Manuscript accepted on :18-03-2024
Published online on: 24-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Mazhar Ozkan
Second Review by: Dr. Abhishek Raj
Final Approval by: Dr. Luis Jesús Villarreal-Gómez
Yulduzkhon T. Mirzayeva1* , Abdisalim A. Zaripov1
, Abdisalim A. Zaripov1 , Inoyat Z. Zhumaev1
, Inoyat Z. Zhumaev1 , Pulat B. Usmanov1
, Pulat B. Usmanov1 , Shavkat Yu. Rustamov1
, Shavkat Yu. Rustamov1 , Sadriddin N. Boboev1
, Sadriddin N. Boboev1 , Shakhnoza B. Qurbonova1
, Shakhnoza B. Qurbonova1 , Eldor B. Ibragimov1
, Eldor B. Ibragimov1 , Madina K. Musaeva1
, Madina K. Musaeva1 , Sardor B. Sobirov1
, Sardor B. Sobirov1 and Shahobiddin M. Adizov2
and Shahobiddin M. Adizov2
1Institute of Biophysics and Biochemistry et the National University of Uzbekistan, Tashkent, Uzbekistan
2Institute of the Chemistry of Plant Substances, Uzbek Academy of Sciences, Tashkent, Uzbekistan
Corresponding Author E-mail: inoyat8585@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/2876
Abstract
Introduction: Using conventional organ bath procedures, the current study sought to determine how vincanine hydrochloride affected vasorelaxation brought on by hypoxia in rat aortic rings. Methods: To induce hypoxia, we used a glucose-free Krebs solution that was infused with 95% N2 and 5% CO2. After 60 minutes of hypoxia, the effect of vincanine was evaluated on aortic rings that were precontracted with either 50 mM KCl or 1 µM phenylephrine (PE). The effect of vincanine was more noticeable in aortic rings that had been precontracted by PE as opposed to KCl. Additionally, when verapamil, a blocker of L-type VDCCs, was preincubated with endothelium-intact aortic rings and KCI was used for precontraction, the effect of vincanine on hypoxia-induced vasorelaxation was significantly reduced. Results: Vincanine inhibited hypoxia-induced vasorelaxation in aortic rings precontracted with PE in a calcium-free buffer. Furthermore, the presence of glibenclamide, a specific inhibitor of ATP-sensitive K+-channels (KATP), and tetraethylammonium chloride (TEA), a nonspecific inhibitor of calcium-activated large conductance K+-channels (BKca), significantly reduced the effect of vincanine on hypoxia-induced vasorelaxation. The removal of the endothelium also had a significant impact on the effect of vincanine on hypoxia-induced vasorelaxation. Conclusion: The present findings showed that alkaloid vincanine isolated from the leaves of Vinca minor H. significantly abolished the hypoxia-induced vasorelaxation in rat aorta. The obtained results suggest that vincanine may protect the rat aorta against hypoxic injuries in the vasculature.
Keywords
Hypoxia; Rat Aorta; Vasorelaxation; Vincanine
Download this article as:| Copy the following to cite this article: Mirzayeva Y, T, Zaripov A. A, Zhumaev I. Z, Usmanov P. B, Rustamov S. Y, Boboev S. N, Qurbonova S. B, Ibragimov E. B, Musaeva M. K, Sobirov S. B, Adizov S. M. The Protective Effect of Indole Alkaloid Vincanine Against Hypoxia-Induced Vasorelaxation Model of Rat Aorta. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Mirzayeva Y, T, Zaripov A. A, Zhumaev I. Z, Usmanov P. B, Rustamov S. Y, Boboev S. N, Qurbonova S. B, Ibragimov E. B, Musaeva M. K, Sobirov S. B, Adizov S. M. The Protective Effect of Indole Alkaloid Vincanine Against Hypoxia-Induced Vasorelaxation Model of Rat Aorta. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3x8N0l7 |
Introduction
The world’s top cause of death is still cardiovascular diseases (CVD), with hypertension being the main factor contributing to this high death rate.1 Approximately one-third of annual worldwide deaths can be attributed to CVD2. Moreover, elevated blood pressure, or hypertension, is closely associated with at least half or more of cases involving ischemic stroke, hemorrhagic stroke, ischemic heart disease, cardiomyopathy, aortic aneurysms, and peripheral vascular disease3. Unfortunately, this burden is increasing, despite the advancements in therapeutic treatments, particularly for elderly individuals4,5,6.
