Hestianah E. P, Widjiati W, Ntoruru J. M, Widyanugraha M. Y. A, Luqman E. M. Administration of Nanocurcumin in Mice Models of Endometriosis as an Effort to Improve Folliculogenesis. Biomed Pharmacol J 2024;17(2). |
| Copy the following to cite this URL: Hestianah E. P, Widjiati W, Ntoruru J. M, Widyanugraha M. Y. A, Luqman E. M. Administration of Nanocurcumin in Mice Models of Endometriosis as an Effort to Improve Folliculogenesis. Biomed Pharmacol J 2024;17(2). Available from: https://bit.ly/3VxkVh3 |
Introduction
The existence of endometrial-like tissue outside the uterus is known as endometriosis, which induces chronic inflammation1. In general, the estimated prevalence of endometriosis in females is between 6 and 10%2. One of the complaints that often arise in women with endometriosis is infertility3. The prevalence is 21% in women with infertility complaints, and increases to 82% in women with pelvic pain complaints4.
The inability to conceive following a year of unprotected sex is known as infertility3 and decreased fertility in women with endometriosis5. One theory that has been published regarding the relationship between endometriosis and infertility is the theory regarding changes in the environment around the uterus and egg cells caused by endometriosis. Endometriosis can cause inflammation and scar tissue formation around the uterus, ovaries, and fallopian tubes. These changes can disrupt the normal function of the female reproductive organs and reproductive processes6,7,8. One key idea about endometriosis is that it’s characterized by a localized inflammatory process in the pelvis, oxidative stress from increased Reactive oxygen species (ROS) production, and altered immune system-related cell function in the peritoneal milieu9,10. Oocytes are shielded from oxidative damage during folliculogenesis by a variety of antioxidants that the body produces. However, excessive and persistent ROS production can interfere with folliculogenesis11.
ROS are generated in response to various environmental stimuli, and excessive ROS production can lead to lipid peroxidation, which is often monitored by measuring malondialdehyde (MDA). MDA is produced as a byproduct of oxidative stress-induced lipid peroxidation12.
Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), derived from the rhizome of Curcuma spp., has antioxidant properties13. Nanocurcumin is curcumin with nanoparticle size so that it has better bioavailability and stability than the original curcumin molecule. The administration of curcumin as a treatment for endometriosis is still controversial. This may be because there have not been many studies and publications on the administration of curcumin as a treatment for endometriosis, especially in infertility problems related to endometriosis. In their article, Swarnakar and Paul state that the benefits of curcumin in various diseases include being an antioxidant. Based on this, curcumin is thought to provide benefits as a therapy in endometriosis14.
This research aims to study the effect of nanocurcumin 10-50 nm on endometriosis as an effort to improve oocyte quality and embryonic development through antioxidant mechanisms by improving folliculogenesis. Considering that nanocurcumin has never been used in humans for the treatment of endometriosis with infertility and human studies to determine the effect of nanocurcumin are ethically constrained, this study used mice as models of endometriosis.
Materials and Methods
This research was carried out through several stages, namely making mice model of endometriosis, providing nanocurcumin 10-50 nm treatment, measuring the MDA of peritoneal fluid and folliculogenesis in the ovaries.
Materials
Curcumin (8.20354-Sigma, Aldrich); alcohol, betadine, sterile gauze, round cotton, silk thread 3-0, catgut, ketamine, xylazine, sodium chloride (NaCl) 0.9% (PT. Widatra Bakti); PMSG (Folligon, Intervet, Boxmeer, Holland); hCG (Chorulon, Intervet, Boxmeer, Holland); ketamine HCL (Ketamil®, Troy Laboratories PTY Limited, Australia); MDA ELISA Kit (MDA Assay Kit competitive ELISA, Abcam- ab238537); 10% Neutraled Buffered Formaldehyde (NBF) (HT-501128-Sigma, Aldrich).
Ethical approval
The ethical permition certificate was obtained from the Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia, with number 1.KE.053.05.2022.
Animal study
The Embryology Laboratory and Histology Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga, are the sites of this laboratory experiment study, which employs a completely randomized design (CRD). 24 Mice (Mus musculus) were employed in this study, and they were split up into 4 treatment groups, each with 6 mice. The experimental animals were acclimated and provided with enough food and drink for seven days prior to the treatment.
