Manuscript accepted on :07-03-2023
Published online on: 04-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Tulika Mishra
Second Review by: Dr. Varsha Galani
Final Approval by: Dr. Ayush Dogra
P. Vishnu Mohan Reddy1, Pulala Raghuveer Yadav2 , A. Lakshmi Devi3
, A. Lakshmi Devi3 , Lepakshi Md. Bhakshu4 and K. Venkata Ratnam1*
, Lepakshi Md. Bhakshu4 and K. Venkata Ratnam1*
1Department of Botany, Rayalaseema University, Kurnool, 518 007, Andhra Pradesh, India.
2Department of Biotechnology, Indian Institute of Technology Hyderabad, Kandi 502 285, Telangana State, India.
3Department of Botany, S.V.B. Government Degree College, 518 134, Andhra Pradesh, India.
4Department of Botany, PVKN Government College (A), Chittoor, 517 002, Andhra Pradesh, India.
Corresponding Author E-mail: drvenkatapkd@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2870
Abstract
The objective of the present investigation is to appraise the qualitative and quantitative phytochemical analysis, DPPH and Hydroxyl radical scavenging activity along with a total antioxidant capacity of water extract obtained from six medicinal plants, i.e., Acalypha alnifolia, Caesalpinia bonduc, Carissa spinarum, Commiphora caudata, Moringa concanensis and Terminalia tomentosa. The phytochemical analysis of the six medicinal plants water extracts revealed total phenolic content (TPC) in the range of 27.77 to 3.35 GAE mg/g dwt. The highest TPC is present in A. alnifolia and the lowest is noticed in T. tomentosa. The total flavonoid content is present in between 1.68 to 1.48 QE mg/g dry weight and a higher amount of flavonoid content was observed in A. alnifolia and lowest present in C. bonduc. Antioxidant activity results of the six medicinal plants showed that the highest total antioxidant capacity was observed in Carissa spinarum and lowest in T. tomentosa. DPPH method was used to know the antioxidant capacity of aqueous extract of the six medicinal plants. Among the tested plants A. alnifolia, Carissa spinarum and Moringa concanensis strongly reduced DPPH purple color by expressing ~80% as maximum inhibition. DPPH was strongly inhibited by T. tomentosa (IC50 value 25 μg/ml). Hydroxyl radical was strongly (~96%) inhibited by T. tomentosa and lowest IC50 value was expressed by A. alnifolia (36.4 μg/ml). The study results indicate that all six medicinal plants are rich sources of natural antioxidant components.
Keywords
Aqueous Extract; Antioxidant Studies; Eastern Ghats
Download this article as:| Copy the following to cite this article: Reddy P. V. M, Yadav P. R, Devi A. L, Bhakshu L. M, Ratnam K. V. Phytochemical Analysis of Selected Medicinal Pslants from Eastern Ghats of Andhra Pradesh. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Reddy P. V. M, Yadav P. R, Devi A. L, Bhakshu L. M, Ratnam K. V. Phytochemical Analysis of Selected Medicinal Pslants from Eastern Ghats of Andhra Pradesh. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3wL9p7y |
Introduction
Free radicals are fragments of molecules with a very short half-life, generated through internal/ external sources, are highly reactive and damage macromolecules like proteins, DNA and lipids of cell membranes of living organisms. The most common reactive oxygen species are hydroxyl (OH), hydrogen peroxide (H2O2), super oxide anion (O2), and peroxyl radicals (ROO) and nitrogen derived free radicals are nitric oxide (NO), peroxyl nitrite anion (ONOO), Nitrogen dioxide (NO2) and Di-nitrogen trioxide (N2O3). The chemical constituents that retard or suppress oxidation or prolong the life of the oxidizable molecules/ inhibit the oxidation process are called antioxidants. Antioxidants from natural/plant sources enhance the endogenous enzymes’ anti-oxidative capability and reduce the risk of many free radical mediated diseases. Traditional medicinal systems use medicinal plants containing various chemical components such as alkaloids, polyphenols, glycosides and terpenoids, which showed pharmacological properties such as antioxidant and antimicrobial activities 1, 2. Of the estimated 2.5 to 5 lakhs plant species; very few have been screened for its biological or pharmacological activities3. In this connection, we selected the following water extract of six medicinal plants to screen phytochemical analysis and antioxidant potential.