Accordingly, one of the most pressing issues facing contemporary medicine is the creation of fresh strategies for the effective treatment of arterial hypertension that are based on the most recent developments in molecular pharmacology. In addition, knowledge regarding the pharmacological characteristics of blood vessel-specific targets is especially crucial as they have a direct bearing on the aetiology of arterial hypertension. A specific focus is on clarifying the mechanisms governing the modulation of smooth muscle Ca2+ transporting systems, as their failure results in the emergence of pathogenic processes inside blood vessels. Disturbances in the contractile activity of SMC-which is controlled by the intracellular Ca2+ ion level upheld by the sarcoplasmic reticulum (SR) and plasmalemma-play a pivotal role in this instance(Jackson, 2000). It is well known that abnormalities in the contractile activity of SMCs associated with arterial hypertension are directly linked to their Ca2+ transport system malfunction7. Simultaneously, particular focus is placed on naturally occurring biologically active chemicals derived from plants. These compounds exhibit a broad spectrum of pharmacological effects and specifically interact with different kinds of ion channels.
The study focused on Vinca erecta Regel & Schmalh, a plant primarily found in Central Asia’s mountainous region of Uzbekistan. Through analyzing the roots and aerial parts of Vinca erecta, it was discovered that the plant contains a high concentration of indole alkaloids. These specific alkaloids, known as Vinca erecta, have gained significant recognition in the medical field for their effectiveness in treating cancer, malaria, and cardiac arrhythmia8. Researchers have recently explored the modification of these alkaloids to generate new bioactive compounds. One particularly promising compound is norfluorocurarine (vincanine), which was extracted from Vinca erecta. By synthesizing new quaternary halide derivatives of vincanine, researchers have expanded the scope of organic synthesis possibilities. Additionally, it is important to note that the quaternary base of norfluorocurarine, fluorocurarine, and its natural derivatives exhibit zwitterionic properties of various natures9. Recently we found that vincanine effectively protects rat aorta against hypoxia- induced vasorelaxation. In the present study, we aim to explore the mechanism by which vincanine protects rat aorta against hypoxia-induced vasorelaxation. This should help to gain novel insight into the mechanisms involved in the cardioprotective effect of vincanine10.
Materials and Methods
Chemicals
All chemicals were of analytical grade commercially available. Phenylephrine, L-NAME, methylene blue, indomethacin, glibenclamide, TEA and, BaCl2 were obtained from Sigma Ltd Co., (St. Louis, MO, USA). Vincanine hydrochloride has been isolated as a Vincanine from the roots of Vinca erecta (fam. Apocynaceae) at the Institute of the Chemistry of Plant Substances of Academy of Sciences of Uzbekistan and was kindly provided by Shahobiddin Adizov.
Tissue Preparation
Our institution’s animal use committee authorized all experimental procedures and preoperative care guidelines. After being given sodium pentobarbital anesthesia, adult male Wistar rats weighing 200–250 g had their thorax opened, the thoracic aorta was swiftly removed, and the rats were then placed in Krebs solution, which contained the following concentrations of salt (in mmol/l): 118 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 1.2 mM MgSO4, 2 mM CaCl2, 1.2 mM KH2PO4, and 11 mM glucose. The endothelium was handled extremely carefully during the entire dissection process to prevent accidental injury. After being cleared of fat and connective tissue, the aorta was divided into rings that measured 2-3 mm in length11.
Aortic-ring contraction studies
Two stainless hooks were used to mount the aortic rings; one was fastened to the organ bath’s bottom (Radnoti Glass, NSW, AUS), and the other was attached to a force transducer. Krebs solution was superfused into the organ bath, which was kept at 37 oC and bubbled with a gas mixture consisting of 95% O2 and 5% СО2. For 60 minutes, the Krebs solution was changed at least twice while the aortic rings were equilibrated in the solution with a tension of 1 g. A force transducer (FT-03; Grass Instrument Company, USA) attached to a computer-based data acquisition system (PowerLab, ADInstruments) connected to a chart recorder Endim 621-02 (Germany) was used to record the aortic ring contraction isometrically.