Preparation and Characterization of Nanocurcumin
This examination is used to ensure that the curcumin used has nano particle size. The mean particle size of the nanocurcumin was observed by Dynamic Light Scattering (DLS). In brief, the sample was prepared by taking 1 mg of the nanocurcumin powder (Curcumin 8.20354-Sigma, Aldrich) in 10 ml distilled water and used in characterization studies. Scanning Electron Microscopy (SEM) (EX-250 System, Horiba, Kyoto, Japan) was performed by spreading the nanoparticle dispersion over a carbon tape and drying it under a nitrogen stream. The sample was then coated in a sputter with a gold layer in a vacuum condition34.
Endometriosis Animal Model
Four groups of endometriosis model rats were created, namely the positive control (C+) as mice model of endometriosis, Treatment 1 (T1) as mice model of endometriosis given nanocurcumin 10-50 nm (2.5 mg/KgBW) PO, Treatment 2 (T2) mice given nanocurcumin (5 mg/KgBW) and Treatment 3 (T3) as mice model of endometriosis treated with nanocurcumin (10 mg/KgBW) 0.5 ml orally (PO) while C (+) was given a placebo. Exposure time is carried out every morning for 14 days.
Sample Collection and Histopathology
All mice received injections of PMSG after 14 days of therapy, and 48 hours later, hCG. The Mice were also mated with male mice for ovulation induction. 17 hours later the vaginal plug were evaluated plug and if positive, the termination was done using ketamine HCL (Ketamil®, Troy Laboratories PTY Limited, Australia) 100 mg/kgBW. After the mice was terminated, Using a scalpel, the incision was created in the ventral midline, and the thorax and abdomen were then prepared with scissors.
Peritoneal fluid was taken using a 1 cc syringe in the abdominal cavity. The collected fluid was checked for MDA levels by ELISA. After being removed, the ovaries were put in a sample pot with 10% Neutraled Buffered Formaldehyde (NBF) (HT-501128-Sigma, Aldrich) inside of them. After that, the organ samples were brought to the Universitas Airlangga Faculty of Veterinary Medicine’s Pathology Laboratory for histological preparations.
MDA Level Estimation
MDA level analysis was done at the Laboratory of Physiology, Faculty of Medicine, Universitas Airlangga using the Colorimetric ELISA method with a commercial kit (MDA Assay Kit competitive ELISA, Abcam- ab238537). MDA levels are expressed in ng/ml. Measure the OD value at 450 nm with a microplate reader. Calculate with standard concentration OD value and sample concentration with formula that according to the assay kits used by the laboratory (Standard Diagnostic, Inc., Yongin, Korea).
Folliculogenesis Profile Examination
Histological preparations of previously prepared ovarian tissue were used to examine the folliculogenesis profile. Ovarian tissue fixated with buffer formalin 10%. Then staining process using Hematoxylin and Eosin. A 400x magnification Olympus® light microscope was used to conduct the inspection and then Graafian follicles, tertiary follicles, primary follicles, secondary follicles, and CL numbers were recorded. Five fields of view are used for the computation, and the results are averaged after that.
Statistical Analysis
The statistical analysis used IBM SPSS 23 application with One-Way ANOVA and post-hoc Duncan to seek for differences between groups of variables in MDA levels, and number of CL, primary, secondary, tertiary, and Graafian follicles.
Results and Discussion
The study was conducted by measuring two parameters, namely folliculogenesis, and MDA levels on mice model of endometriosis given nanocurcumin 10-50 nm. In this study, there were five treatment groups used with each treatment consisting of four replications, namely treatment C+ (positive control) as mice model of endometriosis, T1 (treatment 1) mice model of endometriosis group treated with 2.5 mg/KgBW dose of nanocurcumin, T2 (treatment 2) mice model of endometriosis group treated with 5 mg/KgBW dose of nanocurcumin, and T3 (treatment 3) mice model of endometriosis group treated with 10 mg/KgBW dose of nanocurcumin.