Acalypha alnifolia Klein ex Willd., is a rare medicinal plant found in the forests of South India4. Acalypha belongs to the family Euphorbiaceae and is the fourth largest genus with about 450 species5,6. In traditional medicine, the plant has been used to treat dysentery, diabetes and as mosquito repellent 4,7,8.
Caesalpinia bonduc Roxb. (family: Caesalpiniaceae) is locally known as Lata Karanja, is a prickly woody liana distributed throughout the hotter parts of India and Sri Lanka 9 It is a valuable medicinal plant and its different parts, such as bark, leaves, roots and seeds are utilized in traditional system of medicine. The roots are effective as an antiperiodic and antispasmodic properties10, the bark is a good remedy as anthelmintic and febrifuge and the leaves were reported as an emmenagogue11. The seeds are reported to have various pharmacological actions like antipyretic, antiperiodic, asthmatic and febrifuge 12, 13.
Carissa spinarum L. is a spinous evergreen shrub that belongs to the family Apocynaceae, distributed throughout dry localities in India. The plant has been used in the Ayurvedic medical system to treat liver problems, epileptic disease, microbial infections, cytotoxic, viral diseases, inflammation, arthritis and cancer14. The ripened fruits are edible and reported to have cardiac protective properties.
Commiphora caudata (Arn) Engl. belongs to the family Burseraceae, is a thorny shrub to medium sized tree distributed in dry deciduous forests of Peninsular India of Andhra Pradesh, Karnataka and Tamil Nadu 15, 16. It is commonly known as a hill mango because of the mango smell of its stem bark. Traditionally, the bark has been used to treat diabetes, arthritis and obesity 17, 18. Fruits have been used to prepare pickles and gum is used as incense.
Moringa concanensis Nimmo, is a rare medicinal plant of the family Moringaceae. It is found in dry localities in Konkan, Andhra Pradesh. Telugu is called Konda munaga/ Karumunaga, because of its similar morphological characters of drumstick plant M. oleifera19. Different parts of this plant have been reported to treat different human ailments such as leaves for gynic problems, hyper-tention, constipation, jaundice, skin cancers, diabetes and splenomegaly, stem bark for abortion and fruits for rheumatism, nervous disorders, curing liver and spleen diseases, gum for dental problems and flowers for aphrodisiac, leucorrhea and abortion20.
Terminalia tomentosa is a deciduous tree that belongs to the family Combretaceae. Generally, T. tomentosa is called a “crocodile bark tree” because the bark of this has characteristics feature like the skin of crocodile. It is found in Southern parts of the Indian subcontinent and other Southeast Asian countries21. In Ayurveda, the bark treats rheumatism, fever, urinary diseases and diabetes, vertigo, piles, constipation and chronic dysentery22.
Materials and methods
Plant materials
Selected medicinal plant parts i.e. A. alnifolia (aerial parts), C. bonduc (seeds), C. spinarum (fruits), C. caudata (stem bark), M. concanensis (leaves) and T. toemntosa (stem bark) were collected from Nallamala forests of Kurnool District, Andhra Pradesh, India. (Fig.1). The voucher specimens were deposited in Rayalaseema University Herbarium, Kurnool, Andhra Pradesh, India and were identified with the help of regional 23 and local floras 24.
 |
Figure 1: Photographs of the selected medicinal plants |
Preparation of water extracts
The plant parts were cleaned with tap water, sliced and shade dried. The mixer grinder was used to grind the dried material to fine powder. The powdered material was mixed with double distilled and boiled for 30 min and filtered. The filtrate was concentrated on water both. The phytochemical and antioxidant activities of crude water extract (WE) were analyzed.
