Prathamesh Kale1 , Ashpak Tamboli2
, Ashpak Tamboli2 , Javeed Manure3
, Javeed Manure3 and, Manjusha Dake1*
and, Manjusha Dake1*
1Protein Biochemistry Lab, Dr. D. Y. Patil Biotechnology and Bioinformatics Institute, Dr. D. Y. Patil Vidyapeeth Tathawade, Pune, India.
2Sahyadri College of Pharmacy, Sangola, Solapur, India.
3Appasaheb Birnale College of Pharmacy, Sangli, India.
Corresponding Author E-maul:manjusha.dake@dpu.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2877
Abstract
Proteases produced by various bacterial and fungal pathogens are associated with pathogenicity and cause septic hazards. Thereby inhibition of such proteases by protease inhibitors from natural sources is effective strategy to combat infectious diseases. Protease inhibitors originating from plant source are abundant in nature and play an important role in defense mechanism against virulent microbes. Many protease inhibitors can be isolated and purified from plant sources and formulated as therapeutic drugs to combat human diseases. In spite of their applications in biomedicines, it is necessary to explore the novel biochemical characteristics of protease inhibitors with improved efficacy. The objective of the current study was extraction, purification and biochemical characterization of protease inhibitor from L. acidissima. The protease inhibitor was isolated from L. acidissima by buffer extraction method and confirmed for anti-protease activity using standard trypsin assay. The inhibitor was found to be active between pH ranging from 5-9 with maximal activity at pH 7. The inhibitor showed thermal stability at a temperature of 50-60°C and retained 70% activity at 50°C for 2 hr. Inhibitor activity was enhanced by thermal stabilizers glycine, urea, Calcium chloride (CaCl2), glycerol, and sucrose at 50°C; metal ions Cu2+ and Al3+ and detergents like Sodium dodecyl sulfate (SDS), Triton-X 100, Tween-80. Protease inhibitor was also stable in the presence of oxidizing and reducing agents Dimethyl sulfoxide (DMSO), Hydrogen peroxide (H2O2), β-mercaptoethanol, and Sodium Thioglycolate. Thus, optimization and characterization studies indicate that the isolated inhibitor is active under natural conditions. Protease inhibitor showed positive results for anticancer, anti-diabetic and anti-inflammatory activities indicating the therapeutic potential of inhibitor molecule. Further identification and separation of bioactive molecules from the buffer extract of L. acidissima was carried out by Flash chromatography followed by Liquid chromatography-high-resolution mass spectrometry (LC-HRMS) analysis that revealed the presence of Diisobutylphthalate as bioactive phytoconstituent. Docking studies performed revealed the anti-diabetic, anti-inflammatory, and anti-cancer potential of Diisobutylphthalate. Thus, in silico studies support the activity of our isolated protease inhibitor to ensure a promising future in the development of therapeutics.
Keywords
Biochemical activities; Bbiochemical characterization; Limonia acidissima; Molecular docking; protease inhibitor
Download this article as:| Copy the following to cite this article: Kale P, Tamboli A, Manure J, Dake M. In silico and Biochemical Approach for Isolated, Purified, and Characterized Protease Inhibitor from Limonia acidissima. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Kale P, Tamboli A, Manure J, Dake M. In silico and Biochemical Approach for Isolated, Purified, and Characterized Protease Inhibitor from Limonia acidissima. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/4a5linE |
Introduction
Proteases have been known to serve as multifunctional units of all living species, including viruses, bacteria, plants, and mammals since the early stages of protein evolution. Proteolytic enzymes aid in different biological processes such as protein degradation, defense mechanism, development, cell growth regulation, tissue arrangement, seed germination, and creation of treachery elements1,2. Such multifunctional activity of proteases depends on their position in the cell. The intracellular proteases are critical for protein metabolism whereas extracellular proteases perform the hydrolytic catalysis of bigger protein molecules, making cellular absorption easier.
Proteases can either break specific peptide bonds (limited proteolysis) or digest a polypeptide chain into amino acid residues thus participating in catabolism (unlimited proteolysis) 3. Even though proteases are crucial and indispensable for the maintenance and survival of living entities, their over-expression or over-concentration can cause serious biological malfunctions. If their actions are not properly managed, proteases can be extremely dangerous. Protease dysfunction that disrupts homeostatic balance can cause several lethal diseases like hepatitis, arthritis, pancreatitis, emphysema, systemic inflammatory response syndrome, etc4.
Since proteases are an interdisciplinary part of a cell, their activity is controlled by various factors including zymogen activation, spatial or temporal control, direct protease destruction, or inhibiting protease activity with protease inhibitors. Protease inhibition is the most promising aspect among the above-mentioned approaches since they imitate the shape of tetrahedral intermediates formed during enzyme catalysis6, obstructing the enzyme’s usual operating pathway and so preventing undesired proteolysis. Enzyme inhibition that is selective and specific has long been employed to reduce superfluous enzyme activity7,8. Inhibitors are chemical modulators that lower the reactivity and the rate of enzyme reaction with the substrate molecule.9 The inhibitor tends to control the proteases enzyme, is referred to as a proteases inhibitor (PIs)10. Researchers have proposed methods for developing several drugs to inhibit the pathogens causing severe infectious diseases.10 These inhibitors compounds have gained a prime importance in anti-carcinogenic therapy in both in-vivo and in-vitro models. Along with the healthcare, the inhibitor hold promising relations in agriculture. The two key field concerns, degradation of approximately 25% of post-harvest goods and the spoilage of food by microbial attack can be easily tacked using these inhibitors. This is because protease inhibitors manifest antibacterial, antiviral, antifungal as well as insecticidal effects 10,11.
Based upon the inhibitory characteristics, the inhibitors could be broad-spectrum or target-specific. They may inhibit the action of one or more protease families simultaneously. The plant-based inhibitors of proteases are divided into seven groups named as- Potato, Squash, Cereal, Kunitz, Bowman-Birk, and Mustard. PI can be differentiated based on their characteristic properties such as disulfide bridge topology, three-dimensional structure, and thermal tolerance. Most of protease inhibitors are low molecular weight molecules with a mass of 20 to 22 kDa. The Kunitz family inhibits aspartyl, cysteine, and serine proteases. Other types of protease inhibitors include serine, cysteine, aspartic, and metalloprotease inhibitors. Trypsin and/or chymotrypsin are the most common inhibitors of serine proteases, with a molecular weight ranging from 3 to 25 kDa12, 13. Plant-based inhibitors are found in various parts of plants including leaves, endosperms, tubers, seeds, and so on14. Leguminous plant-based protease inhibitors are categorized into 12 different types of serine protease inhibitors and have numerous applications such as antifungal, antibacterial, bioinsecticidal, nematocidal, and acaricidal that help to sustain the plant in insects, pests, and microbial attacks15.
For different reasons, protease inhibitors are found in all eukaryotes, from bacteria to archaea, and plants to animals. Inhibitors generated from both marine and terrestrial bacteria have the potential to aid agriculture, medical treatment, and laboratory research. Because PIs produced by marine microbial fauna have a lower molecular weight than those obtained from other sources, they offer a novel source of inhibitors for a variety of biotechnological applications5. Higher animals also serve as a source of protease inhibitors. The stefin, kininogen and cystatin families are the three primary forms of cysteine proteases found in mammals whereas the PIs function to limit the proteolysis.
Based on previous studies, Limmonia acidissima has been found to be a good source of plant-based protease inhibitors. Hence, for the current study, L. acidissima was selected as a source for extraction of the protease inhibitor. L. acidissima is a medium-sized tree; belonging to the Rutaceae family. Some of the common names include wood apple, monkey fruit, elephant fruit, curd fruit, kaitha bel, and kaitha. Because of its domestic and medical benefits, it is regarded as a highly attractive plant16. India, Sri Lanka, Bangladesh, and Pakistan are all prominent habitats for L. acidissima. In the western Himalayas, they are common even at 0-450 m altitude. It is also found in central and southern India’s arid woodlands. In several parts of the world, these trees are used as boundary plants17. L. acidissima is planted in a variety of soils, and it thrives best on sandy and loamy soils.
Wood apples is also reported for antidiabetic, anti-diarrhea, anti-cancer, and hepato protective properties18. Wood apple is also useful in the treatment of diseases such as sore throats, asthma, diarrhea, cardiac malfunction, and hepatitis and are utilized in several Ayurvedic medicine. Saponins, flavonoids, tannins, and glycosides are important phytoconstituents present in the fruit of L. acidissima19. The aim of the current study is to reveal the chemical and functional properties of protease inhibitors from L. acidissima and to prove the therapeutic potential of the inhibitor by in silico docking studies.