Effect of treatment on Folliculogenesis
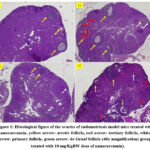 |
Figure 1: Histological figure of the ovaries of endometriosis model mice treated with nanocurcumin. |
Histological picture in fig. 1 shows that the atretic follicle (yellow arrow) is most abundant in the T1 group. The atretic follicle looks pale. It will continue to shrink, eventually forming a small scar on the side of the ovary. Meanwhile, on average, tertiary follicles (red arrows) appeared a lot in the T1 and T3 groups. Tertiary follicle identifying characteristic is a fluid-filled cavity, the antral follicle. The oocyte lies at the edge in a mound made of granulosa epithelial cells, the cumulus oophorus. primary follicle (white arrow) which is shown to consist of a central oocyte surrounded by a single or double layer of follicular cells. Primary follicles were most visible in the T3 group. Meanwhile, de Graaf follicles (green arrow) only appear in the T3 group with large follicles. The follicular fluid fills a single space, called the antrum, which is surrounded by the follicular cells.
Table 1: The average and deviation from the mean of primary, secondary, tertiary, and De Graaf follicles, as well as corpus luteum counts, were assessed in the ovaries of mice with endometriosis treated with nanocurcumin therapy at different concentrations.
|
Group |
Primary Follicle (Mean±SD) |
Secondary Follicle (Mean±SD) |
Tertiary Follicle (Mean±SD) |
Graafian Follicle (Mean±SD) |
Corpus Luteum (Mean±SD) |
|
C(+) |
0.71c±0.75 |
1.71b±0.76 |
0.86b±1.06 |
0.00a±0.00 |
2.14a±0.69 |
|
T1 |
1.57cb±0.78 |
3.71b±1.11 |
1.71ab±1.25 |
0.00a±0.00 |
3.57a±0.53 |
|
T2 |
2.29b±1.38 |
3.71b±1.98 |
0.43b±0.53 |
0.00a±0.00 |
2.71a±2.13 |
|
T3 |
6.71a±3.27 |
7.85a±3.76 |
2.14a±1.57 |
0.14a±0.38 |
2.85a±1.24 |
Note: Different superscripts (a,b,c) in the same column show significant differences between treatments (p<0.05).
The follicle examination results indicated that the T3 group exhibited the highest counts of primary, secondary, tertiary, and Graafian follicles, specifically 6.71±3.27, 7.85±3.76, 2.14±1.57, and 0.14 ± 0.38, respectively. Statistically significant differences (p<0.05) were observed in the counts of primary, secondary, and tertiary follicles compared to the other groups. Although not statistically significant (p>0.05), an increasing trend was noted in the count of Graafian follicles. The number of corpus luteum (CL) in each group did not show statistically significant differences (p>0.05) (Table 1; Figure 1).
Based the result at Table 1, in treatment group 3 (T3), the addition of nanocurcumin at a dose of 10 nm improved the follicle development process, which shows the development of primary, secondary, tertiary, and de Graaf follicles.
In this study, there was an increase in the number of primary follicles in the treatment group with the highest number in the T3 group, which was significantly different from the other groups. Similarly, in calculating the number of secondary follicles, it was found that the T3 group with the highest number and significantly different from the other groups. This demonstrates that in a mice model of endometriosis, the injection of not italic can increase the number of primary and secondary follicles.
Antioxidants including catalase, superoxide dismutase (SOD), glutathione transferase, paraoxanase, heat shock protein (HSP) 27, and protein isomerase shield oocytes from oxidative damage during folliculogenesis. Once generated, ROS have the ability to interact with other molecules to cause cellular functions and various organelles to malfunction. ROS overproduction that persists over time can have detrimental effects on a number of signaling pathways that are necessary for folliculogenesis11.
It is commonly known that in animals, the development of follicles and oocytes starts in the womb. In summary, the primordial germ cells go through mitosis in order to generate primary oocytes. Following that, meiotic development starts at the diplotene phase of the first meiotic division and ends there24. Entering puberty, meiotic development will continue, the chromosomes relax and the resulting nuclear structure is called the germinal vesicle (GV). After the luteinizing hormone (LH) surge, the GV disappears, the chromatin is condensed, the homologous chromosome pairs are separated and the half will be expelled in the first polar body. At this point meiosis stops again (metaphase II – MII). At this time, the oocyte is said to be mature and ready to receive sperm to undergo fertilization25. Additionally, granulosa cells and theca cells generate the peptide hormone inhibin during the process of folliculogenesis, as well as the ovarian hormones progesterone (P) and estradiol (E2). These hormones act as a feedback system to control the production and release of FSH, LH, and GnRH. At the tiny antral follicle stage, the majority of ovarian follicles go through an apoptotic process known as atresia instead of ovulation25. This study succeeded in proving that the administration of nanocurcumin could improve folliculogenesis by increasing the quantity of primary and secondary follicles in the model of endometriosis in mice.
