Sugarjav Enkh-Amar1,2* , Dejidmaa Buyantogtokh2
, Dejidmaa Buyantogtokh2 , Anu Altangerel2
, Anu Altangerel2 , Uuganbayar Baatartsogt2
, Uuganbayar Baatartsogt2 , Irekhbayar Jambal1
, Irekhbayar Jambal1 and Chimedragchaa Chimedtseren2
and Chimedragchaa Chimedtseren2
1School of Arts and Sciences, National University of Mongolia, Ulaanbaatar,14201, Mongolia
2Research Center, Institute of Traditional Medicine and Technology, Ulaanbaatar, 17032, Mongolia.
Corresponding Author E-mail: sugarjave@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2880
Abstract
The objective of the present investigation is to identify the biologically active components of Astragalus galactites (AG) and examine their effect on rats of acute gouty arthritis induced by crystals of the compound monosodium urate (MSU). Analyses of AG chemical constituents and their antioxidant activity were performed using both UV spectrophotometric and HPLC methods. Twenty-five adult Wistar rat males in total were assigned at random to a choice of five groups: AG 160 mg/kg, AG 330 mg/kg, MSU group, control group, and normal group. We reported the contents of Formononetin 1.97 µg/mL, Astragaloside IV 0.56 mg/mL, and total flavonoid 0.22±0.07% in the aerial part of our sample AG. In the DPPH scavenging assay, methanol and ethanol extracts established antioxidant properties with an IC50 concentration of 91.04 µg/mL and 93.13 µg/mL, respectively. In the ABTS scavenging assay, IC50 concentrations of 387.2 µg/mL (methanol extract) and 436.2 µg/mL (ethanol extract) were also shown. This investigation also looked at the histopathological characteristics related to MSU-induced gouty arthritis in order to assess the preventive effects of the AG plant on inflammatory mediator levels. In the AG 330 mg/kg group, the level of PGE2 significantly reduced (p<0.001). Our results showed that the AG 330 mg/kg group was relatively effective in the treatment of gouty arthritis compared to other groups, which appears to be mediated by inhibiting the release of cytokines that pro-inflammation. The main compounds of the AG medicine plant, flavonoids and saponins, are acutely anti-gout due to the resulting decrease in PGE2 levels. We have hypothesized that it is able to treat acute gouty arthritis by reducing levels of UA and PGE2, providing an anti-inflammatory effect. This study contributes to the body of evidence that AG can be utilized in preventing and treating hyperuricemia.
Keywords
Astragalus galactites Pall; Antioxidant Activity; Gouty arthritis; MSU
Download this article as:| Copy the following to cite this article: Amar S. E, Buyantogtokh D, Altangerel A, Baatartsogt U, Jambal I, Chimedtseren C. Investigation of Chemical Compounds and Effect of Astragalus Galactites (Pall.) on MSU Crystal-Induced Acute Gouty Arthritis in Rats. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Amar S. E, Buyantogtokh D, Altangerel A, Baatartsogt U, Jambal I, Chimedtseren C. Investigation of Chemical Compounds and Effect of Astragalus Galactites (Pall.) on MSU Crystal-Induced Acute Gouty Arthritis in Rats. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3HBT7QS |
Introduction
Astragalus is a large genus that is widely distributed in temperate regions throughout the world. Astragalus galactites (Pall.) (AG) is one of the genus Astragalus and belongs to the legume family Fabaceae1. The native range of this species is the northern part of China, Mongolia, and Siberia2. A chronic disease called hyperuricemia is caused by a dysregulation of purine metabolism. In recent years, there has been a significant worldwide rise in the incidence of hyperuricemia, which seems to be related to changes in lifestyle and population aging 3. The total prevalence of adults with hyperuricemia was up to 14.6% in America4. There was a 16.6% prevalence of hyperuricemia in Australia5.In addition to these, a recent report stated that 17.4% of Chinese individuals had hyperuricemia6. Epidemiological studies of gout have not been conducted in Mongolia, but according to health statistics, the number of cases has increased every year: 357 cases in 2014, 456 cases in 2015, 619 cases in 2016, 1071 cases in 2017, and 1343 cases in 20187. In order to address this problem, we must scientifically identify the medicinal plants of our country and find efficient and productive ways to control this illness through researching opportunities to obtain new medicines. The aerial parts of AG are used in traditional Mongolian medicine to treat illnesses related to inflammation or heat. Common biological diseases associated with this ‘heat’, such as myocardial ischemia, are traditionally addressed with treatment through heat reduction and improving cardiac blood supply. Aerial parts of AG are considered to be a rich source of a high concentration of bioactive compounds like flavonoids, alkaloids, polysaccharides, and saponins1. Alkaloids, terpenoids, saponins, and flavonoids—particularly flavones and flavanols—have all been shown to have uric acid-lowering properties. By prohibiting xanthine oxidoreductase and controlling uric acid transporters, these compounds may lower blood uric acid levels8. One of the main active ingredients extracted from Astragalus membranaceus (Fisch) Bge, the saponin astragaloside IV is commonly used in traditional Chinese medicine for the treatment of immune system conditions, such as rheumatoid arthritis9. The total flavonoids of Astragalus (TFA) that have been extracted from Astragalus membranaceus Bunge exhibit strong anti-arthritic properties of arthritis induced by FCA in rats10. The pre-clinical studies for assessing the pharmacology, phytochemical, toxic, and biological properties of any herbal drug are essential before its clinical administration. The chemical analysis and pharmacological study of bioactive plants are of great importance in traditional medicine. Specifically, the flavonoid, and saponin examination, quantitative, found in the following scientific methodology and its comparison with standard compounds is very important for establishing AG’s efficacy. A detailed analysis of the phytochemistry and pharmacology of AG, which is widely used in traditional Mongolian medicine, has not been reported. Therefore, there is a need to determine to reveal the biologically active compounds of ingredients of AG. This study will determine these constituents’ chemical composition and evaluate their effect on gouty arthritis, and the anti-inflammatory effect of AG in traditional medicine establishing the scientific basis.