Materials and Methods
Isolation and purification of Protease inhibitor from Limonia acidissima
About 60-80 grams of Limonia acidissima leaves were carefully rinsed with distilled water and cut into smaller pieces with a knife on a glass plate. 25 grams of these pieces were homogenized in 75 ml of 0.2M phosphate buffer (pH 7.0) using an electric blender. The homogenate was kept in a rotary shaker at 150 rpm for 30 mins. Later, it was filtered with a muslin cloth to separate the residual material. The resultant filtrate was then centrifuged at ±4°C for 20 mins at 8,000 rpm20. The crude extract obtained as the clear supernatant was further analyzed for inhibitor activity while the residual cell debris was discarded. All the above procedure was carried out at 4°C21. The crude extract was further purified by ammonium sulfate precipitation method as described by England and Seifter (1990). Different concentrations of ammonium sulfate, as 30%, 60%, and 80%, were utilized to determine the best concentration of ammonium sulfate to achieve the maximum activity of protease inhibitor. The crude extract was added with 30 %, 60 %, and 80% of ammonium sulfate respectively, and was kept overnight. The precipitate was collected by centrifugation at 10,000 rpm for 20 min at ±4°C, suspended in the minimal volume of 0.1M phosphate buffer (pH 7.0). The inhibitor was dialyzed extensively against using the same buffer for the removal of salt. The dialyzed extract was utilized to verify the activity of protease inhibitors.
Estimation of the inhibitory potential of inhibitor
The protease inhibitor was tested with a variety of proteases to determine and evaluate its inhibitory potential by following the Kunitz method with slight modifications22. The assay process was carried out with trypsin, chymotrypsin, papain, and pronase enzymes using 0.2M phosphate buffer (pH 7.0). In the control tube, 0.5ml of trypsin, and 0.5ml of buffer solution were taken and kept for incubation at 37oC for 15 minutes. To this reaction mixture, 2.5 ml of casein, as well as 2.5 ml of TCA (trichloroacetic acid), were added simultaneously. This was incubated for another 30mins at 37°C before being filtered through Whatman paper42. Sample tube containing 0.5ml of inhibitor was preincubated with 0.5ml of trypsin solution at 37°C for 15mins.To this reaction mixture, 2.5ml of casein was added and kept for incubation at 37oC for 30min. Following the incubation, 2.5 ml of TCA was added to terminate the reaction. The reaction mixture was filtered through the Whatman paper. A suitable control for the inhibitor was also prepared by performing the addition of TCA before incubation of the reaction mixture to inhibit the action of Trypsin. The reaction mixture was assessed to analyze the hydrolyzed reaction product after the addition of 2.5 ml of Na2CO3 and 0.5 ml of Folin’s reagent with further incubation at 37°C for 30 min. Optical density was measured at 670 nm using appropriate control. One unit of protease inhibitor activity was measured as the decrease of absorbance of TCA soluble casein hydrolysis product by one unit at 280 nm liberated by the action of trypsin under the standard assay conditions22.
Characterization of protease inhibitor
Optimal pH for protease inhibitor activity
The optimal pH for the highest activity of the protease inhibitor was analyzed by progressively elevating the pH level ranging from 2.0 to 10.0. 1 % (w/v) casein solution was prepared in the respective buffers with varying pH values. The primary buffer systems used were glycine-HCl buffer (pH-2.2), acetate buffer (pH 4–5), sodium phosphate buffer (pH 6-8), and glycine-NaOH buffer (pH 9-10.6). Furthermore, the impact of various pH levels on the inhibitor was assessed as per the standard assay procedure.
Stability of protease inhibitor at different pH
The pH stability was accessed by incubating the purified protease inhibitor (0.5ml) along with different buffers (0.5ml) with pH ranging from 4.0 to 10.0 at ±4°C for 24 hrs. Following the incubation period, 1 ml of the resulting reaction mixture was assayed for determining the protease inhibition activity.
The optimal temperature for protease inhibitor activity
A preincubated reaction mixture containing 0.5ml of protease inhibitor and 0.5ml of enzyme solution at 37°C for 30 min was assayed further at different temperatures from 10 to 80°C using the standard assay procedure. The suitable temperature for maximum inhibitor activity was determined based on the results achieved.
Stability of protease inhibitor at different temperatures
The temperature stability of the inhibitor was accessed by incubating 0.5ml of protease inhibitor and 0.5ml of enzyme solution at variable temperatures (50°C, 70°C, and 90°C) for 2 hours and 4 hours respectively. The reaction mixture was assessed for temperature stability as per the standard assay.
Effect of stabilizers on the protease inhibitor
The effect of Glycine (1M), CaCl2 (10mM), glycerol (10%), urea (10mM), and sucrose (1%) on the thermal stability of protease inhibitor was evaluated at 50°C and 70°C, respectively. After incubating the inhibitor along with thermal stabilizers at the required temperature, the residual activity was determined using the standard assay technique by withdrawing the samples at different time intervals of 1hr and 3hrs.
Effect of various metal ions on the protease inhibitor activity
Mg+2 (magnesium sulfate), Fe+3 (ferric chloride), Na+ (sodium chloride), Cu+2 (copper sulfate), Ca+2 (calcium chloride), Ba+2 (barium chloride), and Al+3 (aluminum sulfate) were the metal ions used to analyze their effect on the protease inhibitor activity at 10mM concentrations in phosphate buffer (pH 7.0). 0.5ml inhibitor and 0.5ml buffer were added to 0.5ml of the respective metal ion solutions prepared in buffer and incubated at 37°C for 30 minutes. Later, the reaction mixture was assayed to determine the percentage of inhibition.
Effect of detergents on the protease inhibitor activity
The effect of various ionic and non-ionic surfactants like Triton-X 100, Tween-20, and Tween-80 at a concentration of 1 % (w/v) on the inhibitor activity was determined by incubating 0.5ml inhibitor and 0.5ml buffer along with 0.5ml of respective detergents for 30min at 37°C. The percentage of protease inhibition was determined after the assay procedure.
Effect of oxidizing and reducing agents on protease inhibitor activity
Effect of oxidizing agents like dimethyl sulfoxide and hydrogen peroxide (1M, 2M, 3M, 5M) as well as reducing agents such as sodium thioglycolate and β-mercaptoethanol (0.2M, 0.4M, 0.6M, and 0.8M) on the activity of protease inhibitor was analyzed by incubating 0.5ml of each oxidant and reductant with 0.5ml protease inhibitor along with 0.5ml phosphate buffer at 37°C for 30 min. Further, the percentage of inhibition was calculated.
Effect of chemical modifiers on the protease inhibitor activity
The chemical modification of amino acids in purified inhibitor was carried out using various chemical modifiers like succinic anhydride, diethylpyrocarbonate (DEPC), and phenylmethylsulphonyl fluoride (PMSF) which are well-known for modifying lysine, histidine, and serine residues, respectively. The varied concentration of these modifiers ranging between 5, – 25 mM was incubated with 0.5ml protease inhibitor and 0.5ml phosphate buffer. Following the incubation, the protease inhibitor activity was calculated using a conventional enzyme assay.
Biochemical characterization of semi-purified inhibitor
Anticancer activity
Cells were incubated at a concentration of 1×104 cells/ml in a Dulbecco’s Modified Eagle Medium (DMEM) with high glucose, FBS (Gibco, Invitrogen), Antibiotic-Antimycotic 100X solution (Thermo Fisher Scientific) for 24 hr at 37°C. After that MCF7 cells (100μl culture medium) and (10, 40, 100μl) of extracts containing PIs (1mg/ml) were incubated into 96 wells microplates respectively. Cell line and DMSO (0.2 % in PBS) were incubated in control wells. Three duplicates of each sample were cultured. To calculate the percentage of viable cells both cell culture and controls were taken. Cell cultures were kept in a CO2 incubator for 24 hr at 37°C and 5 percent CO2 (Thermo scientific BB150). The medium was completely removed and 20μl of MTT reagent was added (5mg/min PBS). Cells were incubated for 4 hr at 37°C in a CO2 incubator. Wells were monitored for the formation of formazan crystals with a microscope. After the removal of the medium, 200μl of DMSO was added (kept for 10 min) and incubation was carried out at 37°C. Absorbance was measured at 550 nm23.