In this study, the number of tertiary follicles increased significantly in the therapy group as well, with the T3 group having the greatest number. This shows that the administration of nanocurcumin could increase the number of tertiary follicles in endometriosis mice model (Figure 1).
Most of the time, there are two phases of tertiary/antral follicular growth. Early follicle growth can be linked to an increase in granulosa cell numbers and, consequently, an increase in the granulosa cell layer during the first phase, which is marked by a slow growth stage26. For the development of oocyte capacity, this period is crucial27. The second phase is marked by fast growth in follicles larger than 2–5 mm, and it seems that antral development, rather than an increase in granulosa cell count, is the cause of this follicular expansion. Until ovulation takes place in this follicle, the antrum’s diameter increases exponentially28. Sufficient LH pulses and FSH hormones regulate this second stage29. Regarding endometriosis, the study conducted recently demonstrated that endometriosis can decrease the number of antral follicles30. Research has also shown that many factors that may be responsible, including reactive oxygen species, free iron and proteolytic enzymes31 of endometriosis can infiltrate into the tissue surrounding the cyst, which then decreases ovarian reserve30. Thus, this study succeeded in proving that the administration of nanocurcumin could improve folliculogenesis by increasing the number of tertiary follicles in mice model of endometriosis.
Additionally, there was a tendency for the T3 group’s Graafian follicle count to grow, however the results of this study did not show a significant difference across all groups. Likewise, in the corpus luteum group, there were no significantly different numbers.
In mammals, after ovulation, the follicle will develop into the corpus luteum (CL). The primary job of the CL is to synthesize progesterone, which is required for both the development and maintenance of pregnancy as well as the creation of an appropriate uterine environment for the peri-implantation conceptus (embryo and related extra-embryonic membranes). Reactive oxygen species (ROS) have been shown to play a major role in defining the age of CL at which folliculogenesis interference can result in luteal phase abnormalities32. It is suspected that antioxidants play an important role in CL physiology during the estrus/menstrual cycle33. Since luteal phase abnormalities can affect fertility by impeding implantation and the development of early conception, in both humans and animals, nanocurcumin with its antioxidant properties may show a positive effect in improve folliculogenesis in endometriosis. Our findings, which revealed no discernible variation in the quantity of corpus luteum and Graafian follicles, might be the consequence of the insufficient dosage of nanocurcumin used in this investigation to enhance folliculogenesis to this extent. From the previous results, it appears that the increase in the number of primary, secondary and tertiary follicles depends on the dose of nanocurcumin given. Therefore, further studies are needed to determine the optimal dose of nanocurcumin to improve the folliculogenesis process.
Effect of treatment on MDA Level
Table 2: The average and deviation from the mean of MDA levels in mice with endometriosis treated with nanocurcumin therapy were examined.
|
Group |
MDA (Mean±SD) |
|
Control (+) |
39.57c±2.27 |
|
Treatment 1 (T1) |
38.38c±2.00 |
|
Treatment 2 (T2) Treatment 3 (T3) |
20.78b±4.58 13.88a±1.30 |
Notes: Different superscripts (a,b,c) in the same column show significant differences between treatments (p<0.05).
Table 2 exhibits the results obtained from calorimetric ELISA analysis of MDA levels. It was observed that group C(+) demonstrated the highest MDA level, amounting to 39.57±2.27 ng/ml. Conversely, MDA levels decreased in treatment groups T1, T2, and T3, registering as 38.38±2.00 ng/ml, 20.78±4.58 ng/ml, and 13.88±1.30 ng/ml, respectively, with T2 and T3 displaying statistical significance (p<0.05) in comparison to groups C (+) and T1 (Table 2).