Experimental Protocols
The effect of vincamine on hypoxia-induced vasorelaxation was assessed using aortic rings incubated in a glucose-free Krebs solution with a constant supply of 95% N2 / 5% CO2. After a 60-minute period of hypoxia which is commonly used in these studies, aortic rings were precontracted with 50 mm KCl or 1µM phenylephrine (PE). The reduction in force upon hypoxia was described as a percentage of the contraction force caused by 50 mM KCl or 1µM PE just before aeration with 95% N2 / 5% CO2. This maximum hypoxic vasorelaxation occurred at around 60 minutes.
To examine the involvement of the voltage-gated Ca2+-channels (VGCCs) in the effect of vincanineon hypoxia-induced vasorelaxation its effects in the presence of verapamil (0.1-1 µM), an L-type Ca2+– channel inhibitor, were studied.
The effects of vincanine on hypoxia-induced vasorelaxation were examined in relation to its role in Ca2+ release from the sarcoplasmic reticulum (SR). Specifically, the effects were examined in relation to PE-induced contraction of endothelium-denuded aortic rings in Ca2+-free buffer containing EGTA (1 mM). In the second series of experiments, the effects of vincanine on hypoxia-induced vasorelaxation and its effects on caffeine-induced contraction were investigated in order to investigate the role of Ca2+ release from the SR in these processes.
Tetraethylammonium chloride (TEA), a nonspecific inhibitor of the calcium-activated large conductance K+ channel (BKCa), glibenclamide, a specific inhibitor of the ATP-sensitive K+ channel (KATP), and BaCl2, a specific inhibitor of the inward rectifying K+ channels (KIR), were studied in conjunction with vincanine to assess the contribution of the K+-channels to the effect of the drug on hypoxia-induced vasorelaxation. Since the action of medicines on K+-channels is more effective in endothelium-intact aortic rings, these investigations were conducted on these rings after they had been precontracted with 30 mM KCl to depolarize the membrane and activate K+-channels12.
After removing the endothelium from ring specimens by rubbing the intimal surface with a cotton ball, the absence of ACh-induced relaxation was considered as evidence of successful denudation. This allowed researchers to evaluate the involvement of the endothelium in the effect of vincanine on hypoxia-induced vasorelaxation. The effects of PE-induced contraction of endothelium-intact aortic rings preincubated with L-NAME (nitro-L-arginine methyl ester, an inhibitor of NO synthase), methylene blue (a guanylyl cyclase inhibitor), and indomethacin (a cyclooxygenase inhibitor) were investigated in order to better understand the role of the endothelium in the effect of vincanine on hypoxia-induced vasorelaxation.
Statistics
This article uses the mean ± standard error of the mean (s.e.m.) of n observations to represent all data. To conduct statistical analysis, an unpaired Student’s t-test was employed. The concentration-response curve was used to get the EC50 and IC50 values, or the drug concentrations that cause a 50% contraction or relaxation of the maximal response (EMax). The sigmoidal curve fitting method in Origin 7.0 (Microcal, Northampton, MA, U.S.A.) was used to generate these values. When P < 0.05 was reached, the differences between the experimental and control values were deemed significant.
Results and Discussion
Impact of vincanine on vasorelaxation produced by hypoxia
In control experiments, exposure of endothelium-intact aortic rings to Krebs solution gassed with 95% N2 / 5% CO2 for 60 minutes significantly reduced contractile response to KCl or PE. As shown in Fig. 1, the response of aortic rings to KCl (50 mM) and PE (1µM) was decreased by 55.3±4.1% and 51.2±4.4%, respectively, of the control response in normoxia. These results show that stimulated hypoxia-induced vasorelaxation which was more potent in aortic rings precontracted with PE than KCl indicating that the contractile response to PE is more sensitive to hypoxia than is that to KCl. It was found that the pretreatment of the endothelium-intact aortic rings with vincanine significantly reduced the vasorelaxation induced by hypoxia in aortic rings precontracted both with KCl and PE Fig.1. As demonstrated in Fig. 1, A the vincanine maximally inhibited hypoxia-induced vasorelaxation in the aortic rings precontracted with KCl (50 mM) from 44.7±4.1% to 17.4±3.7% at the concentration of 15 µM. Similarly, on the aortic rings precontracted with PE (1 µM) vincanine maximally inhibited hypoxia-induced vasorelaxation from 48.8±4.4% to 11.5±3.2% at the concentration of 10 µM Fig. 1, B.