Anti-inflammatory activity
The reaction mixture contains 0.4 ml of egg albumin, 5.6 ml PBS (pH 6.4), and 100μl of a sample. Control was prepared using distilled water. The reaction mixtures were incubated at (37°C ± 2) for 15 min and further heated at 70°C for 5 min. The absorbance was taken at 660 nm24. (NSAID) Diclofenac sodium (100 μg/ml) was used as a standard nonsteroidal anti-inflammatory drug. The % inhibition of protein denaturation was determined using the following formula.
% inhibition = C –T/ C
Where, T = absorbance of the test sample, C = absorbance of control.
Anti-bacterial activity
The inoculum of bacterial cultures of Shigella spp., Salmonella typhi, Bacillus subtilis, and Staphylococcus aureus was prepared. 100μl inoculum was spread over the nutrient agar plates evenly. Wells of 6mm were made with a sterile cork borer. 100μl of the sample was added to the wells. The Petri plates were incubated at 37°C for 24hr25. Streptomycin (1mg/ml) was used as a positive control and DMSO as a negative control. The diameters of the zone of inhibition were measured to determine the antibacterial activity (ZI).
Anti-Fungal activity
The inoculum was prepared from the fungal cultures of Candida albicans and Aspergillus niger. 100μl of fungal inoculum was spread over the sterile Sabourad Dextrose agar (Hi media) medium. A sterile cork borer was used to drill 6mm diameter wells. 100μl of the sample was added to the wells. The Petri plates were incubated at 37°C for 24hrs25. Miconazole (1mg/ml) and DMSO were taken as the positive and negative control respectively. Antifungal activity was measured based on the diameters of the zone of inhibitions (ZI).
Antioxidant activity
Antioxidant activity was analyzed using DPPH (1, 1-Diphenyl-2, Picryl-Hydrazyl) (George et al., 1996). 100μl each of plant extract (1mg/ml) and 0.1% methanolic DPPH were added to the microtiter plate and incubation was carried out for 30 mins under the dark conditions26. Change in color from purple to yellow and pale pink indicates strong and weak positive results respectively. The absorbance was taken at 490 nm on an ELISA plate reader. Radical scavenging activity (%) was calculated as follows:
[(Absorbance of control – Absorbance of test sample) / (Absorbance of control)] * 100Amylase inhibition (anti-diabetic activity)
500μl each of the sample (1mg/ml) and α-amylase enzyme {fungal diastase (0.5%) in 0.1M phosphate buffer with pH 6.9} were allowed to react. After 10 min incubation at 25°C, 500μl of 1% starch solution was added. The mixture was incubated again at 25°C for 10 min and added with 1ml of DNSA, kept in a boiling water bath for 10min. The absorbance was taken at 540 nm27. % of enzyme inhibition was calculated using the following formula.
[Abs 540 (control) – Abs 540 (extract)] / Abs 540(control) * 100
Flash Chromatography
For the separation of bioactive molecules, the dialyzed buffer extract from Limonia acidissima wassubjected to flash chromatography (Combifash companion). The solvent system was developed and optimized for flash chromatography by using TLC. The linear gradient program was set to isolate the phytoconstituents as per the optimized solvent28.
HR-LCMS
For the HR-LCMS studies Q-ExactivePlus Biopharma, Thermo Scientific was used. For the data Acquisition Thermo Scientific Xcalibur, Version 4.2.28.14 software was used29. For the data processing Compound Discoverer 3.2 software was used. Hypersil Gold 3micron column (100 x 2.1 MM) was used.
Molecular Docking Study of trypsin receptor with potential bioactive compounds
To explore the binding mode of Diisobutylphthalate with trypsin receptors (1TRN.pdb) as a molecular target, the molecular docking was performed using AutoDock432. The crystal structure of the human trypsin receptor (1TRN.pdb) was taken from the protein database (www.rcsb.org). Using the Discovery Studio Visualizer, the atomic coordinates of Diisobutylphthalate were created30. Grid boxes measuring 60×60×60 were constructed all the way around the active region of trypsin for the docking research. The rigidity of receptor protein and flexibility of drug molecules was maintained constant. The Lamarckian Genetic Algorithm was used to produce the docking conformations (LGA). Further clustering of these 100 output conformations was performed using an all-atom RMSD with a 4 cut-off. The clusters were further examined based on the binding energy including van der Waals, electrostatic interactions. The least energy conformation of Diisobutylphthalate was further analyzed for the bonding and non-bonding interactions using the Discovery Studio visualizer30. The images of docked conformation were generated using the PyMol software31.
Molecular docking of human estrogen receptor with anti-cancer compounds
The interaction of estrogen receptors with Diisobutylphthalate was explored using molecular docking by AutoDock432. The crystal structure of the human estrogen receptor (1ERR.pdb) was taken from the protein database (www.rcsb.org). The anti-cancer activity of Diisobutylphthalate against the breast cancer cell line MCF-7 was studied. Breast cancer has estrogen receptors for utilizing estrogen for cellular growth. Treatment with anti-estrogen inhibitors prohibits the growth of cancer cells 34. Therefore, the estrogen receptor is an important molecular target for the design and development of anti-cancer drugs.
In this study, we used the human estrogen receptor (1ERR.pdb) as a molecular target to explore the binding mode of Diisobutylphthalate. The atomic coordinate of Diisobutylphthalate was generated using the Discovery Studio Visualizer30. For the docking study, grid boxes of 40×40×40 were built around the active site of the estrogen receptor. Here, we keep receptor protein as rigid and drugs as flexible molecules. [Docking studies were performed by using LGA, 100 conformations were clustered, and docking interactions were analyzed based upon the binding energy using Discovery Studio visualizer30. Docking images were produced using PyMol31. The images of docked conformation were generated using PyMolsoftware31.
Molecular docking study of potential anti-diabetic compounds with human insulin receptors
To investigate the binding mode of Diisobutylphthalate, we performed molecular docking with human insulin receptors using AutoDock432. A local docking approach was used to explore the binding mode of Diisobutylphthalate with the human insulin receptor as a molecular target. The crystal structure of the human insulin receptor (1IRK.pdb) was taken from the protein database. The insulin receptor is a transmembrane receptor that is activated by insulin, IGF-I, and II33. Insulin receptor belongs to the large class of receptor tyrosine kinase. It plays a key role in the regulation of glucose homeostasis. Insulin signaling controls access to blood glucose in body cells35. Therefore, the insulin receptor is an important molecular target for the development of anti-diabetic drugs. The atomic coordinate of Diisobutylphthalate was generated using the Discovery Studio Visualizer30. For the docking study, grid boxes of 40×40×40 were built around the active site of the insulin receptor. Receptor proteins and Diisobutylphthalate were kept as rigid as flexible molecules respectively. Docking studies were performed by using LGA, 100 conformations were clustered, and docking interactions were analyzed based on the binding energy using Discovery Studio visualizer30. Docking images were produced using PyMol31. The images of docked conformation were generated using the PyMol software31.
Molecular docking study of potential anti-inflammatory compounds with human COX2 receptors
To investigate the binding mode, Diisobutylphthalate, molecular docking was performed with Cyclooxygenases (COX-2) receptor using AutoDock432. The atomic coordinate of Diisobutylphthalate was generated using the Discovery Studio Visualizer30, and the crystal structure of COX2 (2CDU.pdb) was taken from the protein database (www.rcsb.org). COX-2 is the target of the widely used non-steroidal anti-inflammatory drugs, indicating a role for these enzymes in pain, fever, inflammation, and tumorigenesis36.
A blind docking approach was used, to explore the binding mode of Diisobutylphthalate with the COX-2 receptor. Receptor COX-2 protein was considered as rigid and Diisobutylphthalate as flexible molecule. Docking studies were performed by using LGA, Discovery Studio visualizer30, and PyMol software31. The images of docked conformation were generated using the PyMol software31.
Results
A potential protease inhibitor was extracted from the leaves of Limonia acidissima and purified by salt precipitation using ammonium sulfate. Dialyzed and concentrated inhibitor was used to determine the inhibition activity against different proteases.
Effect of Protease Inhibitors on Different Proteases
 |
Figure 1: Percentage of inhibition against various proteases: The graph shows the percent of inhibition of inhibitor against various proteases. |
The protease inhibitor isolated from Limonia acidissima showed the maximum percentage of inhibition against the pronase enzyme (67 ± 1.3%) which is of bacterial in origin. For trypsin and chymotrypsin enzymes with human origin, the percentage inhibition was (59 ± 1.6%) and (47 ± 1.7%) respectively. The least inhibition was observed against papain enzyme (plant origin) i.e., (23 ± 2.6%).