The elevated levels of MDA in the control group led to a disturbance in the follicle development process. Based on the results presented in the below table, an increase in MDA levels is associated with a decrease in the number of pre-antral, antral, and CL follicles. In this research, the MDA levels decreased as the nanocurcumin dosage increased. The decrease in MDA levels was significant in the T2 and T3 groups with the lowest decrease in the T3 group. This shows that nanocurcumin can have a positive effect by reducing oxidative stress in endometriosis as indicated by a decrease in MDA levels. Futhermore, elevated MDA levels in the control group disrupted follicle development, resulting in decreased numbers of pre-antral, antral, and corpus luteum follicles.
The primary active ingredient in turmeric, curcumin, belongs to the curcuminoid family and has been utilized in traditional medicine for many years. Asian cultures have been using curcumin for a long time, and it hasn’t been shown to be poisonous. Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), derived from the rhizome of Curcuma spp., has antioxidant properties13. Curcumin as a nanoparticle has better bioavailability and stability than native curcumin molecules21 and provides a better therapeutic effect efficiency on target tissues, than native curcumin22,23. This study succeeded in proving that the administration of nanocurcumin can cause a decrease in MDA levels in the peritoneal fluid of mice model of endometriosis.
MDA is a dialdehyde compound which is the end product of lipid peroxidation in the body. High MDA concentration indicates an oxidation process in the cell membrane. The chemically stable nature of MDA makes this compound more often used as a marker of oxidative stress than other compounds15.
Endometriosis is known to cause oxidative stress and is a potential factor involved in the pathophysiology of endometriosis6. Excessive ROS, including superoxide, hydroxyl, and hydrogen peroxide, are produced during severe oxidative stress and can harm proteins, DNA, and other parts of the cell16. The body forms a defense in the form of antioxidant complexes that collectively act against free radicals. Additionally, unchecked lipid peroxidation brought on by oxidative stress has the potential to damage DNA and directly inhibit proteins, both of which can harm cells17. Since MDA is a persistent by product of lipid peroxidation, it can be utilized as a proxy for total lipid peroxidation. MDA and SOD are regarded as biological indicators of oxidative stress18.
Like in many other cells, the intraovarian environment is directly harmed by oxidative stress, which is brought on by an imbalance between the synthesis and breakdown of ROS. In addition, by the fifth month of fetal life, all primary oocytes have produced and are inactive until meiosis I is finished, which is a process that lasts for decades leaving the oocyte vulnerable to chronic oxidative damage. Several studies have demonstrated that granulosa cell (GC) apoptosis, corpus luteum degradation, and worsening of oocyte quality are all caused by accumulation of ROS in the ovary. Additionally, it lessens oocyte-GC communication, which impacts the development of preovulatory oocytes19. Folliculogenesis, meiosis, and ovulation are all significantly impacted by the lipid peroxidation cascade reaction, which is typically the cause of oxidative damage to the ovaries20.
Curcumin inhibiting the process of apoptosis while the mechanism may be through multiple pathways including inhibition of activation of the transcription factor NF-kß with various results, suppression of inflammatory activity through the suppression of TNF-α directly or through its antioxidant effects in endometriosis35. High levels of MDA, pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β), angiogenic factors (IL-8 and VEGF), monocyte chemoattractant protein-1 (MCP-1), and oxidized LDL (ox-LDL) were detected in the peritoneal fluid of endometriosis patients36. Curcumin to the culture medium of peritoneal fluid from infertile women with endometriosis resulted in more improved GDF9 and Kit Ligand expression37. Curcumin inhibit the induction of pro-inflammatory cytokines, angiogenic cytokines and macrophage migration inhibitory factor by NF-kB in in vitro model. Several recent studies also found the modulatory effect of curcumin on several important molecular targets (TNF, IL-1, IL-6) and enzyme (COX-2)38,39. In addition, curcumin can also lower anti-apoptotic genes expression, antioxidants and anti-angiogenesis effects40,41.
Conclusion
Administering nanocurcumin for 14 days resulted in a reduction of MDA levels and an enhancement of folliculogenesis. Specifically, the administration of nanocurcumin at a dosage of 10nm/KgBW led to decreased MDA levels and improved folliculogenesis. This investigation effectively demonstrated the ability of nanocurcumin administration to enhance folliculogenesis by increasing the quantity of tertiary follicles in a mouse model of endometriosis.
Acknowledgments
The authors are grateful to the authorities Universitas Airlangga and Faculty of Veterinary Medicine Universitas Airlangga, Surabaya, East Java, Indonesia.