Materials and Methods
Plant Materials
The plant sample was collected as a raw material in the Tahiltayn mountain area (47º 91’ 44” N, 106º 70’ 63” E), Songinokhairhan district, Mongolia, (May 2021). Professor E. Ganbold, Sc.D., of the National University of Ulaanbaatar, Mongolia, confirmed the taxonomic of the plant species’ origin. The herbs were dried in the air and ground to a fine powder suitable for extraction.
Experimental Animals
A total of twenty-five adult Wistar rat males weighing between 220-250 grams were acquired from the Institute of Traditional Medicine and Technology of Mongolia’s Experimental Animal Center. The temperature and humidity levels were kept under-regulated to 20±1c° and about 50–60%, with a 12-hour light/dark cycle and automated air conditioning system 8–15 times per hour. Rats were fed standard nutrients and allowed to drink ad libitum.
Reagents
Formononetin (≥99.0%), Astragaloside IV (≥98.0%), Monosodium urate (MSU) crystals (CAS 1198-77-2) were supplied by Sigma-Aldrich (USA). The cytokine immunoassay kits, Haematoxylin & Eosin (HE), and Masson’s trichrome were purchased from MLBIO Biotechnology Co. Ltd. (Shanghai, China) and Sigma-Aldrich (Germany), respectively. Analytical grade included all other reagents.
Chemical analysis
Assessment of Total Flavonoid Content
Weigh 1.0 g of the dried and powdered sample accurately into a flask, add 50 mL of 70% ethanol, heat under reflux for half an hour, and then filtrate. The test solution was the supernatant. 1mL of 5% NaNO2, 1mL of 10% Al (NO3)3, and 10 mL of 4% NaOH solution were added to the test solution. Measure the absorbance of the test solution at 500 nm as directed under Ultraviolet-visible Spectrophotometry (UV-2102C, UNICO, China). Rutin equivalent was used to express the amount of total flavonoids present in the plant11.
High-performance liquid chromatography (HPLC) method
The samples were homogenized with 80% methanol at 25-300C, sonicated for half an hour, and centrifuged at 3500 rpm followed by filtration. The Welchrom C18 column (250 mm, 4.6 mm, 5 μm) was used for the reversed-phase HPLC. Acetonitrile (B) and 0.1% phosphoric acid (A) in distilled water were used for gradient elution (60:40%v/v, 95:5%v/v). In addition, there was a 0.5 mL/min flow rate. The wavelengths used for detection were 203 nm and 254 nm.
Antioxidant Activity
DPPH assay for free radical scavenging
The method with minor modifications12,13 was used to determine the radical scavenging activity of AG. In methanol (ethanol), a series of extracts were prepared at four distinct concentrations (0.025-0.2 mg/mL). After mixing the 1.5 mL extract and the 1.5 mL 3×10–4 M DPPH solution in methanol, they were left to sit in the dark at room temperature for half an hour. After that, a spectrophotometer was used to measure each plant extract containing DPPH’s absorbance at 517 nm. As a blank and control, the plant extract solution (1.5 mL) plus methanol (1.5 mL) and the DPPH solution (1.5 mL) plus methanol (1.5 mL) were utilized, respectively. Every measurement was carried out three times. The sample’s antioxidant capacity was shown as IC50.
ABTS free radical scavenging assay
In methanol and ethanol solution, a series of extracts were prepared at four various concentrations (0.025-0.2 mg/mL), respectively. By reacting 2.45 mmol/L potassium persulfate with 7 mmol/L ABTS solution, ABTS radical cations were created. For 16 hours, the mixture was kept at room temperature in the dark. After that 3.0 mL of ABTS solution was combined with 0.3 mL of each sample, at different concentrations, and thoroughly mixed. After 6 minutes, measure the absorbance at 734 nm14.