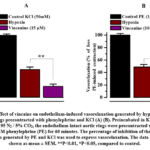 |
Figure 1: Effect of vincaine on endothelium-induced vasorelaxation generated by hypoxia: |
These results show that the effect of vincanine on the vasorelaxation induced by hypoxia is more potent in aortic rings precontracted with PE than with KCl. It was reported that vasorelaxation induced by hypoxia is mainly due to a reduction [Ca2+]i in the smooth muscle cells resulting from the inhibition of Ca2+ influx through VDCCs and calcium release from the SR via IP3Rs13,14.To examine the involvement of the VDCCs in the inhibitory effect of vincanine on hypoxia-induced vasorelaxation its effects in the presence of verapamil, an inhibitor of L-type Ca2+ channels, were studied. In these studies, was found that in aortic rings preincubated with verapamil (0.1 µM) and precontracted with KCl the inhibitory effect of vincanine on the vasorelaxation induced by hypoxia reduced from 27.3±3.7% to 17.4±3.7% Fig. 2. This result suggests that L-type VDCCs may be involved in the inhibitory effect of vincanine on hypoxia-induced vasorelaxation.
 |
Figure 2: Influence of verapamil on the hypoxia-induced vasorelaxation in endothelium in intact rat aortic rings precontracted with KCl was observed. |
Vincanine also inhibited the hypoxia-induced vasorelaxation in the aortic rings precontracted with PE in the Ca2+-free buffer. In these experimental conditions, PE-induced aortic ring contraction is mainly due to the release of Ca2+ from SR through inositol 1,4,5-trisphosphate receptors (IP3Rs)15. Findings displayed in Fig. 3 demonstrate the impact of vincanine on the vasorelaxation induced by hypoxia in these conditions reduced from 24.8±4.1% to 10.8±4.2%. These results indicate that the modulation of calcium release from the SRalso may be associated with the inhibitory effect of vincanine on the vasorelaxation induced by hypoxia.
 |
Figure 3: Effect of vincanine on endothelium-induced vasorelaxation in Ca2+-free Krebs solution with intact rat aortic rings precontracted by phenylephrine. |
This suggestion was confirmed in experiments that examined the effect of vincanine on caffeine-induced contraction in a Ca2+-free buffer which is mediated by Ca2+ released from the SR by the mechanism of Ca2+-induced Ca2+-release (CICR)16,17,18. According to these studies, vincanine decreased the vasorelaxation brought on by hypoxia in intact aortic rings precontracted with caffeine (10 mM) in the Ca2+-free buffer from 12.8±3.2% to 7.8±4.3%. Fig. 4. All of these findings point to the possibility that the modulation of L-type VDCC and the Ca2+-induced Ca2+-release pathway may be the mechanism by which vincanine inhibits hypoxia-induced vasorelaxation.
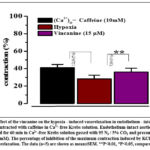 |
Figure 4: Effect of the vincanine on the hypoxia – induced vasorelaxation in endothelium – intact rat aortic rings precontracted with caffeine in Ca2+-free Krebs solution. |
The involvement of the K+ – channels in the inhibitory effect of vincanine on hypoxia-induced vasorelaxation
Numerous studies have demonstrated that the mechanism underlying vasorelaxant response to hypoxia may involve the activation of various types of K+-channels, resulting in vascular hyperpolarization and relaxation. In particular, it was reported that the inward rectifier K+-channels (KIR 2.1) which modulate basal tone in small-diameter coronary and cerebral arteries participated in their hypoxic vasorelaxation19. Also was shown that voltage-gated K+-channels (KV 7) which control the tone of pulmonary and cerebral arteries may also be involved in hypoxic vasorelaxation20. Furthermore, it has been proposed that hypoxia may partially cause vasorelaxation by directly activating ATP-sensitive K+-channels (KATP)21. KATP-channels are essential for vasodilation in response to metabolic demand and for maintaining resting blood flow in a number of vascular beds, most notably the coronary circulation22.