Effect of varying saturations of ammonium sulfate on the inhibition activity
 |
Figure 2: Partial purification of protease inhibitor using ammonium sulfate: The graph shows % of inhibition at different ammonium sulfate saturation for dialyzed protease inhibitor. |
The protease inhibitor activity observed at 30%, 60%, and 80% saturation with ammonium sulfate was (7.38 ± 1.9%), (15.38 ± 1.8%) and (60.97 ± 1.6%) respectively. Thus highest inhibition activity was observed for the inhibitor purified by using 80% saturation with salt. The purified inhibitor was used further for biochemical characterization.
Optimal pH of protease inhibitor activity
 |
Figure 3: Determination of optimum pH of protease inhibitor: The graph shows the percentage of inhibition versus different pH ranging from 2.2 to 10.6 for isolated protease inhibitor. |
To determine the optimum pH of the isolated inhibitor, a broad range of pH from 2.2 to 10.6 was selected. After performing a protease inhibition assay with this wide pH range, the maximum activity of the inhibitor was noted in the range of pH from 6 to 9. The highest inhibition (61 ± 2.9%) was shown at pH 7. The inhibitor was found to be stable at natural pH range and hence can be utilized further in various aspects under natural conditions to give the maximum activity of inhibitor. The lowest inhibition activity at acidic pH suggests the possible inactivation of the isolated of the inhibitor protein.
Stability of protease inhibitor at different pH
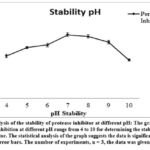 |
Figure 4: Analysis of the stability of protease inhibitor at different pH: The graph shows the percentage of inhibition at different pH range from 4 to 10 for determining the stability of isolated protease inhibitor. |
The stability of the protease inhibitor was noted at wide pH range from 4 to 10. The maximum inhibition (72 ± 2.2%) was observed at neutral pH of 7 followed by pH 8 (70 ± 1.8%) and 9 (64 ± 1.8%). An exponential decrease in inhibition activity was noted at alkaline pH 10 (44 ± 1.1%) and at acidic pH 4. Shifting the pH towards an acidic range from 4 to 6 results in a loss of inhibitor activity due to changes in the ionization status of amino acids and electrostatic interactions.
Optimal temperature of protease inhibitor
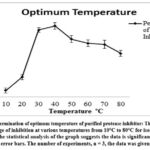 |
Figure 5: Determination of optimum temperature of purified protease inhibitor: The graph shows the percentage of inhibition at various temperatures from 10°C to 80°C for isolated protease inhibitor. |
The potential temperature at which the inhibitor was highly active was determined by standardizing the optimum temperature of the protease inhibitor with consideration of a broad temperature range from 10ºC to 80ºC. The inhibitor displayed a gradual increase in the inhibition activity within the temperature range of 10ºC to 50ºC. A maximum inhibition of (58 ± 2.7%) was noted at 40ºC. The inhibition activity was decreased with a further increase in temperature beyond 50°C due to the thermal denaturation of inhibitor protein at a higher temperature.
Temperature stability for protease inhibitor activity
Further analysis of the temperature stability of protease inhibitor was carried out with respect to time duration.
 |
Figure 6: Analysis of thermal stability of purified protease inhibitor: The graph shows the percentage of inhibition at different temperatures from 30°C to 80°C for determining thermal stability of protease inhibitor. |
The prolonged incubation of the protease inhibitor at various temperatures showed the stability of the inhibitor in the range of 30ºC to 50ºC up to 4 hrs and retained the highest activity. The inhibitor activity at higher temperatures of 70ºC and 80ºC showed a bare minimum increase in the percentage of inhibition. After 2 hr incubation (96 ± 2.7%) at 70ºC and (105 ± 3.5%) at 80ºC and after 4hr incubation (100 ± 4.3%) at 70ºC and (107 ± 3.9%) at 80ºC results indicate false positive inhibition. This is due to the inactivation of protease inhibitor and trypsin enzyme by thermal denaturation.
Effect of stabilizers on the thermal stability of protease inhibitor
The activity of protease inhibitor was accessed in presence of various organic and inorganic stabilizers such as Glycine, Urea, CaCl2, Glycerol, and Sucrose. These thermal stabilizers were used in combination with protease inhibitor to enhance the inhibitor activity.
Effect of thermal stabilizers on protease inhibitor activity at 50ºC:
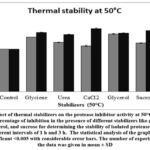 |
Figure 7: Effect of thermal stabilizers on the protease inhibitor activity at 50°C: The graph shows the percentage of inhibition in the presence of different stabilizers like glycine, urea, |
The activity of protease inhibitor in combination with various stabilizers was determined by incubating the combination for a time period of 1hr and 3hr. The observations from the graph indicated that all of the thermal stabilizers including glycine, urea, CaCl2, glycerol, and sucrose enhanced the thermal stability of protease inhibitor at 50°C for 1hr. But for 3hr incubation period, false positive results were observed which may be due to the thermal denaturation of the trypsin protease at 50°C due to prolonged incubation. Among all stabilizers, 10% Glycerol holds promising results with stable inhibition of (36 ± 1.1%) at 50°C coinciding with the temperature stability data for the protease inhibitor activity.
Effect of thermal stabilizers on protease inhibitor activity at 70ºC
The effect of thermal stabilizers on inhibitor activity was also analyzed at a temperature of 70ºC.
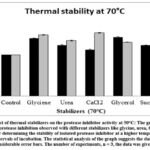 |
Figure 8: Effect of thermal stabilizers on the protease inhibitor activity at 50°C: The graph shows the percentage of protease inhibition observed with different stabilizers like glycine, |
Observations from the graph indicate that all of the thermal stabilizers including glycine, urea, CaCl2, glycerol, and sucrose enhanced the thermal stability of protease inhibitor. The percentage of inhibition was increased after incubation with a thermal stabilizer at 70°C from 1hr to 3hr. Glycerol is an exception where the inhibition was decreased from (43 ± 1.0%) to (31 ± 0.6%) with an increase in incubation period from 1 hr to 3hr.
Effect of various metal ions on protease inhibitor activity
Metal ions contribute to protease inhibitor activity by undergoing reversible oxidation-reduction reactions to stabilize the enzyme-inhibitor transition state complex. Metal ions might serve as a cofactor to promote protease inhibitor activity. Thereby the effect of various metal ions as a possible co-factor on the activity of protease inhibitors was also determined by using various metal salts along with the isolated inhibitor.
 |
Figure 9: Effect of metal ions on the activity of protease inhibitor: The graph shows the percentage of inhibition in the presence of different metal ion salts like CuSO4, AlSO4, MgSO4, FeCl3, |
The inhibitor isolated from Limonia acidissima showed increase in percentage of inhibition in the presence of metal ions. Metal ions such as Cu2+ and Al3+ showed a significant increase in inhibition activity by (27 ± 1.7%) and (22 ± 2.4%) suggesting Cu2+ and Al3+ can be used as a metal ion co-factor to enhance the inhibition activity of the protease inhibitor. Whereas, other metal ions Mg2+, Fe3+, Na+, Ba2+, and Ca2+ showed a slight elevation in inhibition activity as compared to the control of trypsin inhibitor.
Effect detergents on the protease inhibitor activity
Various ionic and non-ionic surfactants tend to lower the activity of protease inhibitor. To analyze their effect, the widely used detergents such as SDS, Triton-X 100, Tween-80, and Tween-20 were added along with the protease inhibitor during the inhibition assay. But as per Fig.10, all the detergents enhanced the activity of protease inhibitor. Graphical data displayed the highest level of protease inhibition with SDS, followed by Triton-X 100, and Tween-80. Elevation in inhibitor activity was (93 ± 1.7%), (22 ± 2.6%) and (13 ± 1.3%) for SDS, Triton-X-100 and Tween-80 respectively. Tween-20 indicated equivalent inhibition as obtained for the control.
 |
Figure 10: Effect of ionic detergents on the activity of purified protease inhibitor: The graph shows the percentage of inhibition observed in presence detergents like SDS, Triton X-100, |
Effect of oxidizing and reducing agents on the percentage of protease inhibition
Oxidizing agents such as DMSO and H2O2 exhibited a significant rise in the percentage of inhibition when applied at 1M to 5 M concentrations. DMSO at 1M concentration increased the percentage of inhibition by (46 ± 1.9%). Slight decrease inhibition was noted at 2M to 5 M DMSO. H2O2 displayed consistent rise in the percentage of protease inhibition from (62 ± 2.2%) to (72 ± 2.7%) when applied at 1M to 5 M concentrations (Table 1).