Effect of AG on MSU Crystals-induced Acute Gouty Arthritis in Rats
Ethics statement
The research was conducted in compliance with the Health Ethics Guidelines that the Ministry of Health in Mongolia (2018) issued. The Institute of Traditional Medicine and Technology of Mongolia (ITMTM) and members of “The Research Ethics Committee” approved the study protocol (№01/13-10-2022).
MSU crystals-induced acute gouty and treatment
In this study, twenty-five adult Wistar rat males weighing between 220-250 grams were used. MSU crystals-induced acute gouty arthritis animal model: MSU injections carried out intra-articularly to rats caused the gouty model15.Rats were given 1.25 mg MSU (in 100 µL phosphate-buffered saline) injected into the left tibiotarsal joint (ankle), and they were given 90 mg/kg of ketamine hydrochloride. The experiment’s injection method was carried out in the same manner as the Jiexi (ST41) intra-articular acupuncture technique, and the injection point corresponds to the Jiexi (ST41) in traditional Chinese, Korean, and Mongolian medicine. Animals were divided into 5 groups: control (n=5), MSU (n=5), Allopurinol (n=5), AG330 mg/kg (n=5), and AG160 mg/kg (n=5), treated groups were orally administered once daily (decoction water extract) for 5 days before MSU crystal injection. The edema was photographed after 24 hours. Ankle tissue and blood samples were taken 24 hours later for further analysis (Figure 1).
Enzyme-linked immunosorbent assay (ELISA)
Afterwards collection the blood was allowed to be kept at room temperature for 15 minutes. Then, the samples were centrifuged for 10 minutes at 3000 rpm in order to distinguish the serum. Using a microplate reader (ChroMate-4300, Awareness Technology Co., USA), the levels of IL-1b, IL-6, IL-10, TNF-a, and PGE2 were estimated by ELISA kits following compliance with the manufacturer’s instructions (Shanghai MLBIO Biotechnology Co. Ltd).
Histopathological examination
Specimens of ankle joints were decalcified in a 10 percent formic acid working solution and stabilized in a 10 percent neutral buffered formalin solution 16–18.
After the completion of the decalcification process, the sample is washed under running water for a full day, dehydrated using progressively stronger alcohols, encased in paraffin wax, sectioned (3-5 μm thick), and stained with Masson’s trichrome and hematoxylin and eosin (HE). Histological images were observed with a light microscope (Nikon Eclipse Ci, Japan)19,20.
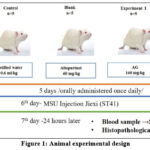 |
Figure 1: Animal experimental design |
The aerial parts of AG are considered to be a rich source of an abundance of bioactive compounds like polysaccharides, flavonoids, alkaloids, and saponins1. We have used aerial parts of AG in all of these experiments.
Statistical analysis
Data was represented as the average ±SD, and the significance levels were established through a one-way analysis of variance (ANOVA) and Tukey’s post hoc test. P-values less than 0.05 were regarded as statistically significant.
Results
Chemical study
Total flavonoid
The flavonoid contents of the extract in terms of rutin equivalent (the standard curve equation: y=0.095x – 0.0008 R2 = 0.9999) were between 4.0 to 40.0. The flavonoid content was determined 0.22± 0.17% in the ethanol extract of the AG plant.
HPLC identification of Formononetin and Astragaloside IV
We have determined Formononetin, Astragaloside IV contained in the AG’s methanol extract based on the studies that determined some isoflavone and triterpenoid saponin contained in other species of Astragalus by the method of HPLC. 20 μL of each of them were injected into the HPLC system according to the chromatographic conditions given in the section on the HPLC method and the chromatograms were recorded. The retention time was 8.56 minutes and 6.53 minutes for formononetin, and Astragaloside IV, respectively. The chromatogram of the AG indicated the presence of Formononetin, and Astragaloside IV with a retention time of 8.37 minutes and 6.37 minutes compared with their standard substances. The chromatograms of Astragaloside IV and Formononetin are displayed in Figures 2 and 3. The regression equation obtained from the calibration curves (Y=8452.83x+177959.6 (R2=0.9964)) and (Y=1809520+971.99 (R2=0.9849)) can be used to determine the formononetin and Astragaloside IV contents in the AG (Figures 4 and 5). From the results of these calculations, it can be seen that the formononetin and Astragaloside IV content in the AG is 0.002±0.004 mg/mL, 0.56 ±0.005 mg/mL.
Method validation
The HPLC method for the Estimation of Formononetin and Astragaloside IV were developed and validated according to ICH Q2 (R1) guideline (Table 1). In the HPLC method, the results of the validation parameters provided above are suitable21.