Preliminary Phytochemical Screening
Qualitative phytochemical screening of the selected six medicinal plant extracts was analyzed using standard methods 25.
Total phenolic content
Folin-Ciocalteu (FCA) reagent method was used to estimate the total phenolic content of the water extracts in the six medicinal plants26. The detailed procedure was followed as described 27.
Total flavonoid content
Aluminum chloride method was used to estimate the total flavonoid content (TFC) of the water extracts of the six medicinal plants28. The detailed procedure was followed as described 27.
Ammonium molybdate dependent antioxidant capacity
Ammonium molybdate dependent total antioxidant capacity of the selected medicinal plant extracts was estimated by29. The detailed procedure was followed as described27.
DPPH and Hydroxyl radical scavenging activity
DPPH and Hydroxyl radical activity of water extracts were measured as mentioned in detail elsewhere 30, 31. The detailed procedure was followed as described 27.
Results
Qualitative Phytochemical Screening
The preliminary phytochemical studies on six selected medicinal plants revealed the presence of ten different secondary metabolites. Among the tested components, alkaloids, flavonoids, terpenoids, phenolic, tannins and glycosides showed strong reactions. At the same time, steroids showed very feeble reactions. Anthraquinones showed negative results in the tested plants.
Quantitative estimation of total phenolic/flavonoid content
The results on total phenolic content in the selected medicinal plants are between 190 to 25 GAE mg/g dwt. The highest TPC is present in TT and the lowest is noticed in CB (Figure 1). The total flavonoid content is between 80 to 12 QE mg/g dwt. The flavonoid content was higher in TT and lowest present in CB (Figure 2). Gallic acid and quercetin were used as standards.
 |
Figure 2: Total Phenolic (A) and Flavonoid content (B) of water extract of selected medicinal plants |
Total antioxidant capacity (TAC)
The results on TAC of the selected medicinal plant extracts indicate that they expressed 240 to 40 ASE mg/g dwt. The highest TAC was observed in TT and the lowest in CB (Figure 3). Ascorbic acid was used as a standard component.
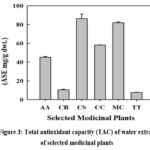 |
Figure 3: Total antioxidant capacity (TAC) of water extract of selected medicinal plants |
DPPH scavenging activity
The results revealed that all the plants expressed concentration dependent DPPH quenching activity. The plants, AA, CS and MC reduced DPPH purple color strongly by expressing ~80% as maximum inhibition. The extracts exhibited IC50 values to inhibit 50% of the DPPH radical between 300 to 25 μg/ml (Table 1).
 |
Figure 4: DPPH quenching activity of water extract of selected medicinal plants |
Hydroxyl radical scavenging activity
All the plants tested exhibited concentration dependent hydroxyl radical scavenging activity (Fig. 5). Among the test plants A. alinifolia showed lowest IC50 value (36.4 ug/ml) and T. tomentosa showed ~96% as maximum inhibition (Table 1).
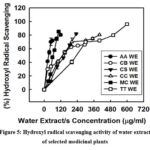 |
Figure 5: Hydroxyl radical scavenging activity of water extract of selected medicinal plants |
Table 1: Qualitative phytochemical analysis of water extracts of selected medicinal plants.