Thus, we investigate in this work if various K+-channel types are involved in the (inhibitory) action of vincamine on hypoxia-induced vasorelaxation. For this purpose, the inhibitory effect of vincamine on hypoxia-induced vasorelaxation was studied in the presence of specific inhibitors of KATP, BKCa and KIR-channels, glibenclamide (GLI), TEA, and BaCl2, respectively. These studies were performed in the aortic rings precontracted with 30 mM KCl since in this condition effect of drugs on K+ channels is more potent23. As shown in Fig. 5 in the intact aortic ring preincubated with glibenclamide (50 μM) and precontracted with KCl (30 mM) the inhibitory effect of vincanine on hypoxia-induced vasorelaxation reduced from 27.2±4.1% to 15.1±4.2%. Similarly, in the presence of TEA (10 mM) the inhibitory effect of vincanine on hypoxia-induced vasorelaxation was reduced from 26.6±3.6% to 9.1±3.6%.By contrast, in the presence ofBaCl2 (100 μM), the effect of vincanine on hypoxia-induced vasorelaxation reduced not significantly (Fig. 5). These results demonstrate that preincubation of aortic rings with glibenclamide significantly abolished the effect of vincanine on hypoxia-induced vasorelaxation suggesting that KATP -channels could be involved in this effect.
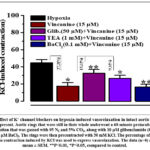 |
Figure 5: Effect of K+ channel blockers on hypoxia-induced vasorelaxation in intact aortic rings when vincanine is present. |
The involvement of the endothelium in the inhibitory effect of vincanine on hypoxia-induced vasorelaxation
It was shown that a critical role in hypoxia-induced vasorelaxation plays changes in the mechanisms of endothelium-dependent contraction and relaxation mediated by several vasoactive factors24,25. Vasoconstrictors like thromboxane (TXA2) and endothelin-1 (ET-1)26, or vasodilators like nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF), are the most significant among them. Thus, the aortic rings with endothelium removed were used to assess the impact of vincanine on hypoxia-induced vasorelaxation in order to examine the participation of endothelium. The vasorelaxation caused by hypoxia in aortic rings precontracted with KCl (50 mM) and PE (1 µM) increased by 5.7±2.4% and 9.1±3.7%, respectively, when the endothelium was removed by rubbing the intimal surface with a cotton ball (Fig. 6). These findings demonstrate that in aortic rings precontracted by PE, the ablation of the endothelium more dramatically increases the vasorelaxation brought on by hypoxia.
In similar experiments in the endothelium-denuded aortic rings precontracted with KCl (50 mM) and PE (1 µM) the effect of vincanine on hypoxia-induced vsorelaxation reduced from 27.2±3.7% to 9.2±3.6% and from 37.3±3.2% to 13.2±3.9%, respectively Fig. 6. These results indicate that removal of endothelium significantly reduced the effect of vincanine on hypoxia-induced vasorelaxation suggesting that this effect of vincanine is endothelium-dependent.
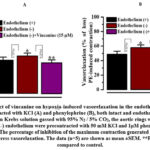 |
Figure 6: Effect of vincanine on hypoxia-induced vasorelaxation in the endothelium – aortic rings precontracted with KCl (A) and phenylephrine (B), both intact and endothelium denuded. |
The effect of vincanine on endothelium-intact aortic rings preincubated with NOS inhibitors (L-NAME), guanylate cyclase-methylene blue, and cyclooxygenase-indomethacin was investigated in order to shed more light on the endothelium’s role in hypoxia-induced vasorelaxation. As shown in Fig. 7,A in the intact aortic ring preincubated with L-NAME (100 µM) and methylene blue (10 µM) and precontracted with KCl (50 mM) the inhibitory effect of vincanine on hypoxia-induced vasorelaxation reduced from 27.3±3.7% to 19.2±3.6% and from 27.3±3.7% to 22.4±4.2%, respectively.At these time in intact aortic ring preincubated with L-NAME (100 µM) and methylene blue (10 µM) and precontracted with PE (1 µM) the inhibitory effect of vincanine on hypoxia-induced vasorelaxation was reduced from 37.3±3.2% to 25.4±4.1% and from 37.3±3.2% to 30.2±3.9% arespectively Fig. 7, B. In contrast preincubation of the intact aortic ring with indomethacine, a cyclooxygenase inhibitor, not significantly inhibited the effect of vincanine on hypoxia-induced vasorelaxation Fig.7. These results demonstrate that L-NAME and methylene blue significantly reduced the effect of vincamine on hypoxia-induced vasorelaxation suggesting that this effect of vincanine is endothelium-dependent and likely is associated with modulation of NO/sGC/cGMP/PKG pathway.