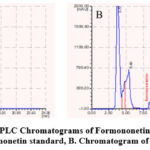 |
Figure 2: HPLC Chromatograms of Formononetin in the AG |
 |
Figure 3: HPLC Chromatograms of Astragaloside IV in the AG |
A. Chromatogram of Astragaloside IV standard, B. Chromatogram of the methanol extract of AG
 |
Figure 4: Calibration curve of Formononetin |
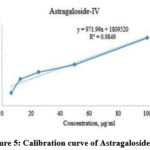 |
Figure 5: Calibration curve of Astragaloside IV |
Table 1: Validation parameters of the proposed HPLC method
|
Parameters |
Formononetin |
Astragaloside IV |
|
Wavelength of detection |
254 nm |
203 nm |
|
Retention time |
8.37 min |
6.37 min |
|
Beer’s law limit (µg/mL) |
6.25-100 |
6.25-100 |
|
Regression formula (y=ax+b) |
Y=8452.83x+177959.6 |
Y=971.99x+1809520 |
|
Slope |
8452.83 |
971.99 |
|
Intercept |
177959.6 |
1809520 |
|
Coefficient of correlation |
0.9964 |
0.9849 |
|
Limit of detection |
2.34 µg/mL |
0.97 µg/mL |
|
Limit of quantification |
7.09 µg/mL |
0.32 µg/mL |
|
Accuracy (% RSD) |
100.3-102.7, |
98.6-101.7, % RSD |
|
Precision (% RSD) |
Inter-day=0.40 |
Inter-day=0.052 |
|
Intra-day=0.78 |
Intra-day=0.083 |
|
|
Mean, mg/mL |
0.002±0.004 |
0.56 ±0.005 |
% RSD-Relative Standard Deviation
Antioxidant activity
The DPPH and ABTS assay were used to evaluate antioxidant activity, and the results were expressed as a percentage of inhibition. Table 2 shows the results of the analysis. The results were contrasted with gallic acid as the standard. For each extract, the concentration that resulted in 50% inhibition (IC50) was found. AG methanol extract had the DPPH and ABTS scavenging effects (IC50 = 91.04 μg/mL, 387.2 μg/mL), AG ethanol extract had the DPPH and ABTS scavenging effects (IC50 = 93.14 μg/mL, 436.3μg/mL), respectively.
Table 2: The results of Antioxidant activity
|
Sample |
DPPH (IC50=µg/ml) |
ABTS (IC50=µg/ml) |
|
Methanol extract |
91.04± 0.23 |
387.2± 0.96 |
|
Ethanol extract |
93.14±0.2 |
436.3±0.1 |
|
Gallic acid standard |
34.8± 0.12 |
67.1 ± 0.2 |
|
% RSD |
0.95 |
1.53 |
Pharmacology study
Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and PGE2 are among the inflammatory mediators created by blood cell infiltration after MSU injection. In the control group, the level of TNFα increased in a gouty model. Allopurinol (Allo) is one of the clinical pharmaceuticals used for the treatment of gouty arthritis. Significant differences (p=0.032) were observed between the AG160 mg/kg and Allo groups or treatment groups. In the current investigation, all treatment groups had a decrease in IL-10 level, while the control group had increased levels (p<0.032). The PGE2 level was significantly reduced in the AG330 mg/kg group (p<0.001) (Figure 6). Through intra-articular injection into the tibiotarsal joint, a model for gout was successfully developed, and the study’s evaluation timeline was established from our preliminary investigation into alterations in the level of uric acid.
Consequently, significant differences (p<0.032) were observed between AG330 mg/kg and AG160 mg/kg and the treatment groups or Allo. As a result of this study, the uric acid levels were increased in control groups and decreased in treatment groups (p<0.001). Therefore, Figure 6A shows that the Uric acid level significantly reduced (p<0.001) in the treatment groups. We examined the edema of the ankle-hind paws of the rats in order to figure out the effects of AG in an animal model of gouty. The images of the hind paws of the rat ankle were taken 24 hours after the MSU injection. The result suggests that AG mitigates MSU crystal-induced paw edema (Figure 7). It can be seen from the edema images of the rear ankle of the rats that the redness and edema in the treatment group indicated the anti-gouty and anti-inflammatory properties of AG. In contrast to the control group, the allopurinol groups showed obvious edema, redness, and an inflammatory process in the ankle joint. In contrast to the allopurinol groups, edema, and redness of the ankle were slightly reduced in the AG160 mg/kg groups, while the AG330 mg/kg groups had a significant anti-inflammatory effect. The results were confirmed by histopathology analysis.
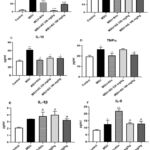 |
Figure 6: The results of pro-inflammatory cytokines in rats (at 24 h). |
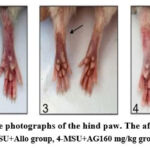 |
Figure 7: Representative photographs of the hind paw. The affected side (arrows). |
Edema of the experimental animal’s paws was used to measure by using a plethysmometer. However, there were no discernible statistically major variances between the groups.