|
Type of the Component |
Selected Medicinal Plants |
|||||
|
AA |
CB |
CS |
CC |
MC |
TT |
|
|
Alkaloids |
+++ |
+ |
++ |
+ |
+ |
+ |
|
Anthraquinones |
|
– |
– |
|
|
|
|
Coumarins |
++ |
+ |
+ |
+ |
++ |
+ |
|
Catecholic compounds |
T |
+ |
+ |
– |
|
++ |
|
Glycosides |
+ |
T |
+ |
– |
+ |
+++ |
|
Flavonoids |
+ |
++ |
++ |
+ |
+++ |
++ |
|
Saponins |
++ |
+ |
+ |
– |
– |
++ |
|
Steroids |
– |
– |
– |
T |
– |
– |
|
Tannins |
|
+ |
+ |
+ |
– |
++ |
|
Terpenoids |
+ |
+ |
– |
++ |
+ |
+ |
|
Phenolic compounds |
++ |
++ |
++ |
++ |
+ |
+++ |
Discussion
Recently researchers have been interested in exploring natural antioxidant principles that are therapeutically potent and with minimum low or no side effects to treat various human ailments and for the food industry. Plants synthesize a wide variety of chemical components such as alkaloids (nitrogenous compounds), terpenes (lipid derivatives) and phenolics (carbohydrate derivatives) with potential pharmacological properties32. Thousands of biologically active phytoconstituents are isolated from higher plants. Of which phenolic compounds are phenolic acids, vitamin E, coumarins, flavonoids, isocoumarins, biflavonols, stilbene, phenols, quinones, betacitie, and chromones etc. reported exhibiting intense antioxidant activity. Scientifically phenolic and flavonoids are reported to have various pharmacological activities such as antioxidant, antiulcer, antispasmodic, cytotoxic anti-inflammatory, antitumor, and antidepressant activities33 to 37.
The Quantity of total phenolic and flavonoids of the water extracts of the selected medicinal plants is estimated using the colorimetric method. The results indicated that T. tomentosa stem bark has more phenolic and flavonoid contents. The Genus Terminalia belongs to the Combretaceae and comprises about 250 species. Of these, only 39 species were studied for its phytochemical composition and 368 phyto-constituents such as terpenoids, flavonoids, tannins, simple phenolics, phenylpropanoids, etc. Quantitative estimation of phenolic and flavonoid content from T. tomentosa stem bark was reported38. They reported higher values of TPC and less TFC than the contents of the present study. Budholiya and Sharma39 estimated total phenolic and flavonoid contents from T. toemntosa leaf extracts.
Vasthi Kennedy and Devarajan Natarajan 40 studied antioxidant and phytochemical analysis of A. alnifolia leaf, especially methanol and aqueous extracts. In the present study, water extract has higher phenolic and flavonoid content than the previous report (Fig. 2A & B). TPC and TFC of C. bonduc seeds showed a lower range than previously reported results 41. This may be due to the variation in the climatic condition in both atmospheric and edaphic conditions.
Phytochemical reports of the genus Commiphora, resulted in the identification of more than 300 chemical constituents 42. Very few and sporadic attempts were made on phenolic and flavonoid contents of C. caudata leaf ethanol extract 43. M. concanensis a rare wild medicinal plant of tropical deciduous forest, received good attention in phytochemical reports of different parts. The results indicated the presence of volatile oils, flavonoids, alkaloids, tannins, and fatty acids 44. Few reports were noticed on quantitative analysis of phenolics and flavonoids from M. concanensis45. A critical review of quantitative estimation of the phytochemical composition of aqueous extracts of C. caudata (stem bark), C. spinarum (fruits), and M. concanensis (leaves) revealed that no previous report was noticed on total phenolic and flavonoid contents, hence, present report on the said species gains importance.
The standard and common method used to estimate the antioxidant capacity of medicinal plant extract is the phosphomolybdenum method. Antioxidant components reduce molybdenum and form a green coloured MO V complex. Revathi et al.,8 reported phosphomolybdenum dependent antioxidant capacity of A. alnifolia leaf extracts i.e., Petroleum ether (38.7 ± 2.2), methanol (139.7 ± 2.8) and hot water extract (82.9 ± 6.4 mg AAE/g). In our current study the total antioxidant capacity of water extract of A. alnifolia aerial parts showed similar values (80.13 ± 1.05 mg AAE/g) to the previously reported values. Based on the review of the antioxidant potential of aqueous extracts on ammonium molybdate dependent antioxidant capacity of C. caudata (stem bark), C. spinarum (fruits), M. concanensis (leaves) and T. tomentosa (stem bark) revealed that no previous report was noticed on ammonium molybdate dependent antioxidant capacity. Hence, the present report on the said species gains importance.