Reducing agents such as β-mercaptoethanol and Sodium Thioglycolate also indicated a positive impact on the protease inhibitor (Table 1). β-mercaptoethanol increased the inhibition activity by 45± 2.0% at 0.2 M concentration while the inhibitor retained 100% activity at 0.8 M concentration of reducing agent. But with Sodium thioglycolate, a steady rise in the inhibition activity was observed from (9 ± 1.4%) to (97 ± 2.1%) with a subsequent increase in the concentration of reducing agent from 0.2 M to 0.8 M.
Table 1: Studying the effect of oxidizing and reducing agents on the percentage of inhibition.
|
Chemical compound |
Concentration (M) |
Percent of inhibition (%) ± S.D. |
|
|
Oxidizing agents |
Dimethyl sulfoxide (DMSO) |
control |
100 |
|
1 |
146 ± 1.9 |
||
|
2 |
144 ± 2.1 |
||
|
3 |
141 ± 2.2 |
||
|
5 |
137 ± 1.1 |
||
|
Hydrogen peroxide (H2O2) |
Control |
100 |
|
|
1 |
162 ± 2.2 |
||
|
2 |
166 ± 2.6 |
||
|
3 |
169 ± 1.5 |
||
|
5 |
172 ± 2.7 |
||
|
Reducing agents |
β-mercaptoethanol |
Control |
100 |
|
0.2 |
145 ± 2.0 |
||
|
0.4 |
115 ± 1.8 |
||
|
0.6 |
109 ± 2.0 |
||
|
0.8 |
100 ± 1.5 |
||
|
Sodium thioglycolate |
Control |
100 |
|
|
0.2 |
109 ± 1.4 |
||
|
0.4 |
171 ± 1.9 |
||
|
0.6 |
186 ± 1.8 |
||
|
0.8 |
197 ± 2.1 |
Effect of chemical modifier on the protease inhibitor
Chemical modifiers tend to modify the structure of protease inhibitor and can terminate the inhibition activity. Chemical modifiers like succinic anhydride, PMSF, and DEPC were used to assess their effect on the isolated protease inhibitor at variable concentrations ranging from 5 mM to 25 mM.
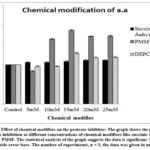 |
Figure 11: Effect of chemical modifiers on the protease inhibitor: The graph shows the percentage of protease inhibition at different concentrations of chemical modifiers like succinic anhydride, |
PMSF showed maximal inhibition activity of (88 ± 1.2%) at 15 mM concentration; whereas DEPC also showed higher inhibition (29 ± 1.7%) at 15 mM concentration. Succinic Anhydride showed slight increase in inhibition activity (11 ± 1.1%) at 20mM concentration. Alteration in the protease inhibitor activity of isolated protease inhibitor in the presence of chemical modifiers PMSF, DEPC, and Succinic anhydride indicate the presence of lysine, serine, or/and histidine as important amino acid residues in the active site of inhibitor.
Anticancer activity against MCF7 cell line
The dialyzed protease inhibitor was analyzed for its anticancer activity. 5-Fluorouracil was used as a standard anticancer drug having 42% (80µl concentration) anticancer activity. Three different concentrations of both standard and inhibitor used were 10µl, 40µl, and 80µl. Protease inhibitor showed (36 ± 2.1%), (41 ± 2.3%) and (49 ± 1.9%) anticancer activity respectively against the MCF7 cell line. 80µl is the optimum concentration at which protease inhibitor had shown the maximum anticancer activity.
Antidiabetic Activity
The dialyzed protease inhibitor was analyzed for its antidiabetic activity. Acarbose (1 mg/ml) was used as a standard to carry outantidiabetic assay which has 78% of activity. The PI exhibited (68 ± 2.4%) inhibition of of α-amylase enzyme thus indicating maximal antidiabetic activity.
Anti-inflammatory activity
The dialyzed protease inhibitor was analyzed for its anti-inflammatory activity. (NSAID) Diclofenac sodium (100 μg/ml) was used as reference drug that has 35% of activity. The PI showed (9 ± 1.3%) anti-inflammatory activity.
Antioxidant activity
This assay for antioxidant activity was performed by using DPPH (2,2-diphenylpicrylhydrazyl) assay. Ascorbic acid was used as the standard antioxidant that denoted 62% activity. The PI has exhibited (13 ± 2.3%) antioxidant activity.
Antibacterial activity
Table 2: The anti-bacterial activity against different bacteria.
|
Zone of Inhibition |
|
|
PI |
|
|
Shigella spp. |
NE |
|
Salmonella typhi |
13mm |
|
Bacillus subtilis |
4mm |
|
Staphylococcus aureus |
NE |
(NE: Not Evaluable)
The dialyzed PI was analyzed against Bacillus subtilis, Staphylococcus aureus, Shigella spp. and Salmonella typhi. Streptomycin (1mg/ml) was used as a standard antibiotic. 13mm of inhibition zone was observed in the case of Salmonella typhi. A 4mm zone of inhibition was observed against Bacillus subtilis. No zone of inhibition was observed against Shigella spp. and Staphylococcus aureus.
Antifungal activity
Table 3: Studying the antifungal activity against fungi.
|
Anti-fungal activity |
|
|
PI |
|
|
Candida albicans |
NE |
|
Aspergillus Niger |
NE |
(NE: Not Evaluable)
The PI was analyzed to assess the anti-fungal activity against Candida albicans and Aspergillus niger. Miconazole (1mg/ml) was used as standard classical antifungal. The PI did not show any zone of inhibition indicating absence of antimicrobial activity against both fungi.
Flash Chromatography
In the present study the buffer extract from Limonia acidissima was subjected to flash chromatography (Combifash companion) for the separation of bioactive molecules. The solvent system was optimized and developed for flash chromatography by using TLC. The linear gradient program was set to isolate the phytoconstituents as per the optimized solvent. The buffer extract was subjected flash chromatography for the separation of phytoconstituents. Observed 4 peaks in the flash chromatogram indicated separation 4 phytochemicals. Fractions 1–5 from peak 1, Fraction 6-11 representing peak 2, Fractions 12–16 contributing to peak 3, Fractions 17-21 representing peak 4, were retrieved, combined, and concentrated under a vacuum
Selection of Component for HR_LCMS Analysis
The percentage of protease inhibition of all four components isolated by flash chromatography was calculated. The inhibition percentage was recorded as (23 ± 2.1%), (46 ± 1.9%), (33 ± 2.2%), and (41 ± 3.1%) for all 4 components respectively. These four components were further analyzed by their λx value. Based on the percentage of inhibition and λx value only component 3 was screened for the HR-LCMS study.
HR-LCMS
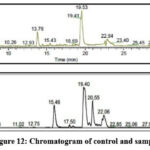 |
Figure 12: Chromatogram of control and sample. |
Based on the retention time, mass, and molecular formula, the high resolution-liquid chromatography-mass spectrometry analysis (HR)-LCMS of the buffer extract of Limonia acidissima was carried out. The results showed the presence of 2 compounds. Among them, the important phytoconstituent confirmed by (HR)-LCMS Analysis is Diisobutylphthalate. This data suggests the possible role of isolated protease inhibitor in therapeutics. Thereby we have performed in silico docking studies to reveal the biological role of isolated protease inhibitor.
Molecular Docking Study of trypsin receptor with potential bioactive compound
For the protease inhibition assay, we used the trypsin enzyme. Diisobutylpthalate showed substantial binding affinity with the trypsin receptor. The least binding energy conformation was found at -6.05 kcal/mol as shown in Figure 12 and Table 2. Furthermore, we analyzed this least binding energy docked complex to explore the nonbonding interactions, as shown in Table 2.
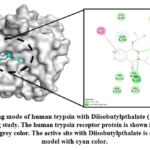 |
Figure 13: Binding mode of human trypsin with Diisobutylpthalate (1TRN.pdb) using molecular docking study. |
The analysis of the trypsin receptor and Diisobutylpthalate docked complex (Figure 13) shows that the Diisobutylpthalate is stabilized by the conventional hydrogen bonding interactions with Asp194 (2.43 Å), Ser195 (2.32 and 2.53 Å) as shown Table 1. Also, His57 forms π-π- T-shaped interactions with Diisobutylpthalate.