Histopathological results of HE staining
Histopathological observation of ankle joint tissues structures in all experimental groups is illustrated in Figure 8. Ankle joint tissues of the control group showed dense inflammatory cell infiltration and new vascularization. Dominant infiltrated cells were macrophage, plasma and multinucleated giant cells. There was edema and an MSU crystal-like structure in the synovial membrane. The synovial lining cell proliferation was observed. The normal group showed that normal histological structure of ankle joint tissue. Allopurinol treated group showed that there was little inflammatory cell infiltration and new vascularization. Dominant infiltrated cells were macrophages and lymphocytes. There was edema and MSU crystal-like structure in the synovial membrane. The synovial lining cell proliferation was observed. AG160 mg/kg treated group showed that less of inflammatory cell infiltration and new vascularization. Mainly infiltrated cells were macrophages, plasma and lymphocytes. There was edema and MSU crystal-like structure in the synovial membrane. The synovial lining cell proliferation was observed. AG330 mg/kg treated group showed that few of inflammatory cell infiltration and new vascularization. Mainly infiltrated were the plasma cells, macrophage and lymphocytes. The synovial lining cell proliferation and numerous capillaries were observed in the joint soft tissue. Histopathological results of HE staining of experimental MSU-induced gouty arthritis are displayed in Figure 8.
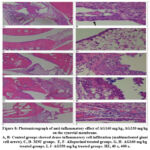 |
Figure 8: Photomicrograph of anti-inflammatory effect of AG160 mg/kg, AG330 mg/kg on the synovial membrane. |
Histopathological results of Masson’s trichrome staining
Histopathological observation of the control group can be seen as a blue color which has infiltrated deep layers of soft tissue and the density of the tissue structure was low. Histopathological examination showed that blue color infiltrated which was significantly weak by allopurinol, AG160 mg/kg, and AG330 mg/kg groups. Inflammatory infiltration was seen in the outer and mid-layer of soft tissue in allopurinol and AG160 mg/kg treated groups. AG330 mg/kg treated group was inflammatory infiltration observed outer layer of the tissue. Histopathological results of Masson’s trichrome staining of experimental MSU-induced gouty arthritis are displayed in Figure 9.
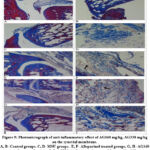 |
Figure 9: Photomicrograph of anti-inflammatory effect of AG160 mg/kg, AG330 mg/kg on the synovial membrane. |
Table 3: Semi-quantitative analysis of AG
|
Groups |
Inflammatory cell infiltration |
Edema |
MSU crystal like structure |
Synovial lining cell proliferation |
New vascularization |
|
Control |
– |
– |
– |
– |
– |
|
MSU |
+++ |
++ |
+ |
+ |
++ |
|
MSU+Allo |
++ |
++ |
+ |
+ |
+++ |
|
MSU+AG160 mg/kg |
++ |
++ |
+ |
++ |
+++ |
|
MSU+AG330 mg/kg |
+ |
++ |
– |
++ |
+++ |
(0- ) none, (1+) mild, (2++) moderate, (3+++) severe
As can be seen from the evaluation table above, the group treated with AG330 mg/kg reduced inflammatory cell infiltration compared to the MSU group, and the MSU crystal-like structure was brought to the same level as the control group. The results of the AG160 mg/kg group were similar to those of the MSU+Allo group.
Discussion
Flavonoids are one of the main compounds in AG, the content was determined at 0.22± 0.17%. These are a class of polyphenolic compounds with anti-inflammatory, and antioxidant properties. For instance, Xin-Yu Liu et al discovered that when Astragalus membranaceus Bunge was isolated, the entire flavonoid had pronounced anti-arthritic effects against rats that had been given FCA-induced arthritis. Signs of FCA-induced rats include increased body weight, reduced paw swelling, inflammatory cell infiltration and synovial hyperplasia, a decreased immune tissues index, and inhibited production of inflammatory mediators10.
We determined that the main biologically active compounds are astragaloside IV and formononetin in Astragalus galactites (Pall.) through qualitative HPLC methods. Formononetin, an active ingredient in Astragalus membranaceus, inhibits the expression of the inflammatory proteins COX-2 and cyclin D1, which can stop the pathogenesis and progression of esophageal cancer 22. In addition, AS-IV was reported to study the molecular mechanisms, including the pathways and target genes, through which AS-IV inhibits the growth of tumors 23. According to a study by Xiaodong Ding et al, the cp genome has 110 complete genes, including 75 protein-coding genes (75 PCGs), 4 ribosomal RNA genes (4 rRNAs), and 30 tRNA genes (30 tRNAs), total plastome length of AG is 126,117 bp. and phylogenetic tree demonstrates that AG and A. laxmannii are the most closely related species 2.