DPPH is a synthetic free radical commonly used to evaluate the antioxidant potential of herbal drugs/pure constituents. The results on DPPH reducing activity revealed that, among the test plant extracts, A. alnifolia strongly (88%) reduced DPPH purple color than other plant extracts. T. tomentosa exhibited the lowest IC50 value (25 μg/ml), indicating its potential as an antioxidant. The intense DPPH quenching activity of T. tomentosa may be responsible for the presence of ellagic acid and gallic acid, which were reported as potential antioxidants/ therapeutics from different traditional medicinal plant sources46. Evangelene and Natarajan47 observed the antioxidant activity of A. alnifolia leaf methanol and water extracts by the DPPH method. Here, we studied the DPPH scavenging activity of water extract of A. alnifolia aerial parts, the results demonstrate that water exhibited IC50 values (42.5µg/ml) higher than leaf extracts. DPPH reducing activity of C. caudata stem bark extracts ethyl acetate, methanol, petroleum ether and chloroform was reported48. Here we reported DPPH quenching capacity of water extract. Vijay Kumar et al., 49 reported DPPH scavenging activity of methanol extract of M. concanensis leaf. The results indicated that ethanol and methanol extracts showed maximum inhibition of 69% and 66 % at 250 µg/ml concentration. In the present study, also reported the DPPH quenching capacity of water extract. It required 60 µg/ ml to reduce 50% DPPH purple color. A critical review of the literature indicated that no previous report was noticed on the DPPH scavenging activity of water extract of C. caudata (stem bark), C. spinarum (fruits), P. toemntosa (leaves) and T. toemntosa (stem bark).
Hydroxyl radicals formed through Fenton’s reaction are the most reactive oxidative molecules capable of damaging biological molecules/ membranes in living cells. In the present experiment, T. tomentosa exhibited maximum hydroxyl radical inhibition as ~96%. A. alnifolia showed the lowest IC50 value (36.4 μg/ml), indicating its potentiality as hydroxyl radical inhibitor. Free radical scavenging property of C. caudata stem bark was reported by DPPH, nitric oxide, SOD methods by in vitro43, 48, 50 and in-vivo studies 51. Antioxidant activity of leaf and fruit oils of C. caudata was reported by Reddy et al.,52. No previous report was noticed on the hydroxyl radical scavenging capacity of C. caudata stem bark extracts by in vitro studies.
The Hydroxyl radical quenching activity of M. concanensis leaf ethanol extract was studied by Balakrishnan & Krishnasamy53. The results showed that, ethanol extract expressed very feeble activity i.e. it expressed a very high IC50 value (400 µg/ ml) and 70% as maximum inhibition at 500 µg/ ml concentration. In the present study, water extract exhibited significant hydroxyl radical scavenging activity by expressing a low IC50 value i.e., 60 µg/ ml. Critical review on hydroxyl radical scavenging activity of A. alnifolia aerial parts54, C. spinarum (fruits), T. tomentosa stem bark55, 56, and P. tomentosa leaves 57 was not reported by earlier workers. Hence, the present work provides additional information on the hydroxyl radical scavenging potential of above mentioned plants.
Conclusion
The current in vitro studies indicated that water extracts of the selected medicinal plants have a high amount of phenolic and flavonoid components, good antioxidant capacity and strongly inhibited Hydroxyl radicals and reduced DPPH, The findings of the study suggest that the selected medicinal plants could be used as a potential source of natural antioxidants.
Acknowledgements
None
Conflict of Interest
Authors declared as no conflict of interest
Funding Sources
Nil
References
- Cowan MM. Plant products as antimicrobial agents. Clinical microbiology reviews. 1999, 12(4): 564-582.
CrossRef - Sulaiman M, Tijani H I, Abubakar B M, Haruna S, Hindatu Y, Mohammed J N, Idris A. An overview of natural plant antioxidants: analysis and evaluation. Advances in Biochem. 2013; 1(4) 64-72.