Conflict of interest
All authors declare that there is no conflict of interest.
Funding
The authors received financial support for the Implementation of Internal Research Universitas Airlangga Number 978/UN3/2022 and publication of this article.
References
- Zieliński K, Drabczyk D, Kunicki M, Drzyzga D, Kloska A, and Rumiński J. Evaluating the risk of endometriosis based on patients’ self-assessment questionnaires. Reprod. Biol. Endocrinol., 2023; 21(1):102-113.
CrossRef - Baldi A, Campioni M, and Signorile P. G. Endometriosis: pathogenesis, diagnosis, therapy and association with cancer (review). Oncol. Rep., 2008; 19(4): 843-846.
CrossRef - Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril., 2012; 98(3): 591-598.
CrossRef - Allaire C, Bedaiwy M. A, and Yong P. J. Diagnosis and management of endometriosis. CMAJ 2023; 195(10): E363–E371.
CrossRef - Broi M. G. D, Ferriani R. A, and Navarro P. A. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist. Reprod., 2019; 23(3):273-280.
CrossRef - Giudice L.C. Clinical practice. Endometriosis. N. Engl. J. Med., 2010; 362(25); 2389-2398.
CrossRef - Burney R.O., and Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil Steril., 2012; 98(3): 511-519.
CrossRef - Macer M. L, and Taylor H. S. Endometriosis and Infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am., 2012; 39(4): 535–549.
CrossRef - Donnez J, Binda M. M, Donnez O, and Dolmans M. M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril., 2016; 106(5): 1011-1017.
CrossRef - Tan Z, Gong X, Wang C. C, Zhang T, and Huang J. Diminished ovarian reserve in endometriosis: insights from in vitro, in vivo, and human studies-a systematic review. Int. J. Mol. Sci., 2023; 24(21):15967.
CrossRef - Agarwal A, Aponte-Mellado A, and Premkumar B. J. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol., 2012; 29 (10):49-53.
CrossRef - Mazaheri-Tirani M, Haghjou M. Reactive oxygen species (ROS), Total antioxidant capacity (AOC) and Malondialdehyde (MDA) make a triangle in evaluation of Zinc stress extension. J Anim. Plant. Sci., 2019; 29(3): 1100-1111.
- Obłoza M, Milewska A, Botwina P, Szczepański A, Medaj A, Bonarek P, Szczubiałka K, Pyrć K, and Nowakowska M. Curcumin-Poly(sodium 4-styrenesulfonate) Conjugates as Potent Zika Virus Entry Inhibitors. ACS. Appl. Mater. Interfaces., 2024; 16(5):5426-5437.
CrossRef - Swarnakar S, and Paul S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J. Biochem. Biophys,. 2009; 46(1): 59-65.
- Grotto D, Maria L. S, and Valentini J. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Química Nova., 2009; 32: 169-174.
CrossRef - Skulachev V. P, Vyssokikh M. Y, Chernyak B. V, Mulkidjanian A. Y, Skulachev M. V, Shilovsky G. A, Lyamzaev K. G, Borisov V. B, Severin F. F, and Sadovnichii V. A. Six Functions of Respiration: Isn’t It Time to Take Control over ROS Production in Mitochondria, and Aging Along with It?. Int. J. Mol. Sci., 2023; 24(16):12540.
CrossRef - Spiteller G. The important role of lipid peroxidation processes in aging and age dependent diseases. Mol. Biotechnol., 2007; 37(1): 5-12.
CrossRef - Mao C, Yuan J. Q., Lv Y-B., Gao X, Gao X, Yin Z. X, Kraus V. B, Si Luo J. S, Chei C. L, Matchar D. B, Zeng Y, and Shi X. M. Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: a community-based cohort study. BMC Geriatrics., 2019; 19(1): 104.
CrossRef - Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y, Chang X, and Zhang Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol., 2020; 11: 617843.
CrossRef - Maclaran K, and Nikolaou D. Early ovarian ageing. Obstet. Gynaecol., 2019; 21(2): 107-116.
CrossRef - Singh A. T, Ghosh M, Forte T. M, Ryan R. O, and Gordon L. I. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk. Lymphoma., 2011; 52(8): 1537-1543.