As a result of our research, the amount of Astragaloside IV and formononetin constituents was determined 0.56 mg/mL and 0.002 mg/mL by using HPLC in the AG, respectively. The developed methods show high reliability and validity, as evidenced by the % RSD values in all validation experiments being less than 2%.
We found that the DPPH and ABTS radical scavenging activity in AG may be the cause of the high concentration of phenolic compounds established from our preliminary investigation. The results of the research showed that AG’s methanolic extract has more antioxidant activity than the ethanolic extract. The compounds ononin and formononetin have been discovered to have extensively reported anti-inflammatory, anticancer, and antioxidant capacities according to a study by Ong SKL et al and Machado Dutra J et al24,25. The compounds astragaloside IV and astragaloside activity for liver protection, as reported by Zhang J et al., have been identified as their anti-inflammatory, anti-apoptotic, and antioxidant capacity and also their significance in improving immunity26. Therefore, the AG medicinal plant we studied has antioxidant and anti-inflammatory properties, we are focused on researching and developing of new herbal medicines for gouty arthritis in the future.
The results of this study indicated that AG prevented gouty arthritis caused by MSU, a protective effect that seemed to be mediated by preventing the release of pro-inflammatory cytokines. Because little is known about the mechanisms underlying crystal formation and growth, gout therapy centered on regulating the size and shape of MSU crystals has not yet been developed27. However, it may have a pharmacological effect of monosodium urate (MSU) in regulating the NLPR3 pathways in the pathogenesis and treatment of MSU-induced gouty arthritis in mice and rats 28. Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and PGE2 are among the inflammatory mediators created by blood cell infiltration after MSU injection 29. By giving AG160 mg/kg and AG330 mg/kg doses to rats that had gouty arthritis created experimentally, we were able to assess their effectiveness against the disease. Comparing the AG330 mg/kg group to other groups, our research suggested that it is comparatively useful for treating gouty arthritis. A report by Feng Lin et al discovered that fermented astragalus significantly lowered plasma concentrations of creatinine and urea nitrogen, preventing kidney disease in hyperuricemic mice. They also found the levels of key inflammatory cytokines that further confirm that fermented astragalus reduced inflammation by inhibiting the process. The higher of astragaloside IV, formononetin, total flavonoids, and other active components may be related to the mentioned previously outcomes through fermentation30. Astragaloside IV was identified to reduce the activation of macrophages while on RA, thereby preventing inflammatory-mediated cartilage and bone erosion by Wang B et al9. According to research by Lima Cavendish R et al and Yang Q et al, astragalosides and formononetin have been shown to be antinociceptive substances for inflammatory diseases31,32. Alkaloids, terpenoids, saponins, and flavonoids—particularly flavones and flavanols—have all been shown to have uric acid-lowering properties33.
We found that noted total flavonoid, formononetin, and astragaloside IV have been proven to have anti-gouty effects in inflammatory pain in AG compared with studies of another astragalus. These biologically active compounds (flavonoids, saponins, and phenolic acids) are explained by the identified UPC2-QTOF-MS analysis in the chloroform fraction and phenolic acids are explained by the identified HPLC analysis of AG that we have previously investigated.
On the other hand, the previously mentioned researcher’s investigations confirmed the anti-gout and anti-inflammatory properties of chemical compounds like formononetin, astragaloside IV, and total flavonoids, which is consistent with what we have discovered. The advantage of the study of Mongolian plants is that the chemical compounds of subject plants are determined by using HPLC and evaluated the effect against MSU-induced gouty arthritis for the first time.
Conclusion
In conclusion, the main compounds of the AG medicine plant are flavonoids and saponin it is an acute anti-gouty due to the result revealed that AG significantly decreased PGE2 and UA decreased amount while experimenting. Therefore, this study contributes to preventing and treating hyperuricemia in AG. We first time generated a hyperuricemia rat model using an MSU-induced in Mongolia. In the future, we are researching and developing new herbal medicines for the treatment of gouty arthritis.
Acknowledgment
The authors would like to thank the team from the Research Center at the Institute of Traditional Medicine and Technology for their help during this study.
Conflict of Interest
The authors have declared no conflicts of interest.
Funding Source
There is no organization that funds/supports the study.
References
- Ligaa U., Davaasuren B., Ninjil N. Medicinal plants of Mongolia used in Western and Eastern medicine. Bayanmongol Capital. CoLtd. 453-454. (2005).