CrossRef - Thakur Bandana, Anthwal Amit, Rawat Devendra, Rashmi Bipin, Rawat MSM. A Review on Genus Alseodaphne: Phytochemistry and Pharmacology. Mini-Reviews in Organic Chemistry.2012; 9: 433-445.
CrossRef - Kovendan K, Murugan K, Vincent S. Evaluation of larvicidal activity of Acalypha alnifolia Klein ex Willd. (Euphorbiaceae) leaf extract against the malarial vector, Anopheles stephensi, dengue vector, Aedes aegypti and Bancroftian filariasis vector, Culex quinquefasciatus (Diptera: Culicidae). Parasitology res.2012; 110(2): 571-581.
CrossRef - Schmelzer GH, Gurib-Fakim A. Plant Resources of Tropical Africa 11(1). Medicinal plants 1. PROTA Foundation, Wageningen, Netherlands, 2008.
- Canales M, Hernandez T, Rodriguez-Monroy M, Flores-Ortiz C, Jiménez-Estrada M, Hernández L, Hernandez-Moreno M, Trejo N, Hernández A, Ramírez J, Orozco J, Eleno M, Martínez K. Evaluation of the antimicrobial activity of Acalypha monostachya Cav. (Euphorbiales: Euphorbiaceae). Afri. J. Pharma. and Pharmacol. 2011;5.
CrossRef - Kamalakannan S, Gopinath C. Interaction of Metathizium anisopliae and Acalypha alnifolia on the mosquitocidal and IGR activity of Dengue vector, Aedes aegypty (L.) (Culicidae: Diptera: Insecta). Inter. J. Advan. Biotech. Res.2013; 3(1): 24-30.
- Revathi P, Thangaraj P, Manian S. Quantification of phenolic compounds, in vitro antioxidant analysis and screening of chemical compounds using GC-MS in Acalypha alnifolia klein ex willd. – A leafy vegetable. Inter. J. Pharma and Bio Sci.2013; 4: B973-B986 .
- Nadkarni A K. Indian Materia Medica. Popular Prakashan, Bombay, India, 1954; pp. 229 .
- Chopra R N, Nayar S L, Chopra I C. Glossary of Indian medicinal plants (Vol. 1, pp. 138-139). New Delhi: Council of Scientific & Industrial Research. 1956.
- Baquar S R. Medicinal and poisonous plants of Pakistan. Medicinal and poisonous plants of Pakistan, 1989.
- Nadkami K, Nadkarni A. DR KM. Nadkarmi’s Indian Materia Medica: With Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic and Home Remedies, Appendices and Indexes: Popular Prakashan 1976.
- Dhar M L, Dhar M M, Dhawan B N, Mehrotra B N, Ray C. Screening of Indian plants for biological activity: Part I. Indian J Exp Biol.1968; 6(4): 232-47.
- Dhatwalia J, Kumari A, Verma R, Upadhyay N, Guleria I, Lal S, Thakur S, Gudeta K, Kumar V, Chao JC, Sharma S, Kumar A, Manicum AE, Lorenzo JM, Amarowicz R. Phytochemistry, Pharmacology, and Nutraceutical Profile of Carissa Species: An Updated Review. Molecules.2021; 26(22): 7010.
CrossRef - Nadkarani AK. Indian materia medica (Vol. II). Mumbai, India: Popular Prakashan 1982.
- Ambasta SP. The useful plants of India publication and information directorate (p. 138). New Delhi: CSIR 1992.
- Al-Harbi M M, Qureshi S, Raza M, Ahmed M M, Afzal M, Shah A H. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J. Ethnopharmacol. 1997; 55(2): 141-150.
CrossRef - Abdul-Ghani R A, Loutfy N, Hassan A. Myrrh and trematodoses in Egypt: an overview of safety, efficacy and effectiveness profiles. Parasitology Inter.2009; 58(3): 210-214.