Table 4: Interaction of Diisobutylpthalate with human trypsin after molecular docking
|
Protein |
Binding energy |
Atoms involved |
Type of interaction |
Distance |
Fig |
|
1TRN-Diisobutylpthalate, |
-6.05 |
ASP194:HN – UNK0:O12 SER195:HN – UNK0:O12 SER195:HG – UNK0:O11 HIS57 – UNK0 |
Conventional H Bond Conventional H Bond Conventional H Bond Pi-Pi T-shaped |
2.43953 2.32678 2.53655 5.41006 |
13 |
The analysis of docking results revealed that the human trypsin receptor interacts with Diisobutylpthalate by showing conventional and non-conventional types of interactions as shown in Table 4.
Molecular docking of human estrogen receptor with anti-cancer compounds
We have studied the anti-cancer activity using breast cancer cell line MCF-7. The estrogen hormone can be used in breast cancer to fuel the growth since it has estrogen receptors. The human estrogen receptor (1ERR.pdb) was used as a molecular target to explore the binding mode and atomic coordinate of Diisobutylpthalate obtained using the Discovery Studio Visualizer. The Diisobutylpthalate showed significant binding affinity with the estrogen receptor, the least binding energy conformation was found at -6.87 kcal/mol as shown in Figure 14 and Table 5. Furthermore, we analyzed these least binding energy conformations to explore the hydrogen bonding and nonbonding interactions as shown in Figure 14.
 |
Figure 14: Binding mode of Diisobutylpthalate with the estrogen receptor (1IRK.pdb). |
Table 5: Interaction of anti-cancer compounds with estrogen receptor after molecular docking
|
Protein |
Binding energy |
Atoms involved |
Type of interaction |
Distance |
Fig |
|
1ERR- Di- isobutlpthalate |
-6.87 |
HIS524:HD1 – :UNK0:O9 GLY521:HA1 – :UNK0:O12 GLY521:HA2 – :UNK0:O12 :UNK0:C17 – A:LEU387 :UNK0:C17 – A:MET388 :UNK0:C17 – A:LEU391 :UNK0:C18 – A:LEU384 :UNK0:C18 – A:LEU387 PHE404 – :UNK0:C17 :UNK0 – A:MET421 :UNK0 – A:ILE424 :UNK0 – A:LEU428 |
Conventional H Bond Carbon H Bond Carbon H Bond Alkyl Alkyl Alkyl Alkyl Alkyl Pi-Alkyl Pi-Alkyl Pi-Alkyl Pi-Alkyl |
2.15522 2.92864 2.63845 5.26455 4.15589 3.65268 5.34296 4.52726 5.49066 3.92056 4.85471 4.81583 |
14 |
The docking results suggest that the estrogen receptor interacts with Diisobutylpthalate by forming hydrogen bonding and non-bonding interactions such as π-alkyl, π-sigma, and π-sulfur type of interactions and hence can act as potential anti-cancer inhibitor targeting the estrogen receptor.
Docking study of potential anti-diabetic compounds with human insulin receptors
To investigate the binding mode of Diisobutylpthalate, we performed molecular docking with human insulin receptors, and the crystal structure of it was taken from the protein database (1IRK.pdb). Diisobutylpthalate showed significant binding affinity with the insulin receptor, the least binding energy conformation was found at -6.17kcal/mol as shown in Figure 15. Furthermore, to explore the bonding and non-bonding interactions of Diisobutylpthalate with the insulin receptor, we analyzed the aforementioned least binding energy docked complex as shown in Figure 15.
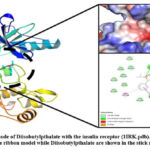 |
Figure 15: Binding mode of Diisobutylpthalate with the insulin receptor (1IRK.pdb). |
The analysis of docked complex of insulin receptor and Diisobutylpthalate showed that Diisobutylpthalate is stabilized by hydrogen bonding interactions involving Phe1151 (1.88 Å), Gly1152 (2.70 Å) and Let1153 (2.94 Å) whereas Phe1152 makes π-anion; Met1153 makes π-sigma and π-alkyl, and Phe1151 and Arg1136 make the π-alkyl type of interactions with Diisobutylpthalate. Insulin receptor interacts with Diisobutylpthalate, by hydrogen bonding and non-bonded interactions such as π-alkyl, π-sigma, and π-sulfur type of interactions
Table 6: Interaction of anti-diabetic compounds with insulin receptor after molecular docking
|
Protein |
Binding energy |
Atoms involved |
Type of interaction |
Distance |
Fig |
|
1ERR- Di-isobutlpthalate |
-6.17 |
PHE1151:HN – :UNK0:O9 GLY1152:HN – :UNK0:O12 MET1153:HN – :UNK0:O12 :UNK0:O9 – A:PHE1151 MET1153:HB2 – :UNK0 MET1153 – :UNK0:C17 MET1153 – :UNK0:C18 PHE1151 – :UNK0:C18 :UNK0 – A:ARG1136 |
Conventional H Bond Conventional H Bond Conventional H Bond Pi-Anion Pi-Sigma Alkyl Alkyl Pi-Alkyl Pi-Alkyl |
1.88119 2.7005 2.94828 4.08331 2.77985 3.90255 4.44008 4.49406 4.68326 |
15 |
Docking study of potential anti-inflammatory compounds with human COX2 receptors
To investigate the binding mode of Diisobutylpthalate, we performed molecular docking with Cyclooxygenases (COX-2) receptor. The clusters were further analyzed based on binding energy, van der Waals, electrostatic energy, etc. The least energy conformation of Diisobutylpthalate was further analyzed for the bonding and non-bonding interactions. The Diisobutylpthalate showed significant binding affinity with the COX2 receptor, the least binding energy conformation was found at -7.96 kcal/mol as shown in Figure 16 and Table 7.
 |
Figure 16: Binding mode of Cox-2 receptor with anti-inflammatory drug Diisobutylpthalate. |
Table 7: Molecular docking of anti-inflammatory compounds after molecular docking
|
Protein |
Binding energy |
Atoms involved |
Type of interaction |
Distance |
Fig |
|
1ERR- Di- isobutlpthalate |
-7.96 |
HIS207:HE1 -UNK0:O10 HIS386:HA – UNK0:O11 HIS388:HA – UNK0:O12 UNK0:H5 – TYR385:O TRP387:HB1 – :UNK0 TRP387:C,O;HIS388:N – :UNK0 HIS207 – UNK0:C18 PHE210 – UNK0:C17 TYR385 – UNK0:C17 HIS386 – UNK0:C18 :UNK0 – ALA202 :UNK0 – LEU390 |
Carbon H Bond Carbon H Bond Carbon H Bond Carbon H Bond Pi-Sigma Amide-Pi Stacked Pi-Alkyl Pi-Alkyl Pi-Alkyl Pi-Alkyl Pi-Alkyl Pi-Alkyl |
1.99561 2.11575 2.69484 2.38031 2.5437 4.66843 4.53501 3.96195 4.63653 4.25299 4.2174 5.39791 |
16 |
The analysis of molecular docking results conclude that the COX-2 receptor interacts with the anti-inflammatory compounds through nonbonding interactions (Figure 16), and showed significant binding affinity. This revealed that these inhibitors prefer to bind at the same binding site with significant binding affinity.
Discussion
Proteolytic enzymes are an integral part of cellular metabolism with a diverse role in biochemical, physiological, and regulatory aspects of cells. Proteases have been implicated in various diseases and pathogenic processes. Hence, effective medications for the treatment of diseases can be created using protease inhibitors that prevent the adverse effects of proteases. Plants represent a potential source of protease inhibitors. In this context in the current study, the Limonia acidissima plant commonly known as the wood apple was screened for the activity of protease inhibitor.
Limonia acidissima commonly known as wood apple and elephant apple with family Rutaceae commonly found in the dry plains of India. The plant has versatile medicinal properties necessitating for verifying the efficacy of the plant through biological screening based on a scientific approach. Thus, the plant has developed a mechanism to resist harsh environmental conditions and to resist plant pathogens. Wood apple is enriched with phytochemicals like alkaloids, flavonoids, phenols, terpenoids, tannins, glycosides, etc.37 Leaves containing stigmasterol, psoralen, bergapten, orientin, and essential oils also represent a promising source of pharmacological activity38.