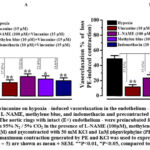 |
Figure 7: Effect of vincanine on hypoxia – induced vasorelaxation in the endothelium – intact aortic rings preincubated with L- NAME, methylene blue, and indomethacin and precontracted with KCl (A) and phenylephrine (B). |
Conclusion
According to the current research, the hypoxia-induced vasorelaxation in the rat aorta was greatly reduced by the alkaloid vincanine that was extracted from Vinca minor H. leaves. Vincanine may shield the rat aorta from hypoxic damage to the vasculature, according to the acquired results. This endothelium-dependent protective effect of vincanine is probably achieved by suppression of L-type VDCC and Ca2+ release from SR in addition to modifying the NO/sGC/cGMP/PKG pathway. New therapeutic approaches for the prevention and treatment of a variety of cardiovascular disorders linked to ischemia/hypoxia may result from further research into the processes behind vincanine’s protective action.
Acknowledgement
This work was supported by the Science and Technology Development Coordination Committee.
Conflict of interest
The authors have declared that no conflict of interest exists.
Funding source
This work was financed by the grant F-OT-2021-154 of the Science and Technology Development Coordination Committee under the Cabinet of Ministers of the Republic of Uzbekistan.
Ethics approval
The experimental protocols complied with the standards and requirements for the humane treatment of animals and the provisions of the Ethical Commission of the IBB at the National University of Uzbekistan. (Protocol No. 7 of 04/07/2022) on the use of laboratory animals. Preparations of isolated aortic segments were obtained using a known method.
References
- V.V. Uzbekov, B.F. Abdullaev, I.Z. Jumayev, Yu.I. Oshchepkova, P.B. Usmanov, and Sh.I. Salikhov. Comparative study of the antiarrhythmic activity of liposomal forms of lappaconitine hydrobromide and its complex with glycyrrhizic acid monoammonium salt in the aconitine arrhythmia model. // Pharmaceutical Chemistry Journal, Vol. 56, No. 10, January, 2023, pp. 1327-1332.
CrossRef - Chen, R., Dharmarajan, K., Kulkarni, VT., Punnanithinont, N., Gupta, A., Bikdeli, B., et al. (2013). Most important outcomes research papers on hypertension. // Circ. Cardiovasc. Qual. Outcomes 6, e26–e35. doi: 10.1161/ circoutcomes.113.000424
CrossRef - Forouzanfar, MH., Liu P, Roth, GA, Biryukov NgM, Marczak,SL., et al. (2017). global burden of hypertension and systolic blood pressure of at least, 165–182.doi:10.1001/jama.2016.19043
CrossRef - Thijssen DH, Carter SE, Green D J. Arterial structure and function in vascular ageing: are you as old as your arteries? // J. Physiol. 594, 2016, 2275–2284.doi:10.1113/JP270597
CrossRef - Harvey, A., Montezano, A. C., and Touyz, R. M. Vascular biology of ageing-Implications in hypertension. // J. Mol. Cell Cardiol. 83, 112–121. doi: 10.1016/j.yjmcc.2015
CrossRef - Gates, PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp. // Physiol. 94, 311–316. doi:10.1113/expphysiol. 2009
CrossRef - Jackson WF. Ion channels and vascular tone. Hypertension. 2000; 35:173-178. doi:10.1161/01.hyp.35.1.173
CrossRef - Azimova SS.; Yunusov MS., Natural Compounds: Alkaloids. Plant Sources, Structure and Properties; Springer, Science & Business Media: New York, NY, USA, 2013
CrossRef - Adizov ShM., Tashkhodzhaev B., Mirzaeva MM., Yuldashev.PKh. Structure of 12-hydroxynorfluorocurarine, its hydrochloride, quaternary salts and a zwitterionic betaine. // Journal of Structural Chemistry. 55, p.1514–1520. 2014
CrossRef - Inoyat Z. Zhumaev, Sadriddin N. Boboev, Pulat B. Usmanov, Shakhnoza B. Qurbonova, Shavkat Yu. Rustamov, Adilbay T. Esimbetov, Gulnaz S. Begdullaeva, Abdisalim A. Zaripov and Shahobiddin M. Adizov. Mechanism of Positive Inotropic Effect of Vincanine on Cardiac Muscle Contraction Activity. // Biomedical & Pharmacology Journal, December 2022. Vol. 15(4), p. 2309-2316.