CrossRef - Malathi R, Chandrasekar S. Research Article Qualitative Phytochemical Analysis, Antimicrobial Activity and Cytotoxic Effect of Moringa concanensis Nimmo Leaves. Res. J. Med. Plants. 2017; 11: 93-99.
CrossRef - Prajapati N D. Handbook of medicinal plants. Agrobios 2003.
- Singh AP. Controversial herbal drugs of Ayurveda. Scientific publishers, 42-43, 2013.
- Meriga B, Naidu PB, Muniswamy G, Kumar GH, Naik RR, Pothani S. Ethanolic fraction of Terminalia tomentosa attenuates biochemical and physiological derangements in diet induced obese rat model by regulating key lipid metabolizing enzymes and adipokines. Phcog Mag, 2017; 3: 385 – 92.
CrossRef - Gamble J. Flora of Madras Presidency. Adlard & Son, London 1935.
CrossRef - Venkata Raju, R. R., & Pullaiah, T. Flora of Kurnool. Bishen Singh Mahendra Pal Singh. Dehra Dun 1995.
- Harborne, J.B. Phytochemical methods. A guide to modern techniques of plant analysis, 3rd Edition, Chapman & Hall, London, UK 1998.
- Singleton V L, Rossi J A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Ameri.J. Enol. Viticul.1965; 16(3): 144-158.
CrossRef - Venkata Ratnam K, Bhakshu MD L, Venkata Raju R.R. Studies on antimicrobial and antioxidant properties of leaf extracts of Syzygium alternifolium (Wt.) Walp. Inter. J. Pharma. & Pharmaceu. Sci.2015;7 (2): 139-143.
- Lakshman Raju B. Phytochemical screening, quantitative estimation of total phenolics and total flavonids, antimicrobial evaluation of Cyamopsis tetragonoloba. Inter. J. Res. Pharmaceu. & Biomed. Sci.2012; 3(3): 1139-42.
- Umamaheswari M, Chatterjee TK: In-vitro antioxidant activities of the fractions of Ccoccinia grandis L. Leaf Extract. Afri. J. Tradit. Comple. & Alter. Med.2008; 5(1): 61-73.
CrossRef - Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol.2002; 79(3): 379-381.
CrossRef - Halliwell B, Gutteridge J M, Aruoma O I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analy. Biochem, 1987; 165(1): 215-219.
CrossRef - Pereira JA, Oliveira I, Sousa A, Valentao P, Andrade PB, Ferreira ICFR, Ferreres F, Bento A, Seabra R, Estevinho L. Walnut (Juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food and Chem. Toxicol.2007; 45: 2287-2295.
CrossRef - Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003; 11 (9):1995-2000.
CrossRef - Araujo C A C, Leon L L. Biological activities of Curcuma longa L. Memórias do Instituto Oswaldo Cruz, 2001; 96(5): 723-728.
CrossRef - Ammon H P T, Anazodo M L. Curcumin: A potent inhibitor of leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (PMNL). Planta Medica.1992; 58: 226.
CrossRef - Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog. Lipid Res. 2004; 43: 3–35.
CrossRef - Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plan. Res.2011; 5(31): 6697-6703.
CrossRef - Srinivasa Reddy Jitta, Daram P, Gourishetti K, Misra C S, Polu P R, Shah A, Lobo R. Terminalia tomentosa bark ameliorates inflammation and arthritis in carrageenan induced inflammatory model and freund’s adjuvant-induced arthritis model in rats. J. Toxicol. 2019; 2019.
CrossRef - Budholiya P, Sharma H K. Comparative phytochemical screening and estimation of bioactive constituents of leaves of Lagerstroemia parviflora, Gardenia latifolia and Terminalia tomentosa. J. Drug Deliv. Therapeu. 2019; 9 (4): 674-678.
- Evanjelene V K, Natarajan D. In vitro antioxidant and phytochemical analysis of Acalypha alnifolia Klein Ex Willd. J. Pharma. Biol. Sci.2012; 1(5): 43- 47.