- Ding X., Zhang C., Gao Y., Bei Z., Yan X. F. Characterization of the complete chloroplast genome of Astragalus galactites (Fabaceae). Mitochondrial DNA B Resour 6, 3278–3279 (2021). DOI: 10.1080/23802359.2021.1993105. https://pubmed.ncbi.nlm. nih.gov/34712811/
CrossRef - Wang R., Lin F., Ye Ch. Multi-omics analysis reveals therapeutic effects of Bacillus subtilis-fermented Astragalus membranaceus in hyperuricemia via modulation of gut microbiota. Food Chem 399, (2023). DOI: 10.1016/j.foodchem.2022.133993 https://pubmed.ncbi.nlm.nih.gov/36029678/
CrossRef - Singh G., Lingala B., Mithal A. Gout and hyperuricaemia in the USA: Prevalence and trends. Rheumatology (United Kingdom) 58, 2177–2180 (2019). DOI: 10.1093/rheumatology/kez196 https://pubmed.ncbi.nlm.nih.gov/31168609/
CrossRef - Pathmanathan, K., Robinson, P. C., Hill, C. L. & Keen, H. I. The prevalence of gout and hyperuricaemia in Australia: An updated systematic review. Semin Arthritis Rheum 51, 121–128 (2021). DOI: 10.1016/j.semarthrit.2020.12.001 https://pubmed.ncbi.nlm. nih.gov/33360648/
CrossRef - Huang J., Ma F. Zh., Zhang Y., Wan Zh., Li Y., Zhou H. Geographical distribution of hyperuricemia in mainland China: a comprehensive systematic review and meta-analysis. Global Health Research and Policy vol. 5, 52 (2020). DOI: https://doi.org/10.1186/s41256-020-00178-9 https://rdcu.be/dpDez
CrossRef - Order of the Minister of Health of Mongolia, Clinical guidelines for the diagnosis and treatment of gout. Ulaanbaatar. Mongolia.(2021).
- Simin Feng., Sijie W., Fei X., Chung S. Y., Ping S. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci Technol 123(8), 87–102 (2022). DOI:10.1016/j.tifs.2022.03.002 https://www.sciencedirect.com/science /article/abs/pii/S0924224422000875
CrossRef - Wang B., Chen, M. Z. Astragaloside IV possesses antiarthritic effect by preventing interleukin 1β-induced joint inflammation and cartilage damage. Arch Pharm Res 37, 793–802 (2014). DOI: 10.1007/s12272-014-0336-2 https://pubmed.ncbi.nlm.nih.gov/ 24469603/
CrossRef - Liu X. Y., Xu L., Wang Y., Li J. X., Zhang Y., Wang Sh. Protective effects of total flavonoids of Astragalus against adjuvant-induced arthritis in rats by regulating OPG/RANKL/NF-κB pathway. Int Immunopharmacol 44, 105–114 (2017). DOI: 10.1016/j.intimp.2017.01.010 https://pubmed.ncbi.nlm.nih.gov/28092862/
CrossRef - Quettier DC., Gressier B., Vasseur J., Dine T., Brunet C., Luyckx MC. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. Journal of Ethnopharmacology vol. 72, 35-42 (2000). https://doi.org/10.1016/ S0378-8741 (00)00196-3 https://www.sciencedirect.com/science/article/ abs/pii/S0378874100001963
CrossRef - Veličković, D. T., Karabegović I. T., Stojičević S. S., Lazić M. L., Valentina D. et al. Comparison of antioxidant and antimicrobial activities of extracts obtained from Salvia glutinosa L. and Salvia officinalis L. Hem Ind 65, 599–605 (2011). DOI:10.2298/HEMIND110412034V https://www.researchgate.net/ publication/259487458
CrossRef - Kaska A., Mammadov R. Antioxidant properties, proximate content and cytotoxic activity of echinophora tournefortii jaub. & spach. Food Science and Technology (Brazil) 39, 875–880 (2019). DOI: 10.1590/fst.09118 https://www.scielo.br/j/cta/a/zRpGLtdwZbQDh8H dxtC3Rrz / ?lang=en
CrossRef - Lan S., Lin J., Zheng N. Evaluation of the antioxidant activity of coreopsis tinctoria nuff. and optimisation of isolation by response surface methodology. Acta Pharmaceutica 64, 369–378 (2014). DOI: 10.2478/acph-2014-0026 https://pubmed.ncbi.nlm.nih.gov/ 25296682/
CrossRef - Goo B., Lee J., Park C., Yune T., Park Y. Bee venom alleviated edema and pain in monosodium urate crystals-induced gouty arthritis in rat by inhibiting inflammation. Toxins (Basel) 13 (9), 661 (2021). DOI:10.3390/toxins13090661 https://www.mdpi.com/2072-6651/13/9/661
CrossRef - Ajong, A. B. et al. Ionised and total hypocalcaemia in pregnancy: An analysis of prevalence and risk factors in a resource-limited setting, Cameroon. PLoS One 17, (2022). DOI: 10.1371/journal.pone.0268643 https://journals.plos.org/plosone/article?id=10.1371/ journal.pone. 0268643