The leaves from L. acidissima showed protease inhibitory activity for Trypsin, chymotrypsin, Pronase, and Papain. The proteinaceous nature of the PI was confirmed by partial purification using ammonium sulfate precipitation. The phosphate buffer with pH 7.0 was used as a standard buffer for the extraction of inhibitor proteins from leaves of L. acidissima. The isolated inhibitor has an optimal pH of 7.0 with an optimum temperature of 37°C; thus an inhibitor is stable component under normal physiological conditions. The physical and chemical characterization indicate that PI is stable over a wide range of temperatures from 30-50°C for up to 4hr and at pH between 6 to 9. Most of the protease inhibitors are active at a wide range of pH from 2 to 1039. pH and temperature stability indicate presence of aromatic amino acid residues that are involved in energy transfer and disulfide bonds contributing to thermal stability of proteinaceous inhibitor.
Most of the plant protease inhibitors are active at temperatures up to 50°C. The stability of pI from wood apple at higher temperatures indicates a rigid and compact structure of the protein structure of the inhibitor stabilized by disulfide linkages similar to protease inhibitor from pea seeds40. Protease inhibitors enriched with cysteine residues exhibit the significant formation of disulfide bridges and thus retain stability under higher pH, temperature, and resistance to Proteolysis41.
The inhibitory protein isolated from L. acidissima was partially purified by ammonium sulfate precipitation whereas the precipitation by 80% saturation by ammonium sulfate exhibited maximal inhibitory activity as compared to other fractions. The efficiency of the isolated protease inhibitor was analysed using trypsin as the standard protease. The physical parameters like pH and temperature as well as the stability in presence of surfactants, detergents, oxidizing, and reducing agents of isolated inhibitor were accessed in this study, and in silico approach for modulating the compatibility of the isolated inhibitor to various compounds supports our analysis.
Cu2+ and Al3+ were found to be co-factors that enhance the inhibitory property of isolated protease inhibitor when their activity was examined in the presence of such chemical modulators. The inhibitor regained stability in the presence of other divalent metal ions Mg2+, Fe3+, Na+, Ba2+, and Ca2+. Metal ions are important for maintaining the structural integrity of the inhibitor protein. Thus, protease inhibitors are metalloproteins engaged in the binding of metal cations via side chains of glutamate and aspartate residues.
Boosting thermal stability is a desirable feature of proteins to explore their biotechnological applications. Amino acids, polyols, and salts are natural osmolytes that prohibit the thermal inactivation of proteins by stabilizing the thermally unfolded proteins42. Almost all the thermal stabilizers glycine, urea, CaCl2, glycerol, and sucrose promoted the stability of protease inhibitor at 50°C where glycerol (36 ± 1.1%) showed a significant increase in the stability of pI at the higher temperature.
Ionic as well as non-ionic detergents display a positive impact on protease inhibitor activity. 1% SDS enhanced the residual inhibitory activity by 93% whereas Triton-x-100 and Tween-80 also increased activity by 22% and 13% respectively. Thus SDS, Triton-x-100, and Tween-80 assist to stabilize the pI. Detergents are also responsible to solubilize proteins from membrane lipids and maintaining protein solubility in solutions. Thus, non-ionic detergents stabilize the activity of inhibitor protein and help to improve the catalytic performance of the inhibitor. Protease inhibitors along with surfactants can be additives to lysis buffer to solubilize membrane proteins by preventing unwanted proteolysis.
Whereas using detergents like SDS supports the efficiency of isolated inhibitors up to higher levels. SDS might expose the hydrophobic residues that bind with detergents by rearranging the conformation of peptide backbone43. Oxidizing agents DMSO and H2O2 enhanced the activity of the protease inhibitor when used between 1% to 5% concentrations. Similarly, the protease inhibitor retained 100% activity at 0.4% β-mercaptoethanol, but displayed elevation in the inhibitor activity by 97% at 0.4% sodium thioglycolate where both used as a reducing agent. Thus, the inhibitor protein is resistant to oxidation by oxidizing agents. Intramolecular disulfide bridges contribute to the functional stability of protease inhibitor in the presence of reducing agents. Examples include Kunitz type protease inhibitor, Peltophorum dubium protease inhibitor44, and Erythrina caffra trypsin inhibitor45.
Chemical modifier covalently binds to specific amino acid side chains of a protein to modify the biological activity. Inhibition of serine protease inhibitors, is associated with a specific active site residue within a loop closed by a disulfide bridge46. Chemical modifiers succinic anhydride, DEPC, and PMSF led to enhancement in the activity of isolated protease inhibitor at 5-25 mM concentrstion. This reverse effect indicates a greater extent of inhibition of trypsin, a serine protease, and validates the chemical nature of the inhibitor binding to serine, lysine, and histidine residues present in the active site of protease.
Positive results achieved for anti-inflammatory, anti-cancer, anti-diabetic, and anti-oxidant activities prove the therapeutic efficacy of the protease inhibitor. Flash chromatography and LC-HRMS analysis revealed the bioactive nature of the isolated inhibitor and identified Di-isobutylpthalate as one of the phytoconstituents contributing to therapeutic activities. Further, the potential effectivity of isolated inhibitor was analyzed via in silico therapeutic approach by performing docking studies. The inhibition of trypsin was confirmed by the formation docked complex between the trypsin receptor and Di-isobutylpthalate (fig.13). Here the analysis of docking studies carried out in such a way that, first the efficiency of our compound to react with insulin receptor was determined; followed by its reactivity with estrogen receptor and COX2 receptor. The data suggest our isolated protease inhibitor has binding efficiency against all three receptors highlighting its possible use in therapeutics. Docking studies, and in vitro antidiabetic, anticancer, and anti-inflammatory assays successfully showed that protease inhibitor isolated from Limonia acidissima holds great opportunity in therapeutics.
Conclusion
Protease inhibitor isolated and purified from Limonia acidissima showed an inhibitory effect on digestive serine protease Trypsin. Thus, the inhibitor is a proteinaceous molecule and exhibits promising biochemical characteristics such as remarkable stability at higher temperatures and a wide range of pH. Compatibility with detergents, oxidizing and reducing agents as well as metal ions reveal the commercial importance of pI. Chemical modification studies designated the presence of lysine, serine, or/and histidine in the reactive site of PI with a key role in the protease inhibition mechanism. Inhibitory activity against trypsin, chymotrypsin, pronase, and papain shows the potential of the protease inhibitor for developing an effective drug in the pharma sector. Inhibition of gram-negative and, to a lesser extent gram-positive bacteria prove the antibacterial activity of pI and its probable use as an antibacterial agent against pathogenic microbes. The inhibitor is a potent therapeutic molecule, thereby showing anti-oxidant, anti-cancer, anti-inflammatory, and anti-diabetic activities. The biochemical nature of the inhibitor was accessed after purification by flash chromatography and LC-HRMS which revealed the presence of the bioactive compound Di-isobutylpthalate. The docking studies confirmed the anti-cancer, anti-inflammatory, and anti-diabetic properties of Di-isobutylpthalate. Thus, the protease inhibitor from L. acidissima has industrial and therapeutic significance offering new opportunities for biotechnological perspectives.
Acknowledgement
We are thankful to Dr. D.Y. Patil Biotechnology and Bioinformatics Institute, Dr. D.Y. Patil Vidyapeeth, Pune for providing the laboratory facilities. We also like to acknowledge Mr. Ashpak Tamboli (Sahyadri College of Pharmacy, Solapur) and Mr. Javeed Manure (Shivaji University, Kolhapur). We heartily acknowledge Sophisticated Analytical Instrument Facility, Indian Institute of Technology, Bombay for providing facilities to perform LC-HRMS analysis.
Conflict of interest
The authors have no conflicts of interest regarding this investigation.
Funding Source
Funding is not taken for this work.
References
- Johnson S, Pellecchia M. Structure and fragment-based approaches to protease inhibition. Curr Top Med Chem., 2006; 6(4):317–29.
CrossRef - Bobbarala V, Rao Vadlapudi V, Chandrasekhar Naidu K. Antimicrobial Potentialities of Mangrove Plant Avicennia marina. J Pharm Res. 2009; 2(6):1019–21.
- Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002;102(12):4501–23.
CrossRef - Greene CM, McElvaney NG. Proteases and antiproteases in chronic neutrophilic lung disease-relevance to drug discovery. Br J Pharmacol. 2009;158(4):1048–58.