CrossRef - Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seed kernels. Food & Chem. Toxicol. 2009; 47(8): 1848-51.
CrossRef - Shen T, Li G H, Wang X N, Lou H X. The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012; 142(2): 319-330.
CrossRef - Sudarshana Deepa V, Kumar P S, Latha S, Selvamani P, Srinivasan S. Antioxidant studies on the ethanolic extract of Commiphora spp. Afri. J. Biotech. 2009; 8(8): 1630- 1636.
- Patil V, Dodiya T. Moringa concanensis an emerging medicinal plant: a phytopharmacological review. Euro. J. Biomed. & Pharmaceu. Sci. 2021; 8 (11): 85-89.
- Santhi K, Sengottuvel R. Qualitative and quantitative phytochemical analysis of Moringa concanensis Nimmo. Int. J. Curr. Microbio. App. Sci, 2016; 5(1): 633-640.
CrossRef - Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res.2005; 579(1-2): 200-13.
CrossRef - Evanjelene VK, Natarajan D. Evaluation of antioxidant, phytochemical and antibacterial properties of Acalypha alnifolia Klein ex Willd. J. Chem. & Pharmaceu. Res.2013; 5(5): 205-212.
CrossRef - Ramesh Kumari, Meyyappan A, Nandi D, Agrawalla B K, Chowdhury A A, Selvamani P. Jaisankar P. Antioxidant and antibacterial activities of bark extracts from Commiphora berryi and Commiphora caudata. Nat. Prod.Res.2011; 25(15): 1454-1462.
CrossRef - Vijayakumar S, Bhuvaneshwari V, Sumathi A. Antioxidant and Anticancer Potential of Methanolic Leaf Extract of Moringa concanensis Nimmo Against Human Breast Cancer Cell Line MCF-7. International Journal of Pharmacognosy and Phytochemical Research, 2017; 9(6); 750-754.
CrossRef - Shaik Azeem Taj, Balakumar BS. Studies on Phytochemical and Antioxidant potential of certain medicinal plants from Udayagiri Hill Range, Andhra Pradesh, India. Sch. Acad. J. Biosci.2014; 2(7): 432-436.
- Anitha K, Sabapathi Mohana Lakshmi, SV. Satyanarayana. Evaluation of Antioxidant Effect of Ethanolic Root Extract of Commiphora caudata in High Fat Diet and Streptozotocin induced Diabetic Rats. Int. J. Pharm. Sci. Rev. Res.2019; 59(1): 84-87.
- Reddy L PA, Narasimha Reddy B, Bhakshu MD L, Venkata Ratnam K, Veeranjaneya Reddy L. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils from Leaves and Fruits of Commiphora caudata Engl. Inter. J. Pharmacog. Phytochem. Res. 2015; 7 (1): 38-44.
- Balakrishnan B B, Krishnasamy K. Evaluation of free radical screening and antioxidant potential of Moringa concanensis nimmo-a medicinal plant used in Indian traditional medication system. Int. J. Pharm. Pharmaceu. Sci, 2018; 10: 91-7.
CrossRef - Seebaluck R, Gurib-Fakim A, Mahomoodally F. Medicinal plants from the genus Acalypha (Euphorbiaceae)–A review of their ethnopharmacology and phytochemistry. J. Ethnopharmacol. 2015; 159: 137-157.
CrossRef - Zhang XR, Kaunda JS, Zhu HT, Wang D, Yang CR, Zhang YJ. The Genus Terminalia (Combretaceae): An Ethnopharmacological, Phytochemical and Pharmacological Review. Nat Prod Bioprospect. 2019; 9(6): 357-392.
CrossRef - Fahmy NM, Al-Sayed E, Singab AN. Genus Terminalia: A phytochemical and Biological Review. Med Aromat. Plants. 2015; 4 (5): 1-21.
- Dianita R, Jantan I.Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus Premna: a review. Pharmaceu. Biol. 2017; 55(1): 1715-1739.
CrossRef