CrossRef - Mirzaeva YuT, Ismailova DS, Shkinev AV, Usmanov P B, Elmuradov BZh. The effect of 5-(4-aminophenyl)-4-amino-1,2,4-triazole-3(2h)-thion on rat aortic smooth muscle cells. Experimental and Clinical Pharmacology 2023; 86 2 Ñ. 3 – 7. doi: 10.30906/0869-2092-2023-86-2-3-7
CrossRef - Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K channels mediate dilatation of cerebral arterioles during hypoxia. // Circ Res 1994 May;74(5):1005-8. doi: 10.1161/01.res.74.5.1005.
CrossRef - Ghosh D, Syed AU, Prada MP, Nystoriak MA, Santana LF, Nieves-Cintrón M, Navedo MF, Calcium Channels in Vascular Smooth Muscle // Advances in Pharmacology, 2017,78:68 doi:10.1016/bs.apha.2016.08.002
CrossRef - Ali H. Eid, Ahmed F. El-Yazbi, Fouad Zouein, et al., Inositol 1,4,5-Trisphosphate Receptors in Hypertension // Front. Physiol., Vascular Physiology 2018, 9: https: // doi.org/10.3389/fphys.2018
CrossRef - Webb RC. Smooth muscle contraction and relaxation. Advances in Physiology Education. 2003;27;4: 201-206.https://doi.org/10.1152/advan.00025.2003.
CrossRef - Collier ML., Ji G., Wang YX., Kotlikoff MI, Calcium-induced Calcium Release in Smooth Muscle // Journal of General Physiology (JGP) 2000, 115(5):653-62. doi :10.1085/jgp.115.5.653 Source PubMed.
CrossRef - Huihui Kong, Peter PJ, Andrea Koop, Lin Zhang, Henry JD, Wayne Chen SR. Caffeine Induces Ca2+ Release by Reducing the Threshold for Luminal Ca2+ Activation of the Ryanodine Receptor // Biochem J. 2009 414(3): 441–452. doi: 10.1042/bj20080489
CrossRef - Laver DR, Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. // Biophys J. 2007;92: 3541–3555. doi: 10.1529/biophysj.106.099028
CrossRef - Park WS, Son YK, Kim NR, Ko Kang JH. Warda SH, M Earm YE, Jung ID, Park YM. Han J Acute hypoxia induces vasodilation and increases coronary blood flow by activating inward rectifier K+- channel // Pflügers Arch. 2007; 454:1023-1030. doi: 10.1007/s00424-007-0269-4.
CrossRef - Hedegaard ER, Nielsen BD, Kun A, Hughes AD, Kroingaard C, Mogensen S, Matchkov VV, Frobert O, Simonsen U, KV7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries. // Brit. J.Pharmacol. 2014; 171;1: 69-82. doi: 10.1111/bph.12424
CrossRef - Michèle Sweeney, Jason X-J Yuan. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels // Respiratory Research 2000; volume 1, p.40–48
CrossRef - Standen NB, KATP Channels in Vascular Smooth Muscle: Structure, Regulation and Functional Roles // J Clin Basic Cardiol 2003; 6: 4; 7-14.
- Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K channels mediate dilatation of cerebral arterioles during hypoxia. // Circ Res 1994 May;74(5):1005-8. doi: 10.1161/01.res.74.5.1005.
CrossRef - Galley HF, Webster NR., Physiology of the endothelium. Br J Anaesth 2004; 93: 105-13. doi:10.1093/bja/aeh163
CrossRef - Cai H., Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000; 87;10: 840-844. https://doi.org/10.1161/01.RES.87.10.840
CrossRef - Pearce WJ, Williams M, Hamade MW, Chang MM, White CR Chronic hypoxia modulates endothelium-dependent vasorelaxation through multiple independent mechanisms in ovine cranial arteries. // Adv Exp Med Biol. 2006; 578:87-92. doi: 10.1007/0-387-29540-2_14.
CrossRef