CrossRef - Boretti A. Curcumin-based fixed dose combination products for cholesterol management: a narrative review. ACS Pharmacol. Transl. Sci., 2024;7(2):300-308.
CrossRef - Wen C, Zhou Y, Zhou C, Zhang Y, Hu X, Li J, and Yin H. Enhanced radiosensitization effect of curcumin delivered by PVP-PCL nanoparticle in lung cancer. J. Nanomater., 2017: 1-8
CrossRef - Bothun A. M, Gao Y, Takai Y, Ishihara O, Seki H, Karger B, Tilly J. L, and Woods D. C. Quantitative proteomic profiling of the human ovary from early to mid-gestation reveals protein expression dynamics of oogenesis and folliculogenesis. Stem. Cells. Dev., 2018; 27(11):723-735.
CrossRef - Ciani F, Cocchia N, d’Angelo D, Tafuri S. Influence of ROS on Ovarian Functions. New Discoveries in Embryology. Edited by Bin Wu. IntechOpen Limited 5 Princes Gate Court, London, SW7 2QJ, United Kingdom. 2015.
CrossRef - Dey P, Monferini N, Donadini L, Lodde V, Franciosi F, Luciano A. M. Method of isolation and in vitro culture of primordial follicles in bovine animal model. Methods. Mol. Biol., 2024; 2770:171-182.
CrossRef - Wang P, Paquet É. R, and Robert C. Comprehensive transcriptomic analysis of long non-coding RNAs in bovine ovarian follicles and early embryos. PLoS One., 2023;18(9):e0291761.
CrossRef - Arroyo A, Kim B, and Yeh J. Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod. Sci., 2020; 27(6): 1223–1252.
CrossRef - Mihm M, and Bleach E. C. Endocrine regulation of ovarian antral follicle development in cattle. Anim Reprod Sci., 2003; 78(3-4), 217-237.
CrossRef - Tian Z, Zhang Y, Zhang C, Wang Y, Zhu H. L. Antral follicle count is reduced in the presence of endometriosis: a systematic review and meta-analysis. Reprod. Biomed. Online., 2021; 42(1): 237-247.
CrossRef - Sanchez A. M, Papaleo E, Corti L, Santambrogio P, Levi S, Viganò P, Candiani M, and Panina-Bordignon P. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum. Reprod., 2014; 29(3): 577-583.
CrossRef - Yan F, Zhao Q, Li Y, Zheng Z, Kong X, Shu C, Liu Y, Shi Y. The role of oxidative stress in ovarian aging: a review. J. Ovarian. Res., 2022; 15:100-120.
CrossRef - Al-Gubory K. H, Garrel C, Faure P, and Sugino N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online., 2012; 25(6):551-60.
CrossRef - Kumar, V., Kumar, R., Jain, V.K. Preparation and characterization of nanocurcumin based hybrid virosomes as a drug delivery vehicle with enhanced anticancerous activity and reduced toxicity. Sci Rep., 2021; 11:368.
CrossRef - Panjaitan, B.C., Sa’adi, A., Hendarto, H., Widjiati. comparison of ovarial malondialdehyde (MDA) level between endometriosis rat given with and without curcumine supplementation. MOGI., 2012; 20(1): 30-34.
- Lu, J., Wang, Z., Cao, J., Chen, Y., Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. RBE., 2018; 16:80.
CrossRef - Hendarto, H., Widyanugraha, MYA., Widjiati, W. Curcumin improves growth factors expression of bovine cumulus oocyte complexes cultured in peritoneal fluid of women with endometriosis. Int. J. Reprod. BioMed. 2018; 16: 775–782.
CrossRef - Wieser F., Cohen M., Gaeddert A. Evolution of medical treatment for endometriosis: back to the roots. Hum.Reprod.Update., 2007; 13(5): 487-499.
CrossRef - Grummer, R. Animal models in endometriosis research. Hum.Reprod.Update., 2006; 12(5): 641-649.
CrossRef - Sharma, R.A., Gescher, A.J., Steward, W.P. Curcumin the story so far. Eu.J.Cancer., 2005; 41: 1955-1968.
CrossRef - Joe, B., Vijaykumarand, M., Lokes, RB. Biological properties of curcumin-celluler and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004; 44(2):97-111.
CrossRef