CrossRef - Ajong, A. B. et al. Adverse maternofoetal outcomes associated with ionised calcaemia, total calcaemia, albuminaemia, and calcium supplementation in pregnancy: Analysis from a resourcelimited setting. PLoS One 17, (2022). DOI: 10.1371/journal.pone.0271525 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0271525
CrossRef - Ajong, A. B. et al. Calcium supplementation in pregnancy: An analysis of potential determinants in an under-resourced setting. PLoS One 18, (2023). DOI: 10.1371/journal.pone.0292303 https://journals.plos.org/plosone/article?id=10.1371/journal.pone. 0292303
CrossRef - MNS 6961:2021. Мал эмнэлэг. Мал, амьтны эрхтнээс эд судлалын шинжилгээ хийх арга. MASM TC 17. 11.220 (2021). ttps://estandard.gov.mn/standard/v/6903
- Suvarna K. S., Layton C., Bancroft J. D., Bancroft’s Theory and Practice of Histological Techniques E-Book. 4th edition (2019). https://www.sciencedirect.com/book/9780702068874/
- INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE. ICH HARMONISED TRIPARTITE GUIDELINE. VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY Q2(R1). (2005). https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
- Li Chen, D. X. L.-R. G. J. J. S. L. Formononetin, an Active Component of Astragalus Membranaceus, Inhibits the Pathogenesis and Progression of Esophageal Cancer Through the COX-2/Cyclin D1 Axis. Clin Lab 69, (2023). DOI: 10.7754/Clin.Lab.2022.220403. https://pubmed.ncbi.nlm.nih.gov/36912303/
CrossRef - Zhou, L. et al. Anticancer effects and mechanisms of astragaloside‑IV (Review). Oncology Reports vol. 49 (2023). DOI: 10.3892/or.2022.8442 https://www.spandidos-publications.com/10.3892/or.2022.8442
CrossRef - Ong, S. K. L. et al. Focus on formononetin: Anticancer potential and molecular targets. Cancers (Basel) 11, (2019). DOI: 10.3390/cancers11050611 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6562434/
CrossRef - Machado Dutra, J., Espitia, P. J. P. & Andrade Batista, R. Formononetin: Biological effects and uses – A review. Food Chemistry vol. 359 (2021). DOI: 10.1016/j.foodchem.2021.129975 https://pubmed.ncbi.nlm.nih.gov/33962193/
CrossRef - Zhang, J., Wu, C., Gao, L., Du, G. & Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. in Advances in Pharmacology vol. 87, 89–112 (2020). DOI: 10.1016/bs.apha.2019.08.002 https://pubmed.ncbi.nlm.nih.gov/32089240/
CrossRef - Matsumoto, M., Wada, Y., Otsu, R., Kobayashi, N. & Okada, M. Controlling nucleation and crystal growth during reactive crystallization of monosodium urate monohydrate from simulated synovial fluid by N2 fine bubble injection. J Cryst Growth 539, (2020). DOI:10.1016/j.jcrysgro.2020.125622 https://www.sciencedirect.com/science/article/abs/pii/S0022024820301457
CrossRef - Ya-Ru Liu, J.-Q. W. J. L. Role of NLRP3 in the pathogenesis and treatment of gout arthritis. Front Immunol 14, (2023). DOI: 10.3389/fimmu.2023.1137822 https://pubmed.ncbi.nlm.nih.gov/37051231/
CrossRef - Aljerf, L. et al. Comparative Study of the Biochemical Response Behavior of Some Highly Toxic Minerals on Selenosis in Rats. Rev. Chim 72, 9–18. DOI:10.37358/RC.21.2.8415 https://www.researchgate.net/publication/351399142
CrossRef - Lin, F. et al. Fermented Astragalus Membranaceus by Bacillus subtilis Ameliorates Hyperuricemia in Mice by Suppressing Inammation via Reducing p38 MAPK and NF-κB Phosphorylation. (2022). DOI:10.21203/rs.3.rs-2075159/v1 https://www.researchgate.net/publication/363785235
CrossRef - Lima Cavendish, R. et al. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J Ethnopharmacol 173, 127–133 (2015). DOI: 10.1016/j.jep.2015.07.022 https://pubmed.ncbi.nlm.nih.gov/26192808/
CrossRef - Q Yang, J. T. L. A. W. Z. B. W. G. W. H. M. Z. C. Antinociceptive effect of astragalosides and its mechanism of action. Acta Pharmacol Sinica 9, 809–812 (2021). https://pubmed.ncbi.nlm.nih.gov/11749861/
- Abebe Tsega Melese 1, D. T. A. 1, L. A. 2, D. F. A.-F. 3, M. L. A. 4. Investigating the phytoavailability of metals in roots of Croton macrostachyus and Phytolacca dodecandra: induced rhizosphere processes. Biometals (2023). DOI: 10.1007/s10534-023-00522 https://pubmed.ncbi.nlm.nih.gov/37474713/
CrossRef