CrossRef - Rawlings ND, Tolle DP, Barrett AJ. Evolutionary families of peptidase inhibitors. Biochem J. 2004;378(3):705–16.
CrossRef - Bode W, Huber R. Structural basis of the endoproteinase-protein inhibitor interaction. Biochim Biophys Acta.2000;1477(1–2):241–52.
CrossRef - Kumar CG, Takagi H. Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv.1999;17(7):561–94.
CrossRef - Sabotič J, Kos J. Microbial and fungal protease inhibitors-current and potential applications. Appl Microbiol Biotechnol. 2012;93(4):1351–75.
CrossRef - Gettins PGW. The F-helix of serpins plays an essential, active role in the proteinase inhibition mechanism. FEBS Lett. 2002;523(1–3):2–6.
CrossRef - Johnson S, Pellecchia M. Structure and fragment-based approaches to protease inhibition. Curr Top Med Chem.2006;6(4):317–29.
CrossRef - Satheesh LS, Murugan K. Antimicrobial activity of protease inhibitor from leaves of Coccinia grandis (L.) Voigt. Indian J Exp Biol. 2011;49(5):366–74.
- Tamhane VA, Chougule NP, Giri AP, Dixit AR, Sainani MN, Gupta VS. In vivo and in vitro effect of Capsicum annum proteinase inhibitors on Helicoverpa armigera gut proteinases. Biochim Biophys Acta. 2005;1722(2):156–67.
CrossRef - Rachel, K Vijaya. Exploring the defensive role of a serine protease inhibitor from sapindus trifoliatus. Shodhganga. 2016.
- Carrillo-Montes JP, Arreguín-Espinosa R, Muñoz-Sánchez JL, Soriano-García M, et al. Purification and Biochemical Characterization of a Protease Inhibitor II Family from Jalapeño Pepper (Capsicum annuum L.). Adv Biosci Biotechnol.2014;5(7):661–8.
CrossRef - Green TR, Ryan CA. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972;175(4023):776–7.
CrossRef - Lallemand J, Bouché F, Desiron C, Stautemas J, Esteves F de L, Périlleux C, et al. Extracellular peptidase hunting for improvement of protein production in plant cells and roots. Front Plant Sci. 2015; 6:37.
CrossRef - Khare, C.P. Indian Medicinal Plants-An Illustrated Dictionary. 2007; 717-718.
CrossRef - Imada C. Enzyme inhibitors of marine microbial origin with pharmaceutical importance. Mar Biotechnol (NY). 2004;6(3):193–8.
CrossRef - Saima Y, Das AK, Sarkar KK, Sen AK, Sur P. An antitumor pectic polysaccharide from Feronia limonia. Int J Biol Macromol. 2000;27(5):333–5.
CrossRef - Pichare MM, Kachole MS. Protease inhibitors of Pigeon pea (Cajanuscajan) and its wild derivatives. Physiol Plant. 1996;98:845–851
CrossRef - M. Asif-Ullah, K.-S. Kim, Yu. Yeon Gyu. Purification and characterization of a serine protease from Cucumis trigonus Roxburghi Phytochemistry. 2006;67:870-875
CrossRef - Kunitz MJ. Crystalline soyabean trypsin inhibitor II. General properties. Gen Physiol. 1947;30:291–310.
CrossRef - Hemanth K. Manikyam, Sunil K. Joshi, Spandana Vakadi, Sandeep B.alavant Patil. Anticancer activity of terpenoid saponin extract of Psidium guajava on MCF-7 cancer cell line using DAPI and MTT assays. African Journal of Pharmacy and Pharmacology. 2021;15(12):206-211.
CrossRef - Dattatraya G. Raut, Sandeep B. Patil, Prafulla B. Choudhari, Vikas D. Kadu, Anjana S. Lawand, Mahesh G. Hublikar and Raghunath B. Bhosale. POCI3 Mediated Syntheses, Pharmacological Evaluation and Molecular Docking Studies of Some Novel Benzofused Thiazole Derivatives as a Potential Antioxidant and Anti-inflammatory Agents. Current Chemical Biology. 2020;14:58-68(11).
CrossRef - D. Moonmun, et al. Quantitative Phytochemical estimation, and Evaluation of antioxidant and antibacterial activity of methanol and ethanol extracts of Heliconiarostrata. Indian journal of pharmaceutical sciences. 2017;79(1): 79-90.
CrossRef - Kavitha Vijayaraghavan, S.Mohamed Ali. Studies on Phytochemical screening and antioxidant activity of chromolaena odorata and annona squamosa. International journal of innovative research in science Engineering and technology. 2013; 2(12): 7315-7321.
- A.A. Muchandi, A. S. Jadhav, S. B. Patil, S. A. Patil, N. B. Jadhav. Antioxidant and In Vitro Antidiabetic Activity of Methanol Extract of Piper cubeba L. International Research Journal of Pharmacy and Medical Sciences.2018:1638-18.
- Petra Webera, Matthias Hamburgera, Nina Schafrothb, Olivier Potterat . Flash chromatography on cartridges for the separation of plant extracts: Rules for the selection of chromatographic conditions and comparison with medium pressure liquid chromatography. Fitoterapia.2011; 82(2): 155-161.
CrossRef - Neupane P, Lamichhane J. Phytochemical profiling using HRLCMS and evaluation of antioxidant and antibacterial activities of Nepalese medicinal plants. Vegetos. 2020; 33(4):628-40.
CrossRef - Cascades, E. R. X-ray crystallography in Biovia Discovery studio. 2016.
- DeLano WL. The PyMOL Molecular Graphics System, Version 1.1. Delano Scientific, San Carlos. 2002.
- Morris a. L, MacArthur MW, Hutchinson EG, Thornton JM. Stereochemical quality of protein structure coordinates. Proteins Struct Funct Genet. 1992; 12:345–364.
CrossRef - Ward CW, Lawrence MC. Ligand-induced activation of the insulin receptor: A multi-step process involving structural changes in both the ligand and the receptor. BioEssays. 2009.
CrossRef - Wang, Z. Y. & Yin, L. Estrogen receptor alpha-36 (ER-α36): a new player in human breast cancer. Molecular and cellular endocrinology. 2015; 15: 1–34.
CrossRef - Belfiore A, Frasca F, Pandini G, et al. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009; 30(6):586-623.
CrossRef - Rouzer CA, Marnett LJ. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, Apr;50 Suppl(Suppl):S29-34.
CrossRef - Ilango K, Chitra V. Wound Healing and Anti-oxidant Activities of the Fruit Pulp of Limonia Acidissima Linn (Rutaceae) in Rats. Trop J Pharm Res., 2010; 9: 223-230.
CrossRef - Patra A, Misra SK, Chaudhury SK. Constituents of Limonia acidissima application of two-dimensional NMR spectroscopy in structure elucidation. J. Indian. Chem. So, 1988; 65: 205-208.
CrossRef - Bijina B et al. Process Biochemistry. 2011a, 46:2291.
CrossRef - Li de la Sierra et al. Dimeric Crystal Structure of a Bowman-Birk Protease Inhibitor from Pea Seeds. J Mol Biol. 1999, 285:1195.
CrossRef - Macedo MLR, Freire MGM. Invertebrate Survival Journal. 2011, 8:190.
- Jamal S, Poddar NK, Singh LR, Dar TA, Rishi V, Ahmad F. Relationship between functional activity and protein stability in the presence of all classes of stabilizing osmolytes. FEBS J. 2009; 276:6024–32.
CrossRef - P. Bressollier, F. Letourneau, M. Drdaci, et al., Purification and characterization of keratinolytic serine protease from Streptomyces albidoflavous. Appl. Environ. Microbiol. 1999;65: 2570–2576.
CrossRef - Macedo MLR, Freire MGM, Cabrini EC, Toyama MH, Novello JC, Marangoni SA. Trypsin inhibitor from Peltophorum dubium seeds active against pest protease and its affect on the survival of Anagasta kuehniella. Biochim Biophys Acta 2003;1621:170–82.
CrossRef - Lehle K, Konert U, Stern A, Propp A, Jaenicke R. Effect of disulfide bonds on the structure, function and stability of trypsin/tPA inhibitor from Erythrina caffra: site-directed mutagenesis, expression and physiochemical characterization. Nat Biotechnol 1996;14:476–80.
CrossRef - Mosolov VV, Valueva TA. Proteinase inhibitors and their function in plants: a review. Prikl Biokhim Mikrobiol 2005;41:261–82.
CrossRef







